Nitroalkanes as Central Reagents in the Synthesis of Spiroketals
Abstract
:1. Introduction
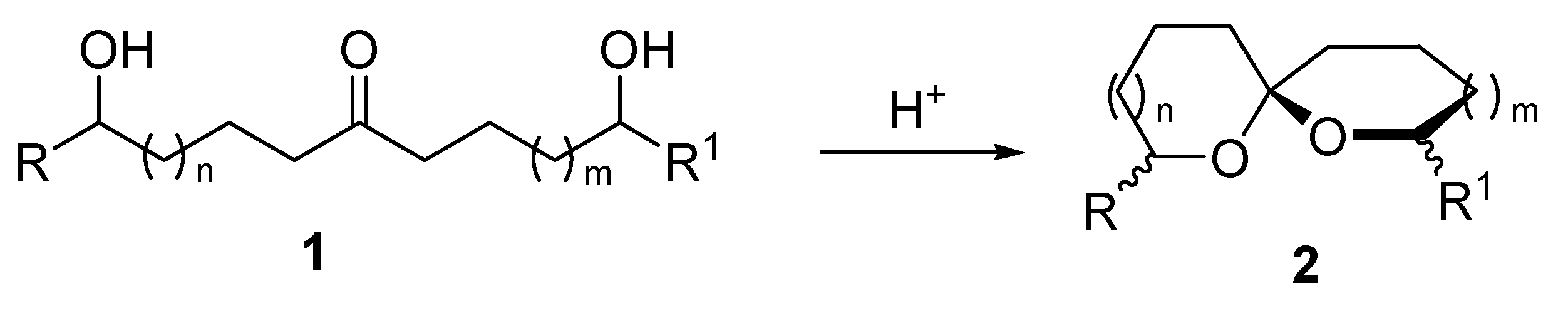
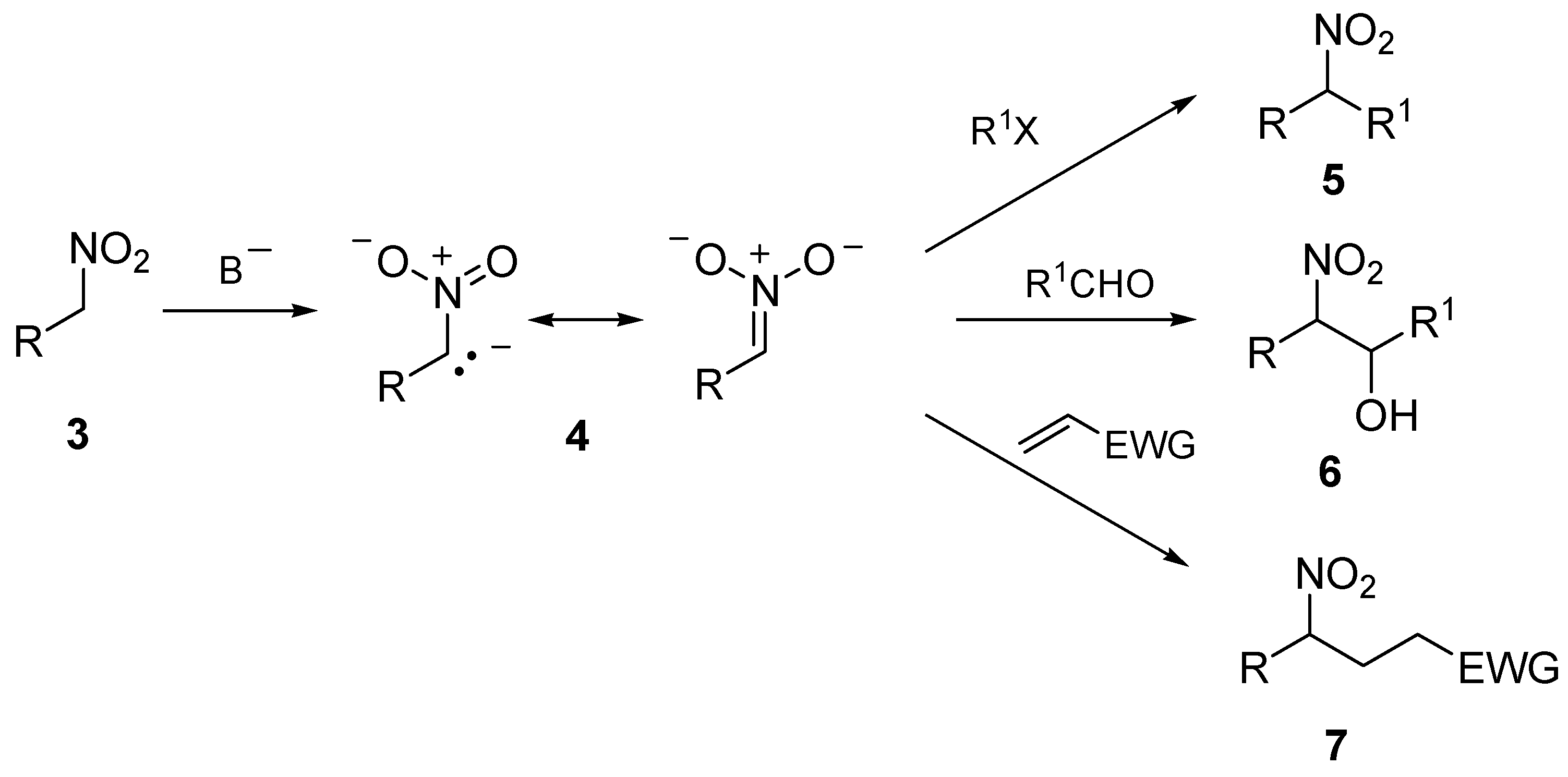

2. 1,6-Dioxaspiro[4.4]nonanes
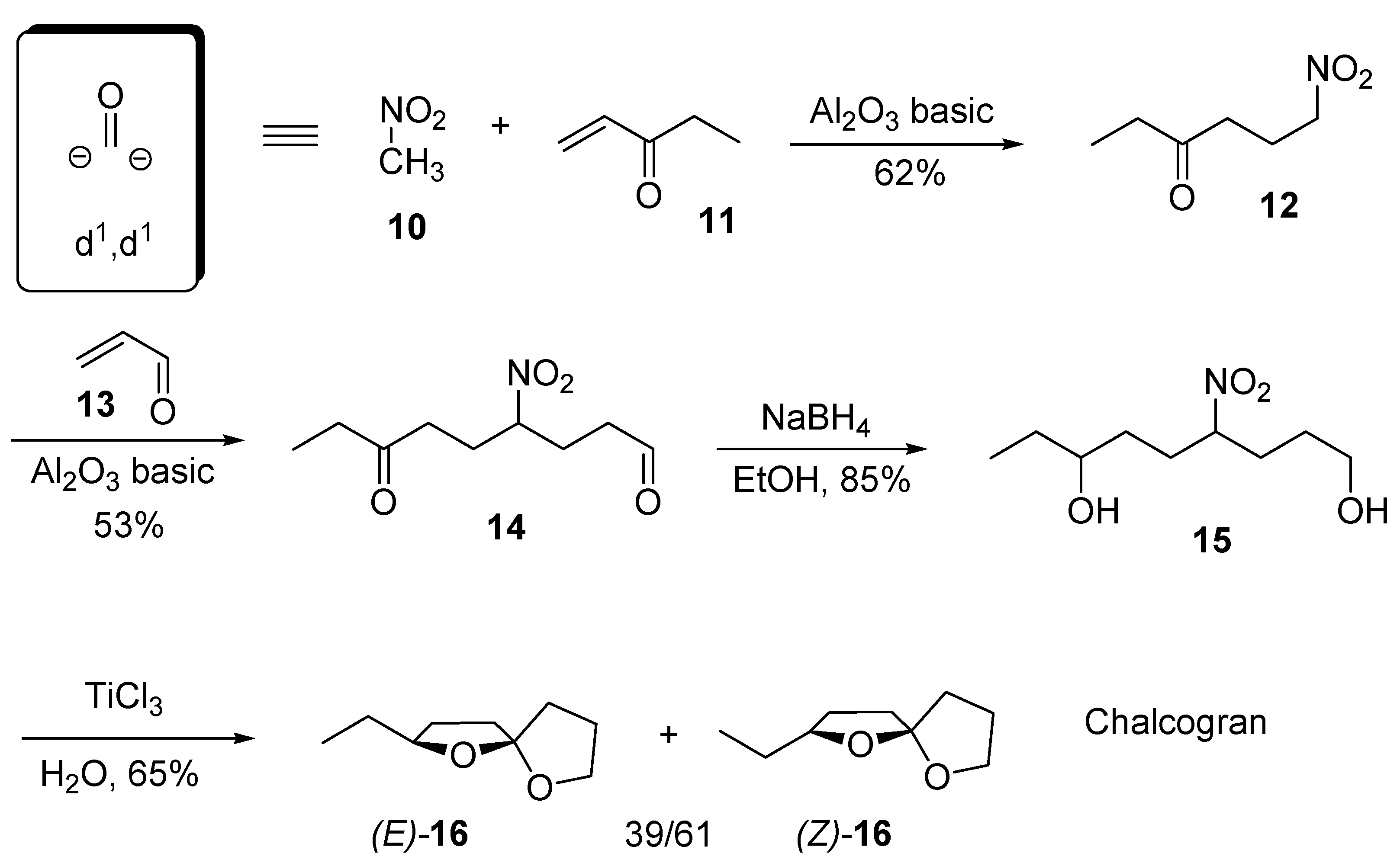

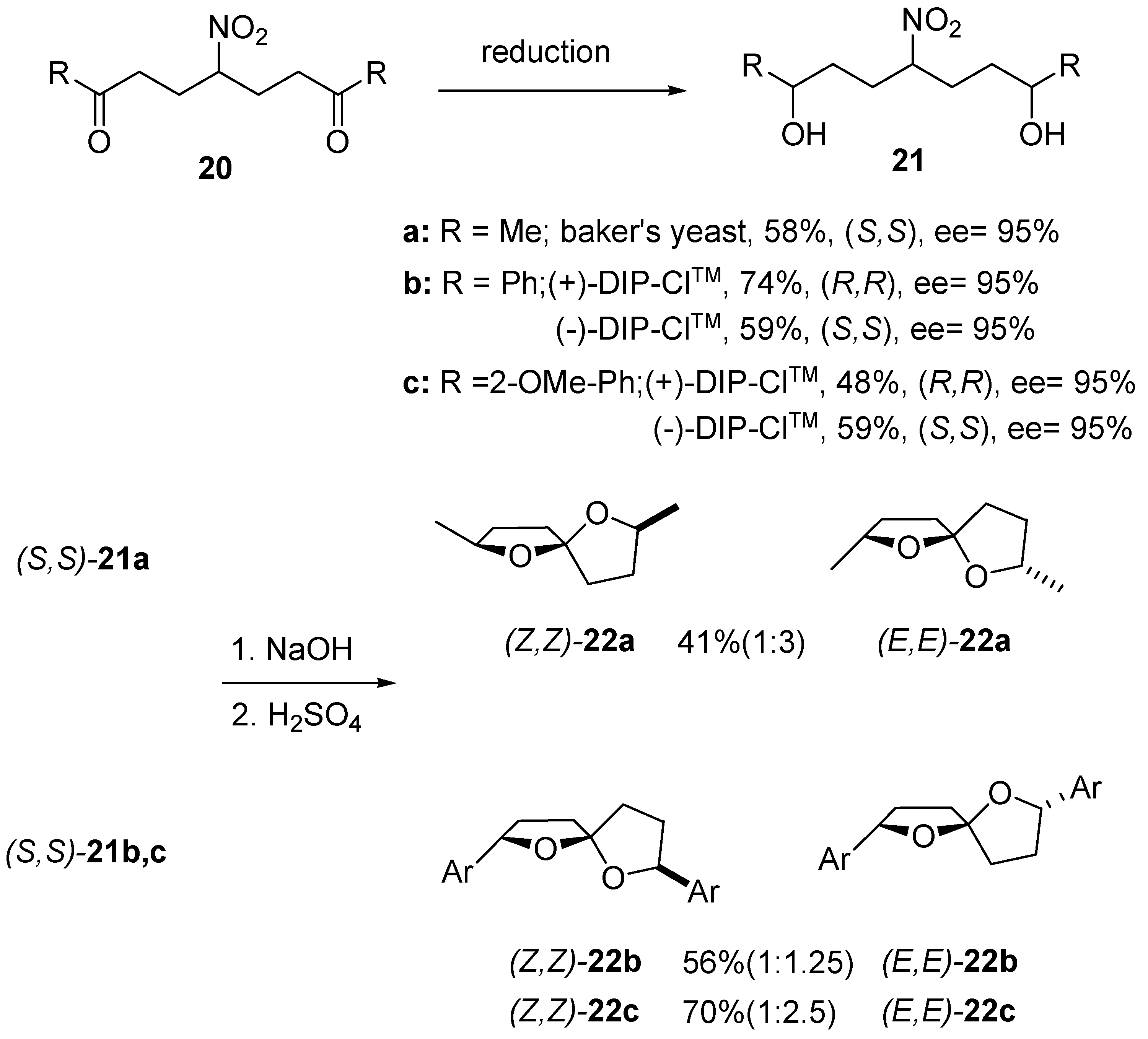
3. 1,6-Dioxaspiro[4.5]decanes and 1,6-dioxaspiro[4.6]undecanes
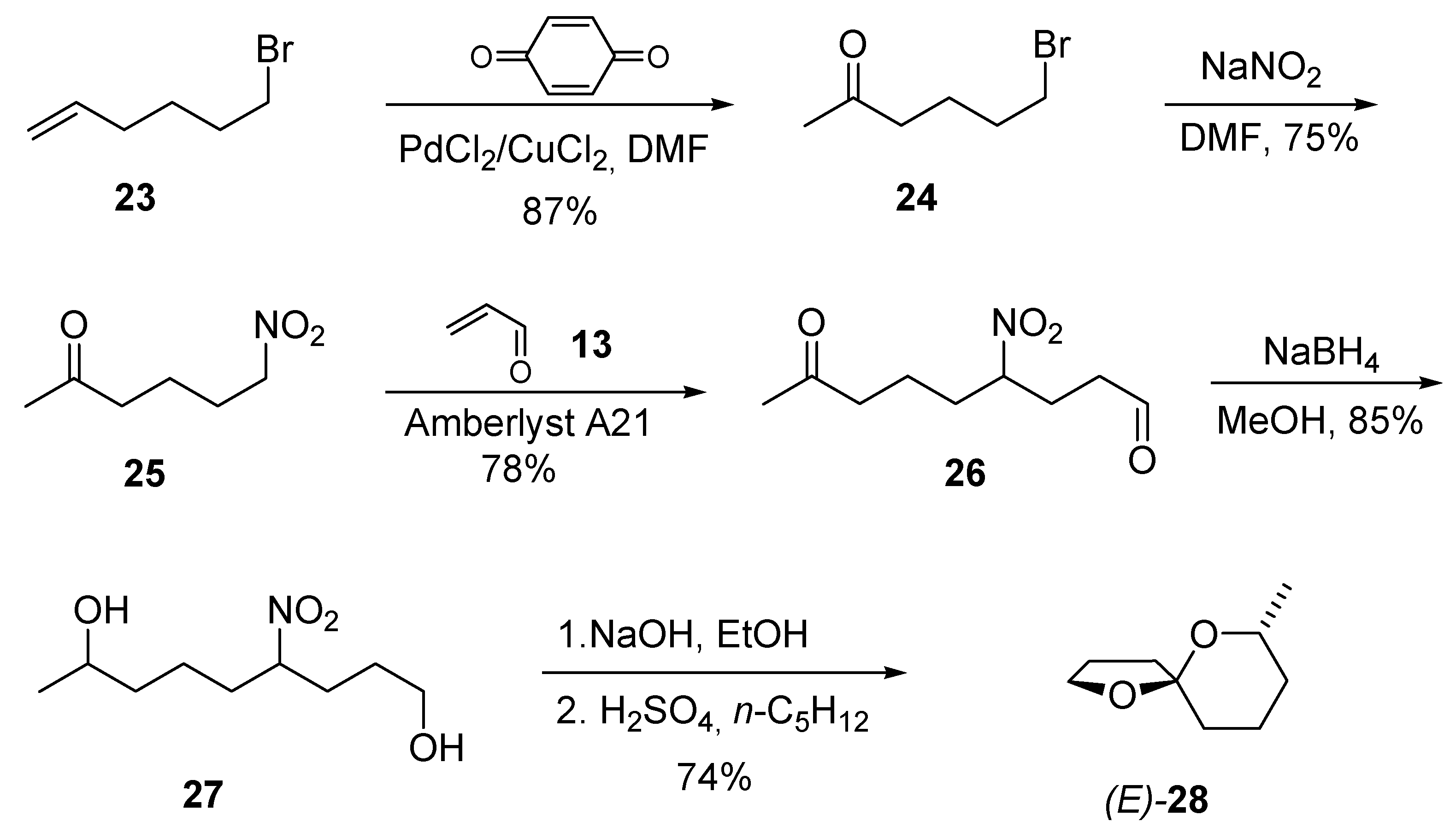
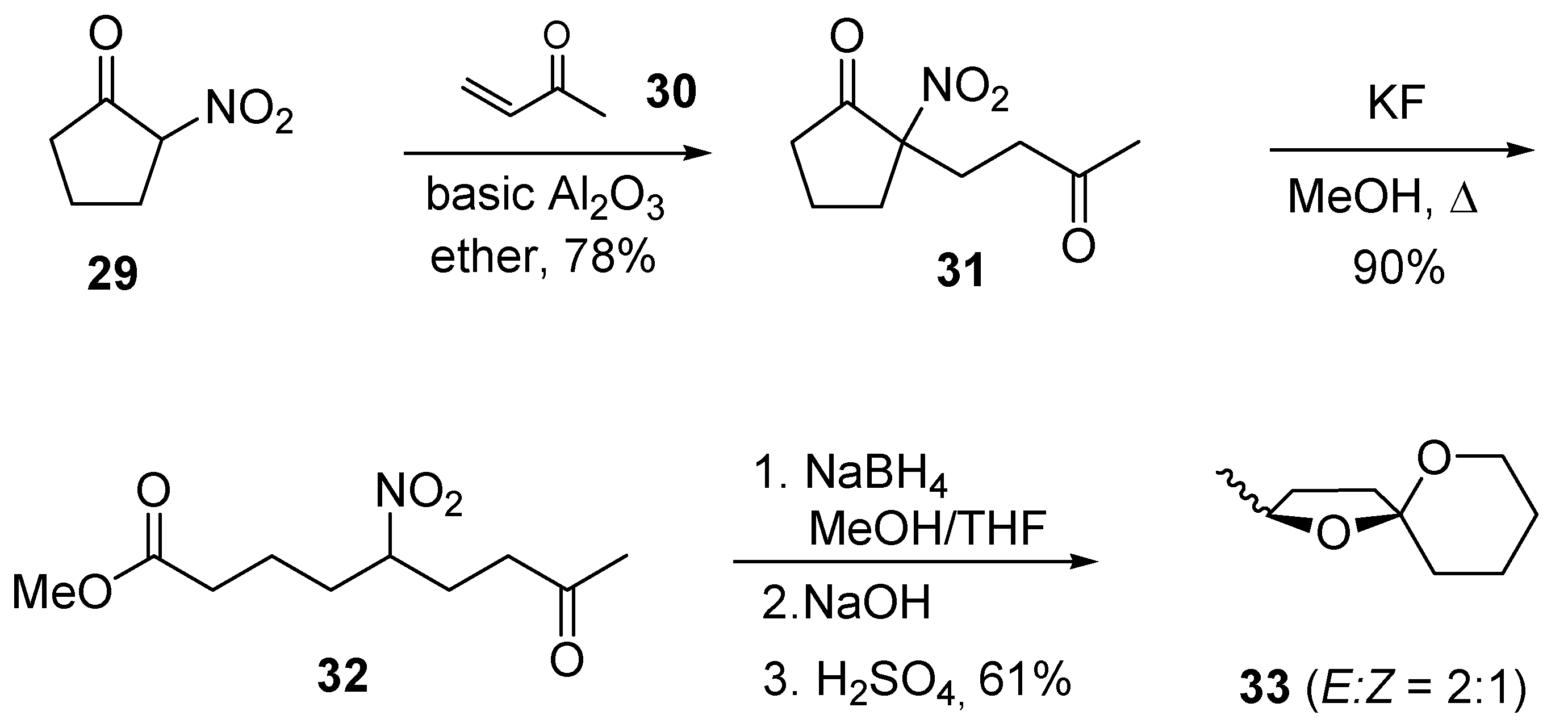



4. 1,7-Dioxaspiro[5.5]undecanes and 1,7-Dioxaspiro[5.6]dodecanes
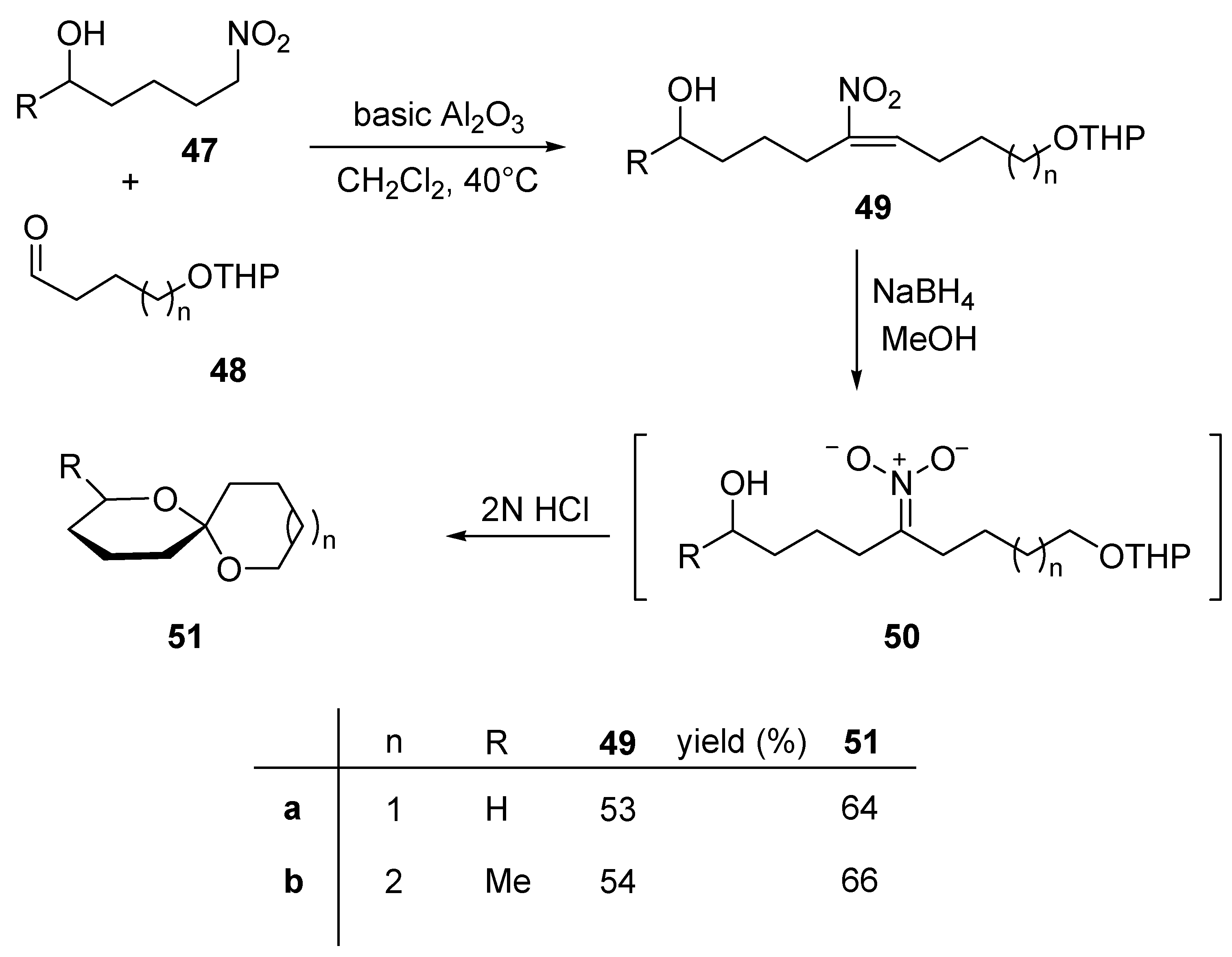
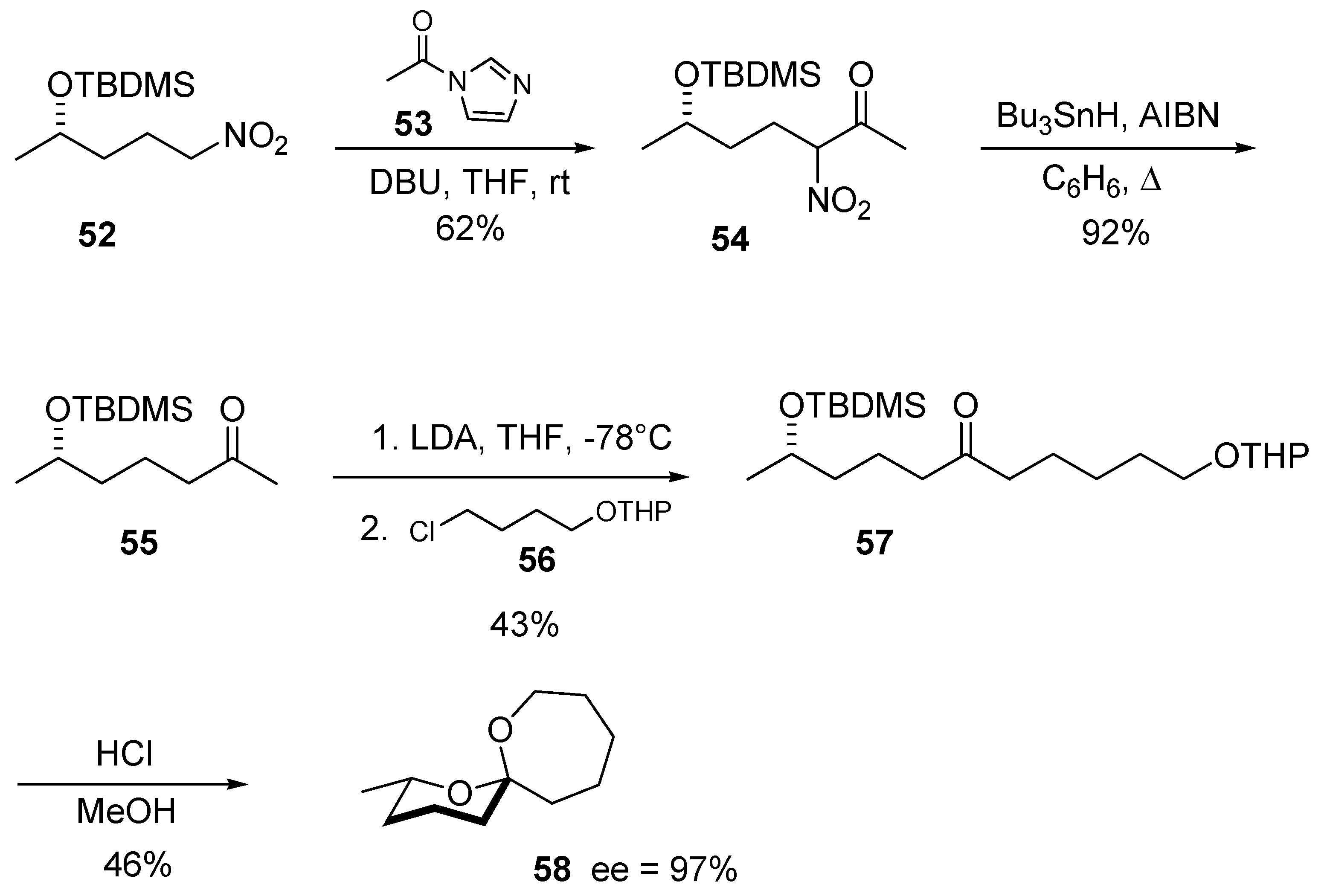
5. Conclusions
Acknowledgements
References
- Perron, F.; Albizati, K. F. Chemistry of Spiroketals. Chem. Rev. 1989, 89, 1617–1661. [Google Scholar] [CrossRef]
- Brimble, M. A.; Farès, A. F. Synthesis of Bis-Spiroacetals Ring Systems. Tetrahedron 1999, 55, 7661–7706. [Google Scholar] [CrossRef]
- Franke, W.; Kitching, W. Spiroacetals in Insects. Curr. Org. Chem. 2001, 5, 233–251. [Google Scholar] [CrossRef]
- Rodríguez, S.; Wipf, P. Oxidative Spiroacetalizations and Spirolactonizations of Arenes. Synthesis 2004, 2767–2783. [Google Scholar]
- Aho, J. E.; Pihko, P. M.; Rissa, T. K. Nonanomeric Spiroketal in Natural Products: Structures, Sources and Synthetic Strategies. Chem. Rev. 2005, 105, 4406–4440. [Google Scholar] [CrossRef]
- Mead, K. T.; Brewer, B. N. Strategies in Spiroketal Synthesis Revisited: Recent Applications and Advances. Curr. Org. Chem. 2003, 7, 227–256. [Google Scholar] [CrossRef]
- Perlmutter, P. Conjugate Addition Reactions in Organic Synthesis; Pergamon: Oxford, 1992. [Google Scholar]
- Yus, M.; Nájera, C.; Foubelo, F. The Role of 1,3-Dithianes in Natural Product Synthesis. Tetrahedron 2003, 59, 6147–6212. [Google Scholar] [CrossRef]
- Ono, N. The Nitro Group in Organic Synthesis; Wiley-VCH: New York, 2001. [Google Scholar]
- Luzzio, F. A. The Henry Reaction: Recent Examples. Tetrahedron 2001, 57, 915–945. [Google Scholar] [CrossRef]
- Rosini, G. The Henry (Nitroaldol) Reaction. In Comprehensive Organic Synthesis; Trost, B.M., Fleming, I., Heathcock, C.H., Eds.; Pergamon: Oxford, 1991; pp. 321–340. [Google Scholar]
- Ballini, R.; Bosica, G.; Fiorini, D.; Palmieri, A.; Petrini, M. Conjugate Additions of Nitroalkanes to Electron-Poor Alkenes: Recent Results. Chem. Rev. 2005, 105, 933–971. [Google Scholar] [CrossRef]
- Ballini, R.; Petrini, M. Recent Synthetic Developments in the Nitro to Carbonyl Conversion (Nef Reaction). Tetrahedron 2004, 60, 1017–1047. [Google Scholar] [CrossRef]
- Seebach, D.; Knochel, P. 2'-Nitro-2'-propen-1'-yl 2,2-dimethylpropanoate (NPP), a Multiple Coupling Reagent. Helv. Chim. Acta 1984, 67, 261–283. [Google Scholar] [CrossRef]
- Rosini, G.; Ballini, R.; Petrini, M.; Marotta, E. Nitromethane as d1,d1 Multiple Coupling Reagent for the Carbonyl Dianion Synthon. Practical Synthesis of Chalcogran. Angew. Chem. Int. Ed. Engl. 1986, 25, 941–942. [Google Scholar] [CrossRef]
- Ballini, R.; Bosica, G.; Uselli, A. A Simple, Efficient, Two-Step Synthesis of Symmetric 2,7-Dialkyl-1,6-Dioxaspiro[4,4]nonanes. J. Heterocycl. Chem. 1994, 259, 259–260. [Google Scholar] [CrossRef]
- Occhiato, E. G.; Guarna, A.; De Sarlo, F.; Scarpi, D. Baker’s Yeast Reduction of Prochiral γ-Nitroketones. II. Straightforward Enantioselective Synthesis of 2,7-Dimethyl-1,6-dioxaspiro[4.4] nonanes. Tetrahedron: Asymmetry 1995, 6, 2971–2976. [Google Scholar] [CrossRef]
- Occhiato, E. G.; Scarpi, D.; Menchi, G.; Guarna, A. Synthesis of Enantiopure 2,7-Diaryl-1,6-dioxaspiro[4.4]nonanes via Enantioselective Reduction of Prochiral γ-Nitroketones by Diisopinocampheylchloroborane (DIP-ClTM). Tetrahedron: Asymmetry 1996, 7, 1929–1942. [Google Scholar] [CrossRef]
- Rosini, G.; Ballini, R.; Marotta, E. Functionalized Nitroalkanes in Synthesis of 1,6-Dioxaspiro[4.5]decane Components of Paravespula Vulgaris Pheromone. Tetrahedron 1989, 45, 5935–5942. [Google Scholar] [CrossRef]
- Deslongchamp, P. Stereoelectronic Effects in Organic Chemistry; Pergamon: Oxford, 1983; pp. 5–53. [Google Scholar]
- Aono, T.; Bieri, J. H.; Hesse, M.; Kostova, K.; Lorenzi-Riatsch, A; Nakashita, Y.; Prewo, R. Structures of Ring-Enlargement Products. Helv. Chim. Acta 1985, 68, 1033–1053. [Google Scholar] [CrossRef]
- Ballini, R.; Petrini, M.; Rosini, G. New and Efficient Synthesis of ω-Nitroalcohols and Spiroketals by Chemio- and Regioselective Reductive Cleavage of 2-Nitrocycloalkanones. Tetrahedron 1990, 46, 7531–7538. [Google Scholar]
- Capecchi, T.; de Koning, C.B.; Michael, J.P. Nitroalkenes as Precursors to the Aromatic Spiroketal Skeleton of γ-Rubromycin. A Nef-type Reaction Mediated by Pearlman’s Catalyst. Tetrahedron Lett. 1998, 39, 5429–5432. [Google Scholar] [CrossRef]
- Capecchi, T.; de Koning, C.B.; Michael, J.P. Synthesis of Bisbenzannellated Spiroketal Core of the γ-Rubromycins. The Use of a Novel Nef-type Reaction Mediated by Pearlman’s Catalyst. J. Chem. Soc. Perkin Trans. 1 2000, 2681–2688. [Google Scholar] [CrossRef]
- Ballini, R.; Petrini, M. Hydroxy-functionalized Conjugated Nitroolefins as Intermediate Precursors of Spiroketals. A New Synthesis of 1,7-Dioxaspiro[5.5]undecane and (E)-2-Metil-1,7-dioxaspiro[5.6]dodecane. J. Chem. Soc. Perkin Trans. 1 1992, 3159–3160. [Google Scholar] [CrossRef]
- Ballini, R.; Bosica, G.; Schaafstra, R. Nitro Ketones in Organic Synthesis: A New, Short Synthesis of Racemic trans-2-Methyl-1,7-dioxaspiro[5.5]undecane, trans,trans- and trans,cis-2,8-Dimethyl-1,7-dioxaspiro[5.5]undecane by Henry Reaction. Liebigs Ann. Chem. 1994, 1235–1237. [Google Scholar] [CrossRef]
- Nakamura, K.; Kitayama, T.; Inoue, Y.; Ohno, A. Asymmetric Synthesis of a Pheromone for Andrena Haemorrhoa F from a Chiral Nitro Alcohol Obtained by the Yeast Reduction of a Nitro Ketone. Tetrahedron 1990, 46, 7471–7481. [Google Scholar]
- Samples availability: Contact the author.
© 2008 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Ballini, R.; Petrini, M.; Rosini, G. Nitroalkanes as Central Reagents in the Synthesis of Spiroketals. Molecules 2008, 13, 319-330. https://doi.org/10.3390/molecules13020319
Ballini R, Petrini M, Rosini G. Nitroalkanes as Central Reagents in the Synthesis of Spiroketals. Molecules. 2008; 13(2):319-330. https://doi.org/10.3390/molecules13020319
Chicago/Turabian StyleBallini, Roberto, Marino Petrini, and Goffredo Rosini. 2008. "Nitroalkanes as Central Reagents in the Synthesis of Spiroketals" Molecules 13, no. 2: 319-330. https://doi.org/10.3390/molecules13020319



