Synthesis of Novel Hybrid Molecules from Precursors With Known Antiparasitic Activity
Abstract
:Introduction


Results and Discussion
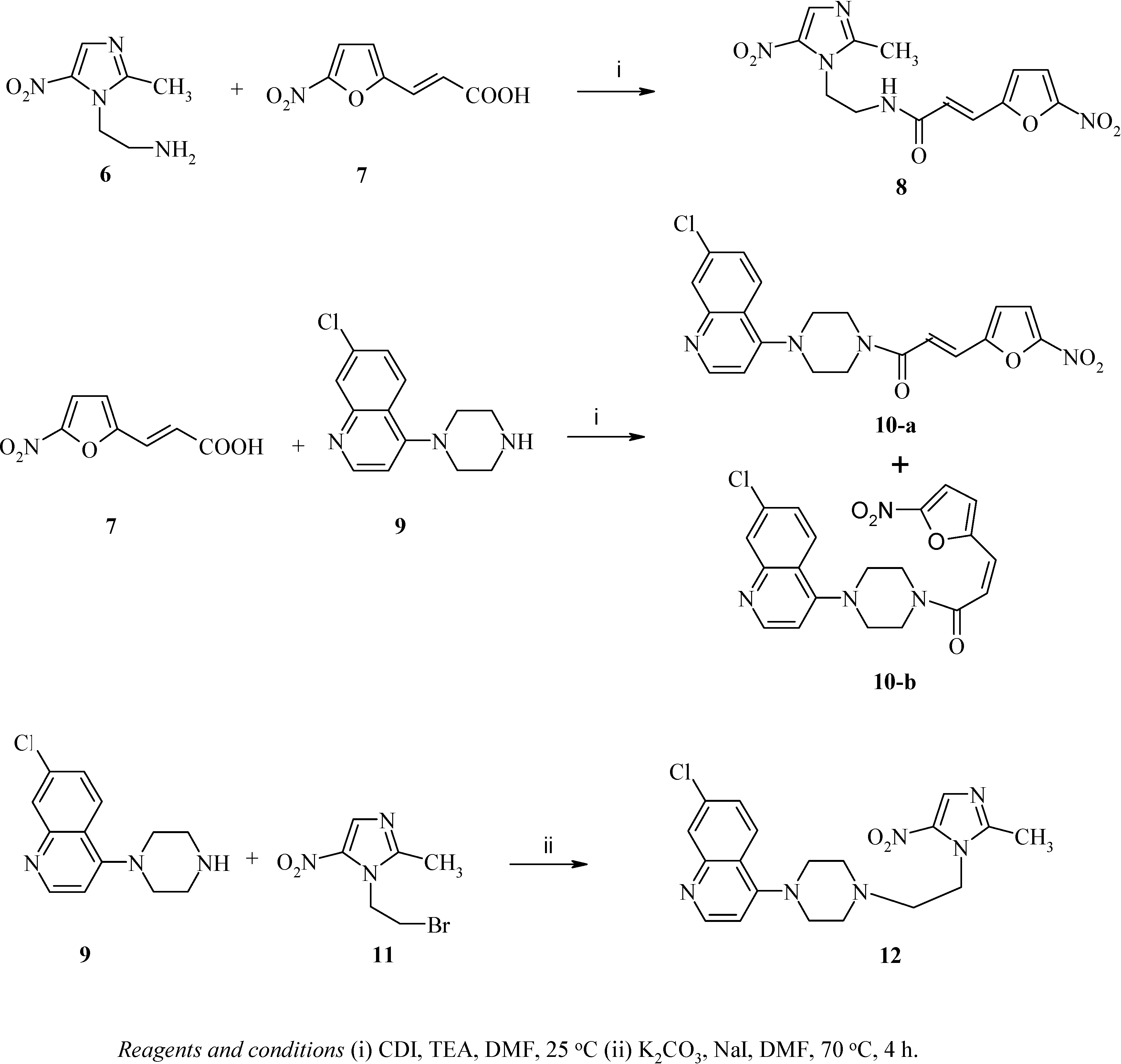
Chemistry
Antiamoebic and antigiardial activity
| Mean IC50 ± SD (n) | ||||
|---|---|---|---|---|
| (µM) | ||||
| Compound | Entamoeba histolytica | Giardia intestinalis | Hep-2 cells | Vero cells |
| 8 | 5.32 ± 0.21 | 5.56 ± 0.37 | 108.46 ± 8.02 | 90.55 ± 7.83 |
| 10-a | 139.96 ± 6.37 | 144.82 ± 5.37 | 574.03 ± 7.77 | 593.04 ± 8.71 |
| 12 | 0.96 ± 0.23 | 0.97 ± 0.32 | 604.52 ± 12.69 | 616.25 ± 16.94 |
| Metronidazole | 5.20 ± 1.03 | 5.70 ± 0.78 | 1460.86 ± 18.31 | 1495.13 ± 17.79 |
| 6 | 18.20 ± 2.28 | 18.50 ± 1.43 | ||
| 7 | 94.26 ± 9.76 | 88.42 ± 7.10 | ||
| 9 | 55.70± 5.00 | 67.51 ± 8.67 | ||
| Chloropip/furan | 22.53/22.53 ±1.07 | 25.75/25.75 ± 2.23 | ||
| Chloropip/metro | 1.04/1.04 ± 0.21 | 1.17/1.17 ± 0.26 | ||
| Furan/metroamine | 3.19/3.19 ± 0.21 | 3.77/3.77 ± 0.33 | ||
Experimental
General
1-(2-Bromoethyl)-2-methyl-5-nitro-1-imidazole (11)
7-Chloro-4-(piperazine-1-yl) quinoline (9)
3-(5-nitrofuran-2-yl)-N-[2-(5-nitroimidazol-1-yl)ethyl]acrylamide (8) and E-Z isomers of 1-[4-(7-chloroquinolin-4-yl)piperazin-1-yl)]-3-(5-nitrofuran-2-yl)propenone (10-a and 10-b)
Synthesis of 7-chloro-4-(4-[2-(5-nitroimidazol-1-yl)ethyl]piperazin-1-yl)quinoline (12)
Biological Activity
Test organisms
Antiamoebic and antigiardial activity
Cytotoxicity assay
Conclusions
Acknowledgements
References
- Bisi, A.; Rampa, A.; Budriesi, R.; Gobbi, S.; Belluti, F.; Ioan, P.; Valoti, E.; Chiarini, A.; Valenti, P. Cardiovascular hybrid drugs: New benzazepinone derivatives as bradycardic agents endowed with selective beta1-non-competitive antagonism. Bioorg. Med. Chem. 2003, 11, 1353–1361. [Google Scholar] [CrossRef]
- Schellenberg, D.; Abdulla, S.; Roper, C. Current issues for anti-malarial drugs to control P. falciparum malaria. Curr. Mol. Med. 2006, 6, 253–260. [Google Scholar] [CrossRef]
- Burgess, S.J.; Selzer, A.; Kelly, J.X.; Smilkstein, M.J.; Riscoe, M.; Peyton, D.H. A Chloroquine-like Molecule Designed to Reverse Resistance in Plasmodium falciparum. J. Med. Chem. 2006, 49, 5623–5625. [Google Scholar] [CrossRef]
- Greenwood, B.M.; Bojang, K.; Whitty, C.J.; Targett, G.A. Malaria. Lancet. 2005, 365, 1487–1498. [Google Scholar] [CrossRef]
- O’Neill, P.M.; Posner, G.H. A Medicinal Chemistry Perspective on Artemisinin and Related Endoperoxides. J. Med. Chem. 2004, 47, 2945–2964. [Google Scholar] [CrossRef]
- Ashley, E.A.; White, N.J. Artemisinin-based combinations. Curr. Opin. Infect. Dis. 2005, 18, 531–536. [Google Scholar] [CrossRef]
- Walsh, J.J.; Coughlan, D.; Heneghan, N.; Gaynor, C.; Bell, A. A novel artemisinin- quinine hybrid with potent antimalarial activity. Bioorg. Med. Chem. Lett. 2007, 17, 3599–3602. [Google Scholar] [CrossRef]
- Al-Zghoul, K.H.A.; Salih, K.S.M.; Ayoub, M.T.; Mubarak, M.S. A Convenient Procedure for the Synthesis of Substituted 4-Methylaminocoumarin. Heterocycles 2005, 65, 2937–2947. [Google Scholar] [CrossRef]
- Salih, K.S.M.; Al-Zghoul, K.H.A.; Mubarak, M.S.; Ayoub, M.T. Synthesis of Coumarinsulfonamides With Potential Pharmacological Interest. J. Saudi Chem. Soc. 2006, 9, 623–630. [Google Scholar]
- Salih, K.S.M.; Ayoub, M.T.; Saadeh, H.A.; Al-Masoudi, N.A.; Mubarak, M.S. Synthesis, Characterization, And Biological Activities Of New Benzofuran Derivative. Hetrocycles 2007, 71, 1577–1587. [Google Scholar] [CrossRef]
- Al-Soud, Y.A.; Al-Sa’doni, H.H.; Amajaour, H.A.S.; Salih, K.S.M.; Mubarak, M.S.; Al-Masoudi, N.A.; Jaber, I.H.Z. Synthesis, Characterization and anti-HIV and Antitumor Activities of New Coumarin Derivatives. Naturforsch. B 2008, 63, 83–89. [Google Scholar]
- Knight, R. The chemotherapy of amebiasis. J. Antimicrob. Chemother. 1980, 6, 577–593. [Google Scholar] [CrossRef]
- Majewska, A.C.; Kasprzak, W.; De Jonckheere, J.F.; Kaczmarek, E. Heterogeneity in the sensitivity of stocks and clones of Giardia to metronidazole and ornidazole. Trans. R. Soc. Trop. Med. Hyg. 1991, 85, 67–69. [Google Scholar] [CrossRef]
- Farbey, M.D.; Reynoldson, J.A.; Thompson, R.C. In vitro drug susceptibility of 29 isolates of Giardia duodenalis from humans as assessed by an adhesion assay. Int. J. Parasitol. 1995, 25, 593–599. [Google Scholar] [CrossRef]
- Johnson, P.J. Metronidazole and drug resistance. Parasitol. Today 1993, 9, 183–186. [Google Scholar] [CrossRef]
- Orozco, E.; Lopez, C.; Gomez, C.; Perez, D.G.; Marchat, L.; Banuelos, C.; Delgadillo, D.M. Multidrug resistance in the protozoan parasite Entamoeba histolytica. Parasitol. Int. 2002, 51, 353–359. [Google Scholar] [CrossRef]
- Samarawickrema, N.A.; Brown, D.M.; Upcroft, J.A.; Thammapalerd, N.P. Involvement of superoxide dismutase and pyruvate:ferredoxin oxidoreductase in mechanisms of metronidazole resistance in Entamoeba histolytica. J. Antimicrob. Chemother. 1997, 40, 833–840. [Google Scholar] [CrossRef]
- Wassmann, C.; Hellberg, A.; Tannich, E.; Bruchhaus, I. Metronidazole resistance in the protozoan parasite Entamoeba histolytica is associated with increased expression of iron-containing superoxide dismutase and peroxiredoxin and decreased expression of ferredoxin 1 and flavin reductase. J. Biol. Chem. 1999, 274, 26051–26056. [Google Scholar] [CrossRef]
- Pozas, R.; Carballo, J.; Castrob, C.; Rubio, J. Synthesis and in vitro antitrypanosomal activity of novel Nifurtimox analogues. Bioorg. Med. Chem. Lett. 2005, 15, 1417–1421. [Google Scholar] [CrossRef]
- Benkli, K.; Karaburun, A.; Du-Karaburun, N.; Demi, E. Synthesis and antimicrobial activities of some new nitroimidazole derivatives. Arch. Pharm. Res. 2003, 26, 773–777. [Google Scholar] [CrossRef]
- Musonda, C.C.; Little, S.; Yardleyb, V.; Chibalea, K. Application of multicomponent reactions to antimalarial drug discovery. Part 3: Discovery of aminoxazole 4- aminoquinolines with potent antiplasmodial activity in vitro. Bioorg. Med. Chem. Lett. 2007, 17, 4733–4736. [Google Scholar] [CrossRef]
- Clark, C.G.; Diamond, L.S. Methods for cultivation of luminal parasitic protists of clinical importance. Clin. Microbiol. Rev. 2002, 15, 329–341. [Google Scholar] [CrossRef]
- Neal, R.A. Antiamoebic activity of drugs given singly and in combination against axenically grown Entamoeba histolytica. Arch. Invest. Med. 1978, 9, 387–392. [Google Scholar]
- Aley, S.B.; Zimmerman, M.; Hetsko, M.; Selsted, M.E.; Gillin, F.D. Killing of Giardia lamblia by cryptdins and cationic neutrophil peptides. Infect. Immun. 1994, 62, 5397–5403. [Google Scholar]
- Sample Availability: Samples of the compounds 8-12 are available from the authors.
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Saadeh, H.A.; Mosleh, I.M.; Mubarak, M.S. Synthesis of Novel Hybrid Molecules from Precursors With Known Antiparasitic Activity. Molecules 2009, 14, 1483-1494. https://doi.org/10.3390/molecules14041483
Saadeh HA, Mosleh IM, Mubarak MS. Synthesis of Novel Hybrid Molecules from Precursors With Known Antiparasitic Activity. Molecules. 2009; 14(4):1483-1494. https://doi.org/10.3390/molecules14041483
Chicago/Turabian StyleSaadeh, Haythem A., Ibrahim M. Mosleh, and Mohammad S. Mubarak. 2009. "Synthesis of Novel Hybrid Molecules from Precursors With Known Antiparasitic Activity" Molecules 14, no. 4: 1483-1494. https://doi.org/10.3390/molecules14041483
APA StyleSaadeh, H. A., Mosleh, I. M., & Mubarak, M. S. (2009). Synthesis of Novel Hybrid Molecules from Precursors With Known Antiparasitic Activity. Molecules, 14(4), 1483-1494. https://doi.org/10.3390/molecules14041483





