Total Antioxidant Capacity of Some Commercial Fruit Juices: Electrochemical and Spectrophotometrical Approaches
Abstract
:1. Introduction
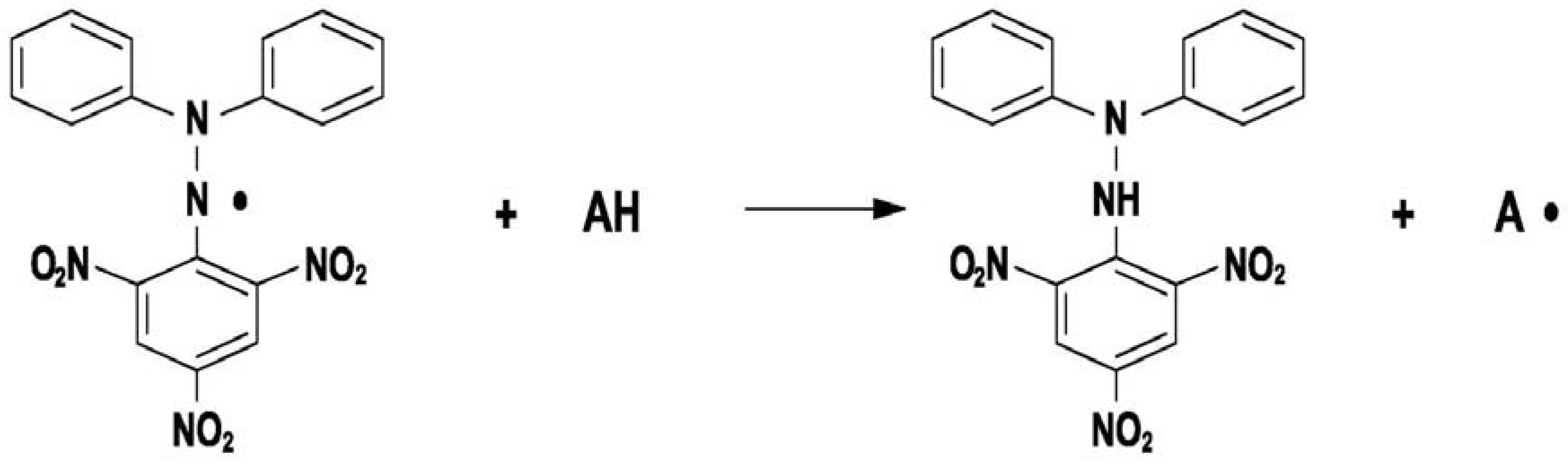
2. Results and Discussion
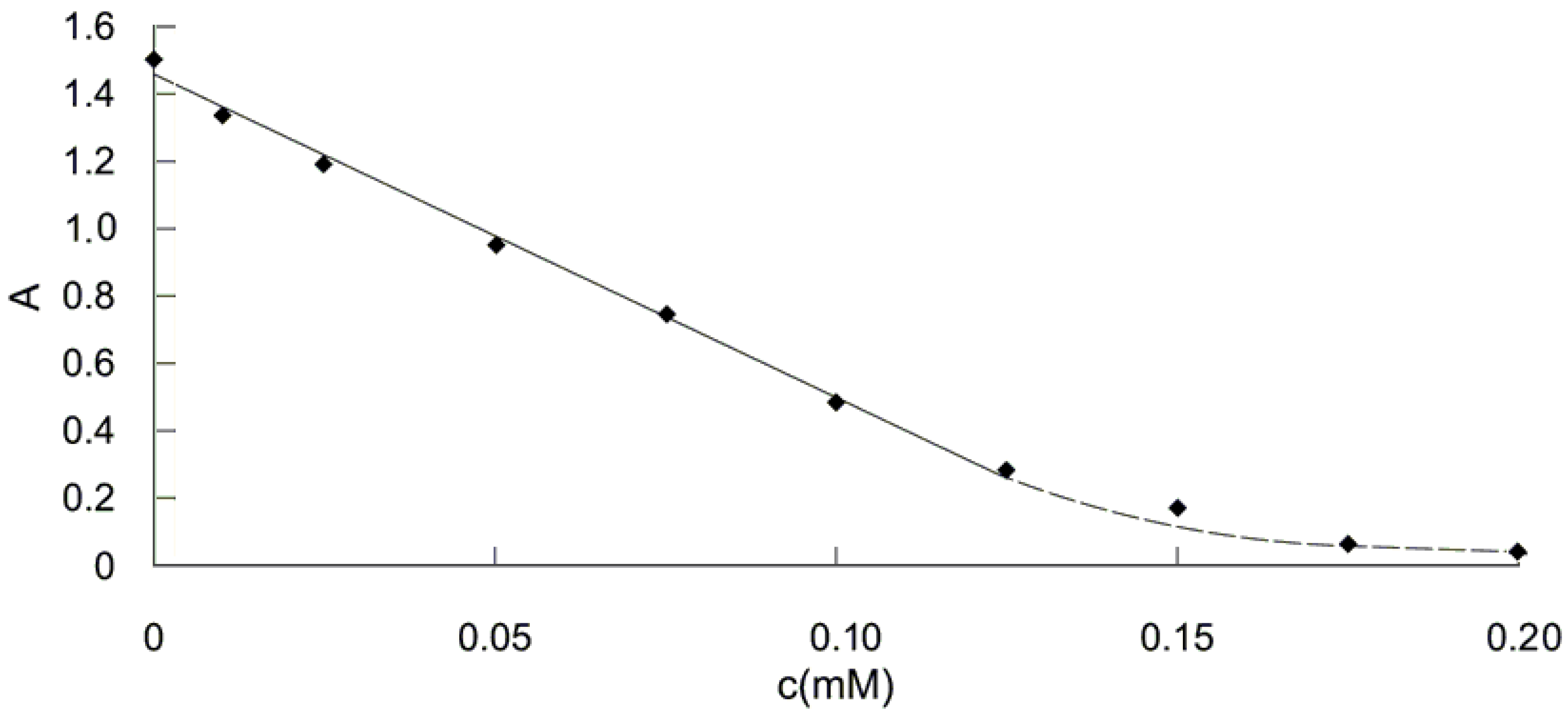
2.1. Spectrophotometry: the study of DPPH· absorbance decrease in the presence of antioxidants
2.2. Electrochemical method
2.2.1. Study of the reversibility of the DPPH·/DPPH redox couple

2.2.2. Study of the influence of the potential difference, ΔE, applied between the two electrodes on the analytical signal
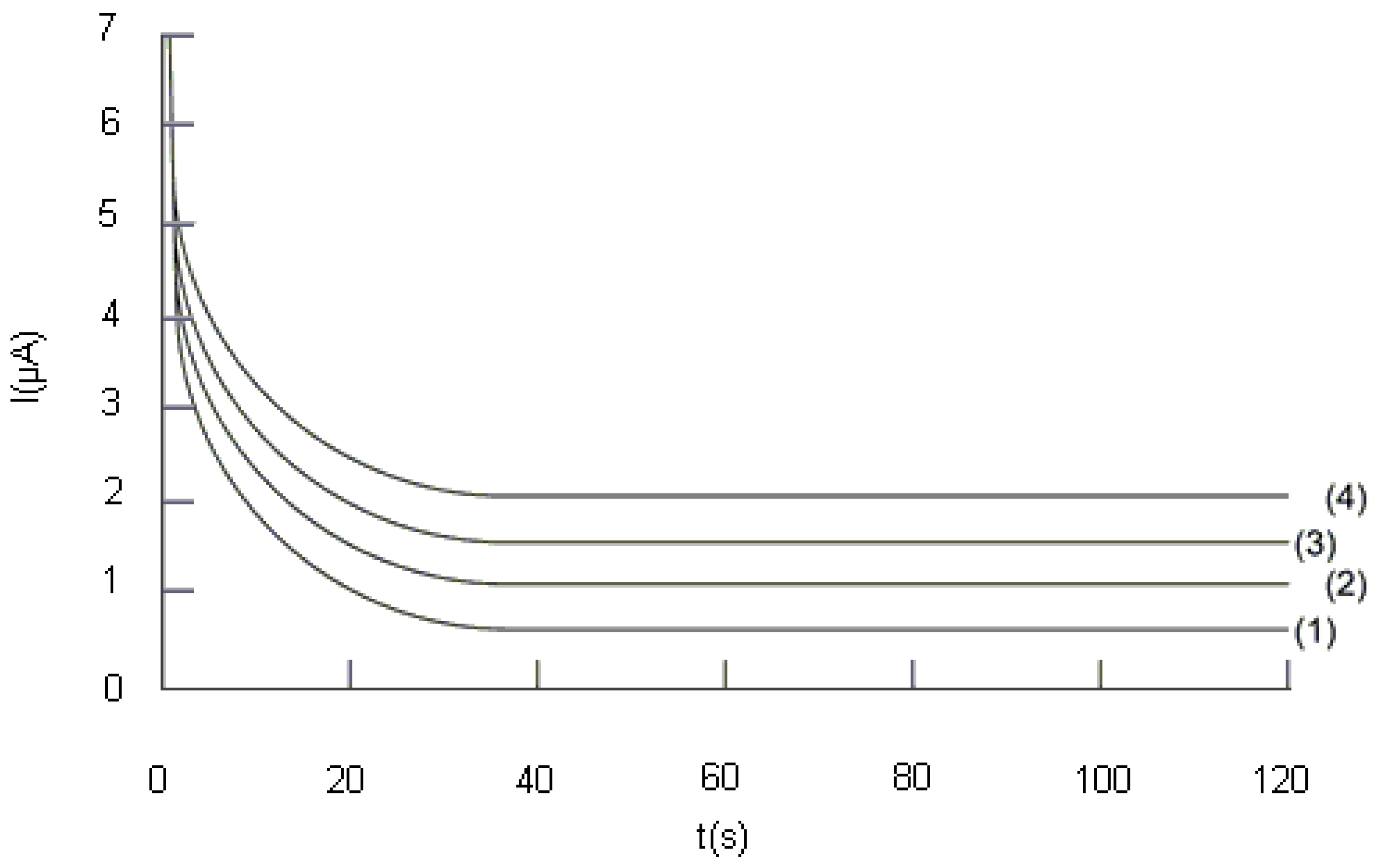
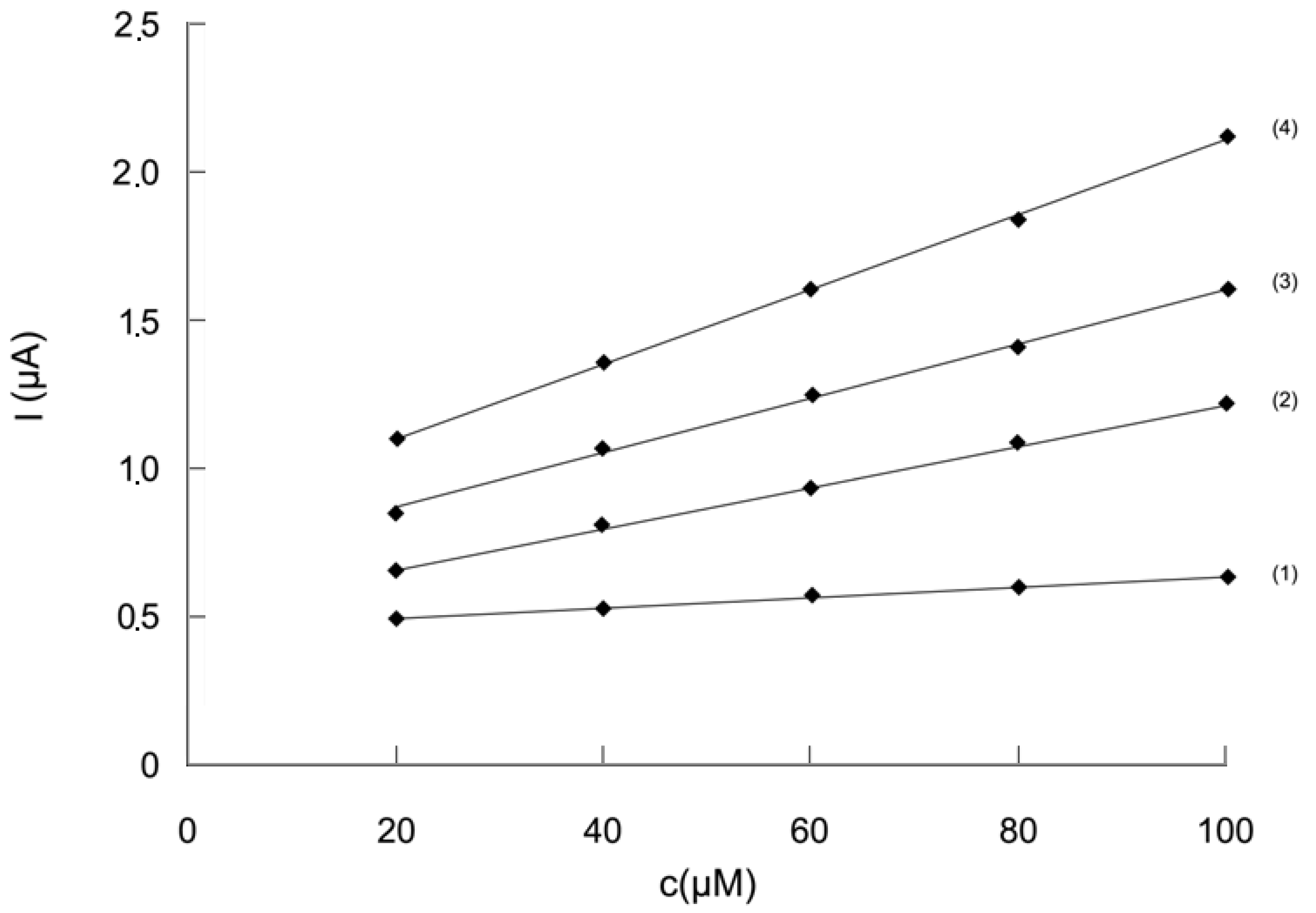
2.2.3. Study of the influence of the Trolox® concentration on the analytical signal
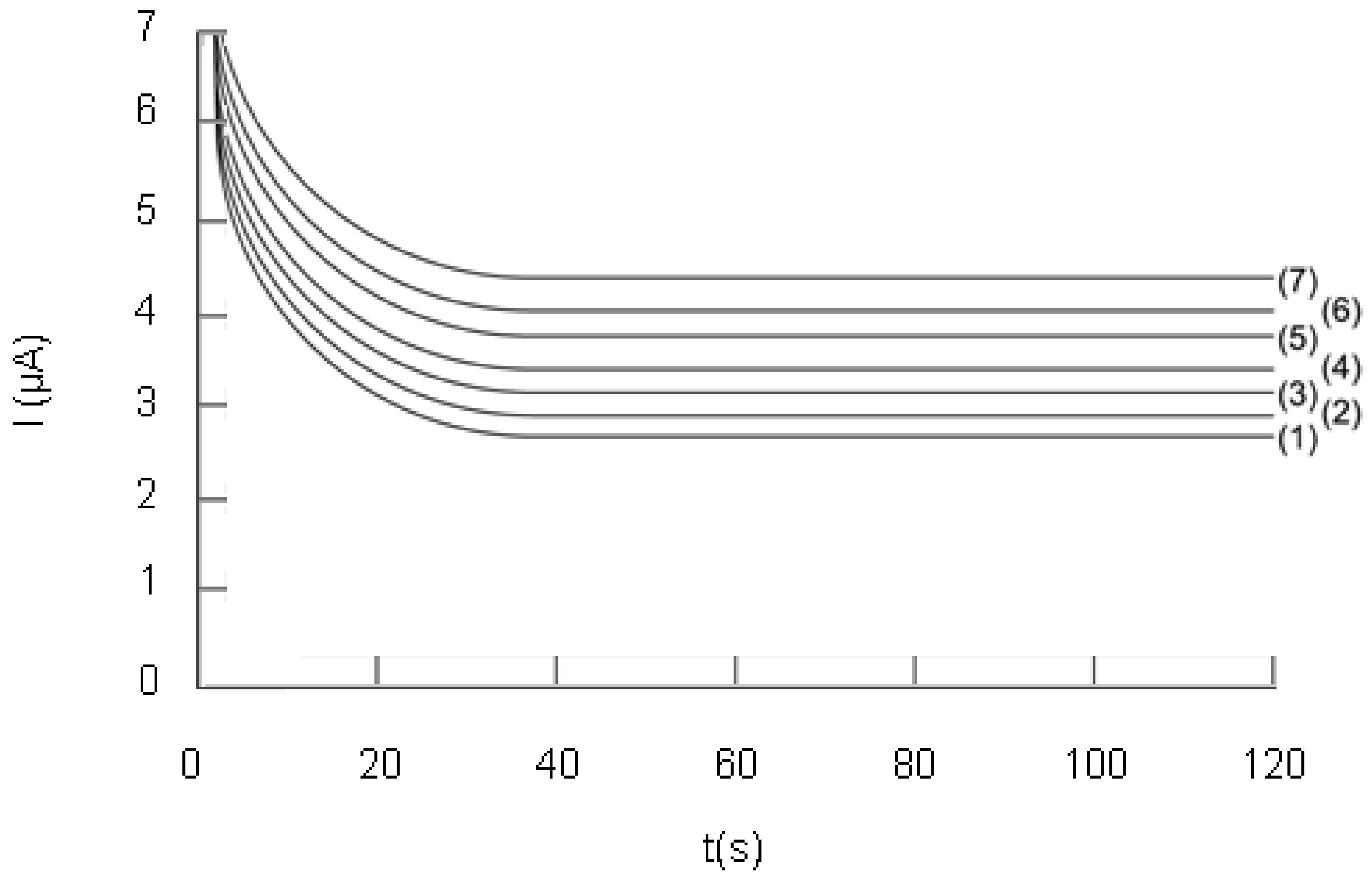
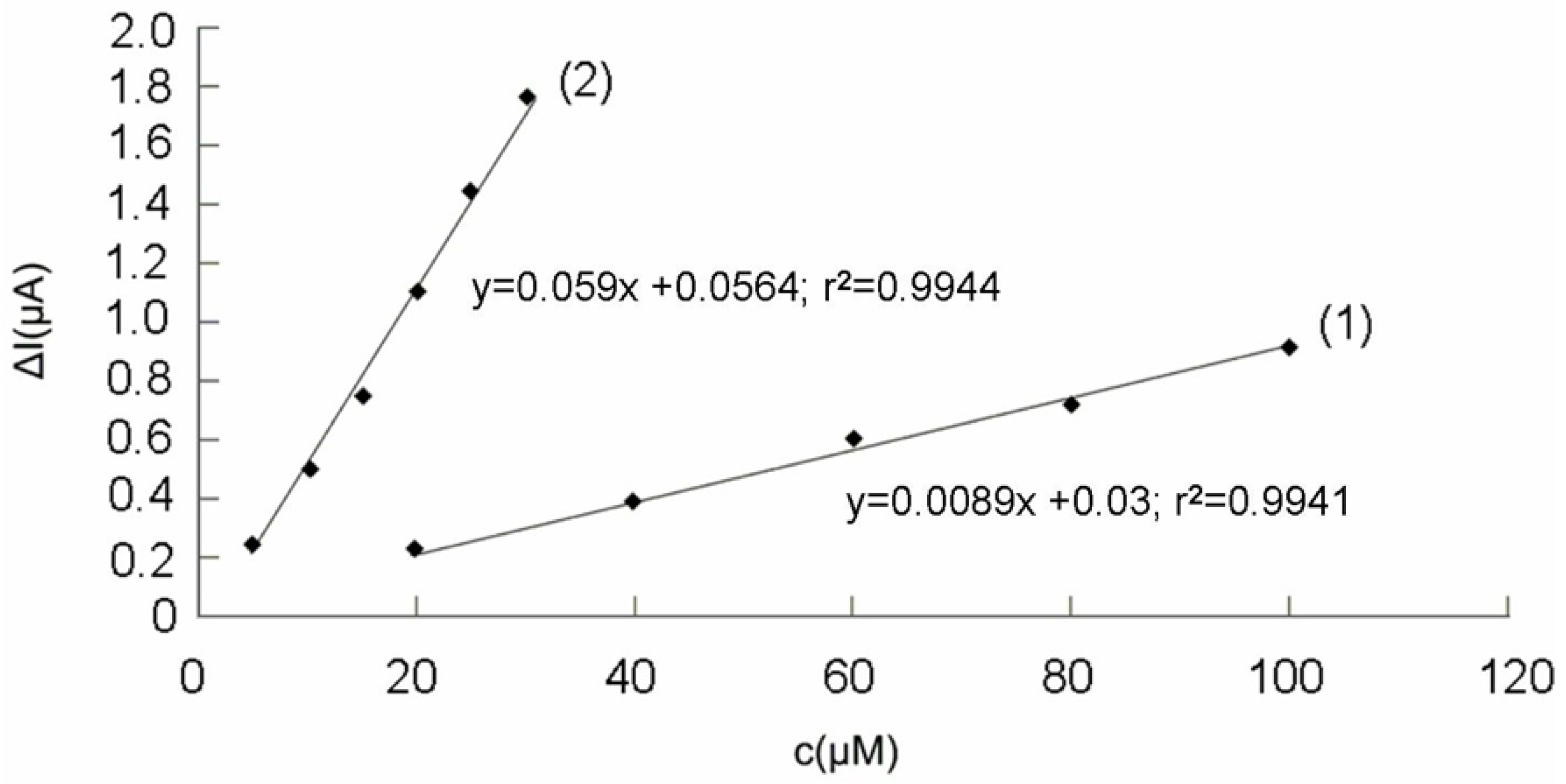
2.3. Analysis of real samples
| Spectrophotometry | Biamperometry | Literature Data | |||
|---|---|---|---|---|---|
| Product | TEAC value (spectrophotometry) mM Trolox | Absorbance value | TEAC value (biamperometry) mM Trolox | ΔI value (biamperometry) µA | TEAC values (mmoles Trolox/kg or mM Trolox) |
| Orange juice | 9.25 | 0.89 | 9.07 | 0.64 | 8.74 mmoles/kg fresh weight orange by ABTS+. method [13] |
| 8.88 mM sweet oranges extract* by DMPD**/FeCl3 method [32] | |||||
| Lemon juice | 6.5 | 0.86 | 6.25 | 0.68 | 7.0 mmoles/ kg fruit (limes) by FRAP*** [7] 7.3 mmoles Kg fruit (limes) by FRAP [33] |
| Fanta orange | 0.71 | 1.03 | 0.70 | 0.69 | - |
| Cappy grapefruit | 0.064 | 1.15 | 0.0615 | 0.67 | - |
| Frutti fresh Tutti Frutti | 4.0 | 0.732 | 4.32 | 0.51 | - |
| Fanta lemon | 1.54 | 0.870 | 1.45 | 0.74 | 2.20 mM lemon soft beverage by ABTS+. method [13] |
| Prigat orange | 2.40 | 0.790 | 2.46 | 0.67 | 3.02 mM orange soft beverage by ABTS+. method [13] |
| Prigat peach | 1.24 | 0.795 | 1.20 | 0.63 | 2.51 mM peach soft beverage by ABTS+. method [13] |
| Product | Measured TEAC value (mM Trolox) after 1st addition | Recovery % after 1st addition | Measured TEAC value (mM Trolox) after 2nd addition | Recovery % after 2nd addition |
|---|---|---|---|---|
| Prigat peach | 1.73 | 101.76 | 2.16 | 98.18 |
| Prigat orange | 3.50 | 101.15 | 4.45 | 99.77 |
| Fanta lemon | 2.0 | 102.56 | 2.48 | 101.22 |
| Fanta orange | 0.97 | 102.10 | 1.21 | 100.83 |
| Cappy grapefruit | 0.0875 | 101.15 | 0.11 | 98.65 |
| Frutti freshTutti Frutti | 6.16 | 97.47 | 8.14 | 97.83 |
| Lemon juice | 8.7 | 99.42 | 11.2 | 99.55 |
| Orange juice | 13.05 | 101.80 | 16.80 | 101.39 |
3. Conclusions
4. Experimental
4.1. Reagents and solutions
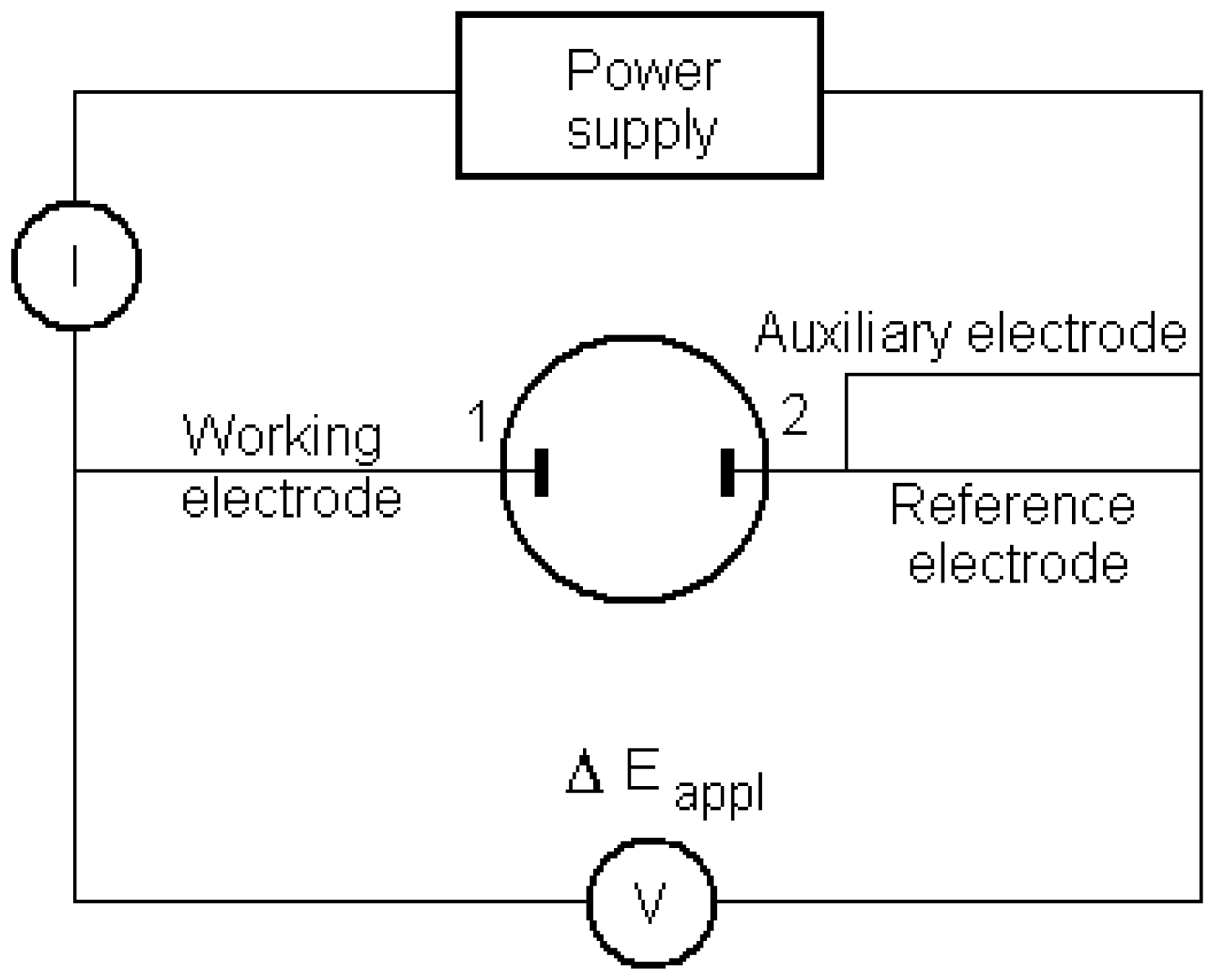
4.2. Instrumentation
Acknowledgements
References
- Ye, Z.; Song, H. Antioxidant vitamins intake and the risk of coronary heart disease: meta-analysis of cohort studies. Eur. J. Cardiovasc. Prev. Rehabil. 2008, 16, 26–34. [Google Scholar]
- Trible, D.L. Antioxidant consumption and risk of coronary heart disease: emphasis on vitamin C, vitamin E and β-carotene: a statement for healthcare professionals from the American Heart Association. Circulation 1999, 99, 591–595. [Google Scholar] [CrossRef]
- Jalil, A.M.M.; Ismail, A. Polyphenols in cocoa and cocoa products: is there a link between antioxidant properties and health? Molecules 2008, 13, 2190–2219. [Google Scholar] [CrossRef]
- Jipa, S.; Zaharescu, T.; Gorghiu, L.M.; Dumitrescu, C.; Setnescu, R. Kinetic aspects concerning the thermal oxidation of LDPE stabilized with vitamins. Rev. Chim. (Bucuresti) 2004, 55, 514–518. [Google Scholar]
- Jipa, S.; Zaharescu, T.; Gigante, B.; Santos, C.; Setnescu, R.; Setnescu, T.; Dumitru, M.; Gorghiu, L.M.; Kappel, W.; Mihalcea, I. Chemiluminescence investigation of thermo-oxidative degradation of polyethylenes stabilized with fullerenes. Polymer Degrad. Stabil. 2003, 80, 209–216. [Google Scholar] [CrossRef]
- Gorghiu, L.M.; Jipa, S.; Zaharescu, T.; Setnescu, R.; Mihalcea, I. The effect of metals on thermal degradation of polyethylenes. Polymer Degrad. Stabil. 2004, 84, 7–11. [Google Scholar] [CrossRef]
- Blomhoff, R. Dietary antioxidants and cardiovasular disease. Curr.Opin. Lipidol. 2005, 16, 47–54. [Google Scholar] [CrossRef]
- Bourgeois, C.F. Antioxidant vitamins and health: cardiovascular disease, cancer, cataracts, and aging; HNB Publishing: New York, USA, 2003. [Google Scholar]
- Gey, K.F.; Puska, P.; Jordan, P.; Moser, U.K. Total antioxidant capacity of plant foods. Inverse correlation between plasma vitamin E and mortality from ischemic heart disease in cross-cultural epidemiology. Am. J. Clin. Nutr. 1991, 53, 326S–334S. [Google Scholar]
- La Vecchia, C.; Altieri, A.; Tavani, A. Vegetables, fruit, antioxidants and cancer: a review of Italian studies. Eur. J. Nutr. 2001, 40, 261–267. [Google Scholar] [CrossRef]
- Schaffer, S; Schmidt-Schillig, S; Muller, W.E.; Eckert, G.P. Antioxidant properties of mediterranean food plant extract: geographical differences. J. Physiol. Pharmacol. 2007, 56, 115–124. [Google Scholar]
- Yizhong, C.; Luo, Q.; Mei, S.; Corke, H. Antioxidant activity and phenolic compounds of 112 traditional Chinese medicinal plants associated with anticancer. Life Sci. 2004, 74, 2157–2184. [Google Scholar] [CrossRef]
- Pellegrini, N.; Serafini, M.; Colombi, B.; Del Rio, D.; Salvatore, S.; Bianchi, M.; Brighenti, F. Total antioxidant capacity of plant foods, beverages and oils consumed in Italy assessed by three different in vitro assays. J. Nutr. 2003, 133, 2812–2819. [Google Scholar]
- Lam, R.Y.Y.; Woo, A.Y.H.; Leung, P.S.; Cheng, C.H.K. Antioxidant actions of phenolic compounds found in dietary plants on low-density lipoprotein and erythrocytes in vitro. J. Am. Coll. Nutr. 2007, 26, 233–242. [Google Scholar] [CrossRef]
- Halvorsen, B.L.; Calrsen, M.H.; Philips, K.M.; Bohn, S.K.; Holte, K.; Jacobs Jr, D.R.; Blomhoff, R. Content of redox-active compounds (ie, antioxidants) in foods consumed in the United States. Am. J. Clin. Nutr. 2006, 84, 95–135. [Google Scholar]
- Sakkiadi, A.V.; Stavrakakis, M.N.; Haroutounian, S.A. Direct HPLC assay of five biologically interesting phenolic antioxidants in varietal greek red wines. Lebensm.-Wiss. u.-Technol. 2001, 34, 410–413. [Google Scholar] [CrossRef]
- Tung, Y.T.; Wu, J.H.; Kuo, Y.H.; Chang, S.T. Antioxidant activities of natural phenolic compounds from Acacia confusa bark. Bioresour. Technol. 2007, 98, 1120–1123. [Google Scholar] [CrossRef]
- Jayaprakasha, G.K.; Girennavar, B.; S. Patil, B.S. Radical scavenging activities of Rio Red grapefruits and Sour orange fruit extracts in different in vitro model systems. Bioresour. Technol. 2008, 99, 4484–4494. [Google Scholar] [CrossRef]
- Thaipong, K.; Boonprakob, U.; Crosby, K.; Zevallos-Cisneros, L.; Byrne, D.H. Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating antioxidant activity from guava fruit extracts. J. Food Compos. Anal. 2006, 19, 669–675. [Google Scholar] [CrossRef]
- Rivero-Perez, M.D.; Muniz, P.; Gonzalez-Sanjose, M.L. Antioxidant profile of red wines evaluated by total antioxidant capacity, scavenger activity, and biomarkers of oxidative stress methodologies. J. Agric. Food Chem. 2007, 55, 5476–5483. [Google Scholar] [CrossRef]
- Molyneux, P. The use of the stable free radical diphenylpicrylhydrazyl (DPPH) for estimating antioxidant. Songklanakarin J. Sci. Technol. 2004, 26, 211–219. [Google Scholar]
- Gil, M.I.; Tomas-Barberan, F.A.; Hess-Pierce, B.; Kader, A.A. Antioxidant capacities, phenolic compounds, carotenoids, and vitamin C contents of nectarine, peach, and plum cultivars from California. J. Agric. Food Chem. 2002, 50, 4976–4982. [Google Scholar] [CrossRef]
- Ruiz, M.A.; Garcia-Moreno, E.; Barbas, E.; Pingarron, J.M. Determination of phenolic antioxidants by HPLC with amperometric detection at a nickel phthalocyanine polymer modified electrode. Electroanalysis 1999, 11, 470–474. [Google Scholar] [CrossRef]
- Imbayashi, S.; Kong, Y.T.; Watanabe, M. Amperometric biosensor for polyphenol based on horseradish peroxidase immobilized on gold electrodes. Electroanalysis 2001, 13, 408–412. [Google Scholar] [CrossRef]
- Blasco, A.J.; Gonzalez, M.J.; Escarpa, A. Electrochemical approach for discriminating and measuring predominant flavonoids and phenolic acids using differential pulse voltammetry: towards an electrochemical index of natural antioxidant. Anal. Chim. Acta. 2004, 511, 71–81. [Google Scholar] [CrossRef]
- Milardovic, S.; Ivekovic, D.; Rumeniak, V.; Grabaric, B.S. Use of DPPH·/DPPH redox couple for biamperometric determination of antioxidant activity. Electroanalysis 2005, 17, 1847–1853. [Google Scholar] [CrossRef]
- Milardovic, S.; Kerekovic, I.; Rumenjak, V. A flow injection biamperometric method for determination of total antioxidant capacity of alcoholic beverages using bienzymatically produced ABTS +. Food Chem. 2007, 105, 1688–1694. [Google Scholar] [CrossRef]
- Zielinska, D.; Szawara-Nowak, D.; Zielinski, H. Comparison of spectrophotometric and electrochemical methods for the evaluation of the antioxidant capacity of buckwheat products after hydrothermal treatment. J. Agric. Food Chem. 2007, 55, 6124–6131. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. Lebensm.-Wiss. u.-Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Milardovic, S.; Ivekovic, D; Grabaric, B.S. A novel amperometric method for antioxidant activity determination using DPPH free radical. Bioelectrochemistry 2006, 68, 175–180. [Google Scholar] [CrossRef]
- Nenadis, N.; Tsimidou, M. Observations on the estimation of scavenging activity of phenolic compounds using rapid 1,1-diphenyl-2-picrylhydrazyl (DPPH*) tests. J. Am. Oil Chem. Soc. 2002, 79, 1191–1195. [Google Scholar] [CrossRef]
- Asghar, M.N.; Khan, I.U.; Arshad, M.N.; Sherin, L. Evaluation of antioxidant activity using an improved DMPD radical cation decolorization assay. Acta Chim. Slov. 2007, 54, 295–300. [Google Scholar]
- Halvorsen, B.L.; Holte, K; Myhrstad, M.C.W.; Barikmo, I.; Hvattum, E.; Remberg, S.F.; Wold, A.B.; Haffner, K.; Baugerod, H.; Andersen, L.F.; Moskaug, J.O.; Jacobs, D.R.; Blomhoff, R. A systematic screening of total antioxidants in dietary plants. J. Nutr. 2002, 132, 461–471. [Google Scholar]
- Sample Availability: Samples are available from the authors.
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Pisoschi, A.M.; Cheregi, M.C.; Danet, A.F. Total Antioxidant Capacity of Some Commercial Fruit Juices: Electrochemical and Spectrophotometrical Approaches. Molecules 2009, 14, 480-493. https://doi.org/10.3390/molecules14010480
Pisoschi AM, Cheregi MC, Danet AF. Total Antioxidant Capacity of Some Commercial Fruit Juices: Electrochemical and Spectrophotometrical Approaches. Molecules. 2009; 14(1):480-493. https://doi.org/10.3390/molecules14010480
Chicago/Turabian StylePisoschi, Aurelia Magdalena, Mihaela Carmen Cheregi, and Andrei Florin Danet. 2009. "Total Antioxidant Capacity of Some Commercial Fruit Juices: Electrochemical and Spectrophotometrical Approaches" Molecules 14, no. 1: 480-493. https://doi.org/10.3390/molecules14010480




