Introduction
Radix Astragali is the dried root of both
Astrugalus membrunaceus (Fisch.) Bge. var.
mongholicus (Bunge) Hsiao and
Astragalus membranaceus (Fisch.) Bge., which belongs to the Fabaceae family. It is a crude drug widely used in Traditional Chinese Medicine for thousands of years to treat various renal diseases. Polysaccharides from
Radix Astragali are a class of macromolecules that have been shown strong anti-tumor [
1] and anti-glomerulonephritis activities [
2]. Studies have also shown that
Astragalus polysaccharides (APS) have an effect on nephrotic syndrome [
3], possess immunopotentiatiive functions [
4] and cause improvement of early diabetic nephropathy [
5]. In the present study, we extracted, isolated and purified the polysaccharide components from
Radix Astagali and determined their molecular weight as well as their monosaccharide composition so as to further investigate its mechanism of action.
Results and Discussion
Though there are numerous literature reports on the extraction and purification of polysaccharides from
Radix Astragali, only a few have studied the determination of its molecular weight and monosaccharide composition [
6]. In the present work, the extraction and isolation of
Astragalus polysaccharides was performanced by boiling-water decoction and ethanol precipitation to yield crude polysaccharide, then the Sevag method was used to remove protein components after re-dissolution of the crude polysaccharides. The solution was then dialyzed against distilled water for 2 days and precipitated by adding ethanol until the concentration of ethanol reached 80%. The precipitate was collected by centrifugation and washed successively with absolute ethanol and acetone to give a light yellow powder. For additional purification the
Astragalus polysaccharides were subjected to DEAE-52 cellulose column chromatography, eluting with distilled water. Each 50 mL eluted fraction was collected and the content of sugar was monitored using the phenol-sulfuric acid method [
7]. Fractions 8~14 were combined, concentrated to 100 mL and designated as the APS fraction.
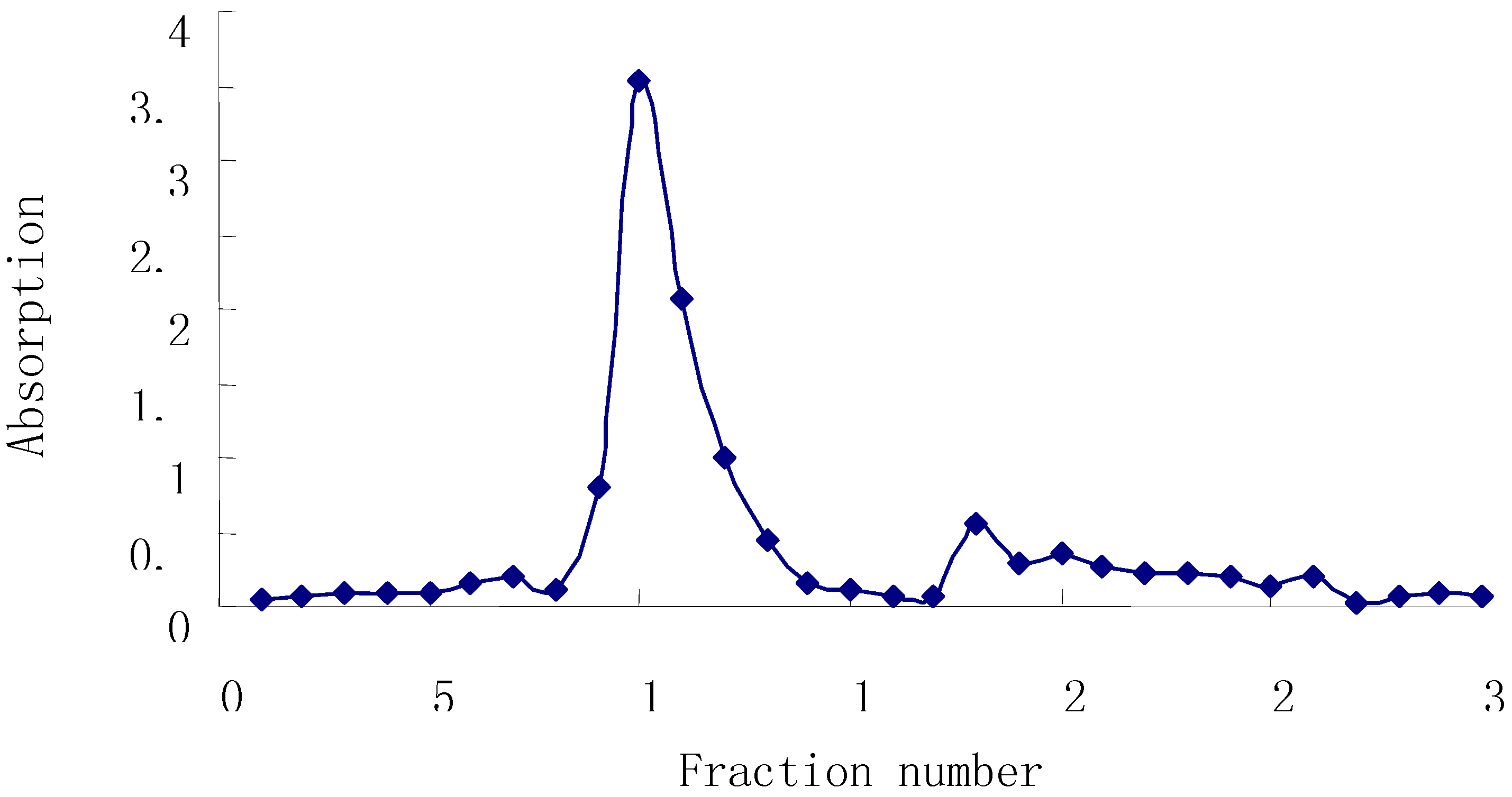
Figure 1 shows that one main peak was found in the elution trace by this method.
Figure 1.
Elution Curve of Astraglus Polysaccharide on DEAE-52 Cellulose.
Figure 1.
Elution Curve of Astraglus Polysaccharide on DEAE-52 Cellulose.
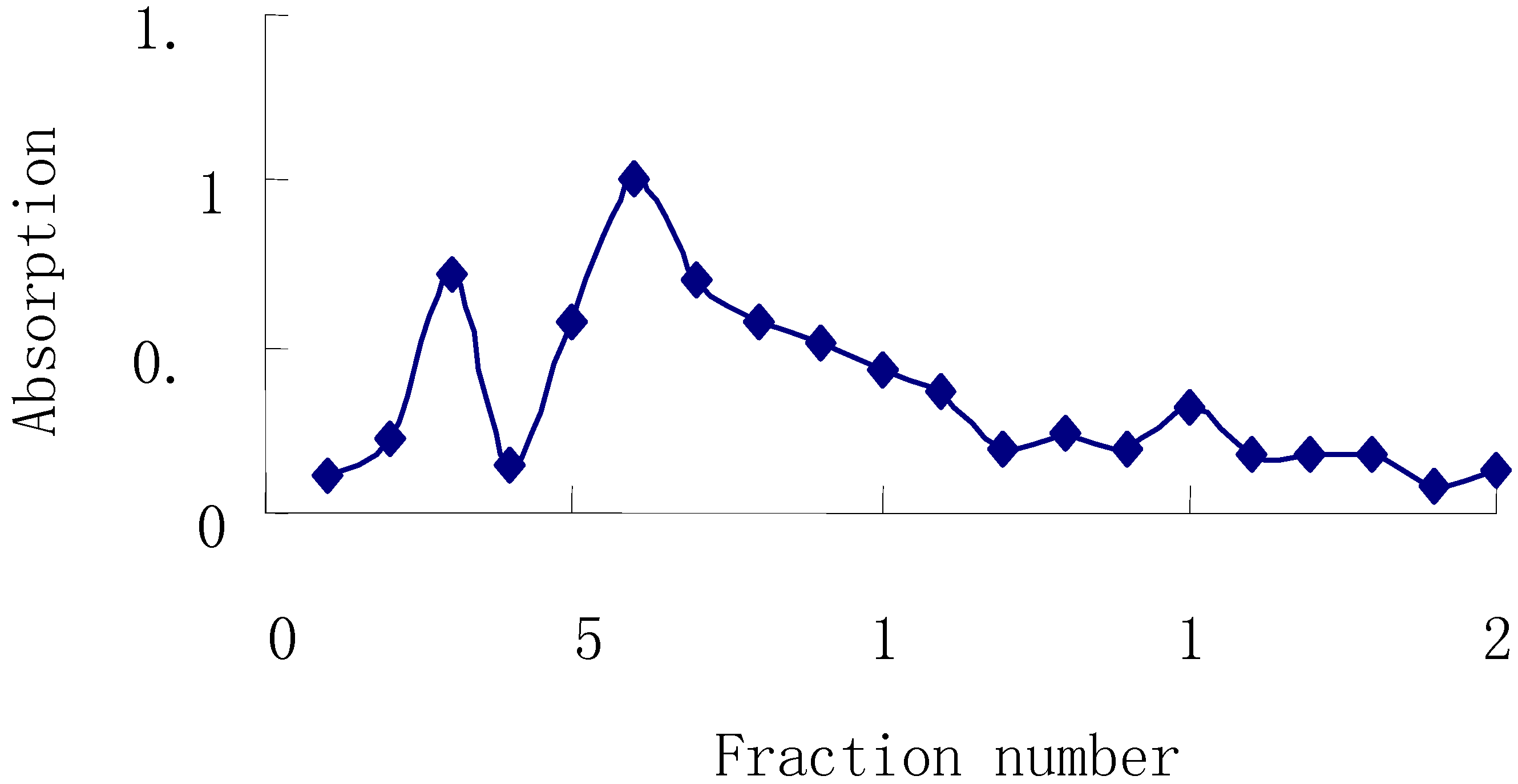
Then, this material was subjected to Sephadex G-150 column chromatography (2×60 cm), eluting with distilled water collecting 50 mL fractions with monitoring by the phenol-sulfuric acid method. Fractions 2~4 and 5~7 were combined, concentrated, and named APS-I and APS-II, respectively.
Figure 2.
Elution Curve of APS on Sephadex G-150 column.
Figure 2.
Elution Curve of APS on Sephadex G-150 column.
A HPLC-GPC method was used to determine the molecular weight of APS-I and APS-II.
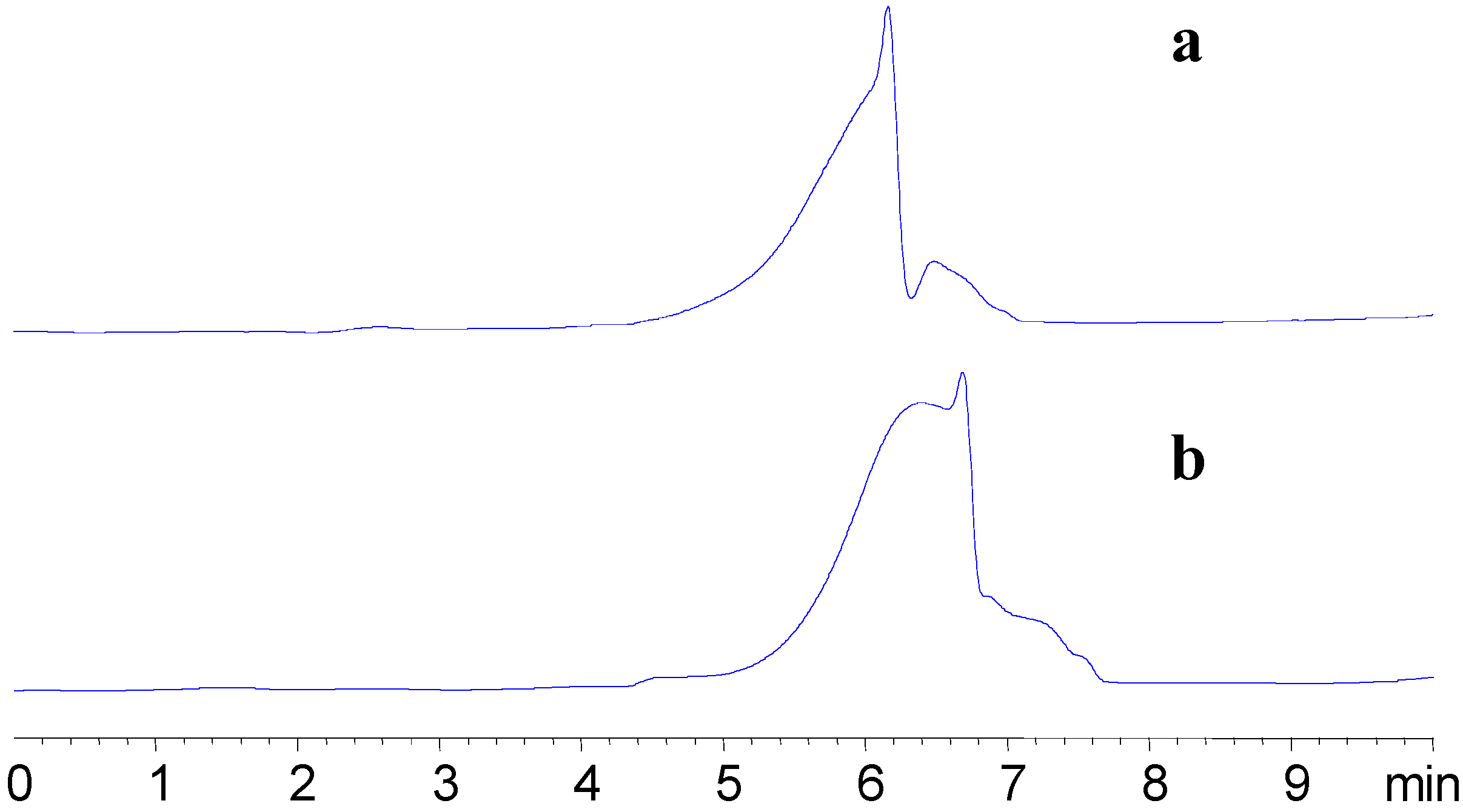
Figure 3 shows the chromatograms of APS-I and APS-II. The molecular weight (Mw) of APS-I and APS-II, calculated using GPC software, were 1,699,100 and 1,197,600, respectively. The Dextran calibration curve of was Log (Mw) = 5.5324 – 0.4704 RT – 0.05829 RT
2 (RT: retention time of Dextran).
Figure 3.
HPLC-GPC chromatography of APS-1 and APS-II.
Figure 3.
HPLC-GPC chromatography of APS-1 and APS-II.
Determination of the molecular weight of ASP-I and APS-II by HPLC-GPC run in isocratic mode. Mobile phase: 100% water; the column and optical unit of RID were set at 30°C and 35°C, respectively; flow rate, 1.0 mL/min; injection volume, 10 mL; (a) APS-I, retention time 6.162 min; (b) APS-II, retention time 6.397 min.
Monosaccharide composition analysis was carried out by TLC and HPLC methods. The TLC results presented in
Figure 4 shows that the hydrolyzates of APS-I, APS-II and five mixed standard monosaccharides share the same arabinose and glucose spot.
Figure 4.
Monosaccharide composition analysis of APS-I and APS-II by TLC.
Figure 4.
Monosaccharide composition analysis of APS-I and APS-II by TLC.
Lane 1 correspond to five standard monosaccharides, Lanes 2 and 3 correspond to hydrolyzates of APS-I and and ASP-II, respectively, Lanes 4 and 5 correspond to aqueous solutions of APS-I and ASP-II, respectively.
The chromatogram in
Figure 5 shows that using an HPLC-RID method, APS-I was composed of two monosaccharides – arabinose and glucose – in a molar ratio of 1:3.45; APS-II was composed of three monosaccharides – rhamnose, arabinose and glucose – in a molar ratio of 1:6.25:17.86.
Figure 5.
HPLC-RID analysis of monosaccharide composition.
Figure 5.
HPLC-RID analysis of monosaccharide composition.
(a) Mixed standard monosaccharides, rhamnose, xylose, arabinose, fructose and glucose, about 2 mg/mL for each monosaccharide. (b) APS-I hydrolyzates. (c) APS-II hydrolyzates.
Experimental
General
Standard monosaccharide (glucose, xylose, arabinose, rhamnose and fructose) and Dextran standards (12,000, 40,000, 70,000, 150,000, 500,000 and 2,000,000 Da) were purchased from Sigma. DEAE-Cellulose 52, Sephadex G-150, Sephadex G-200 and Sephacryl S-300HR were from Sinopharm Chemical Reagent. Co, Ltd. and Amersham Pharmacia Biotech, respectively. The polysaccharide content of the different eluting fractions was monitored using the phenol-sulfuric acid method, with an UV-Vis spectrophotometer (Beijing Purkinje General Instrument Co., Ltd., P.R. China).
Plant material
Radix Astragali was purchased from Anhui GuoTou Chinese Medicine Co.Ltd. and authenticated by Professor Liu Shoujin, Department of Medicinal Plants, Anhui University of Traditional Chinese Medicine, as radix of Astagalus membranaceus (Fisch.) Bge. The Radix Astragali was cut into small slices (about 3 cm2 × 2 mm thick), kept in a vacuum drying oven at 60°C for 12 hours and then stored for further use.
Isolation and Purification
The crude polysaccharide was subjected to ion-exchange on a DEAE-52 cellulose column (4×60 cm), eluted with distilled water (1500 mL) and each 50 mL was collected with monitoring by the phenol-sulfuric acid method. The eluting fractions 8 to 14 were combined and concentrated to 100 mL, and named APS. The APS fraction was then subjected to gel-filtration chromatography on a Sephadex G-150 column (2 × 30 cm), eluting with distilled water (1000 mL) and collecting 50 mL fractions, using the same method to monitor the polysaccharide content. Fractions 2 to 4 and fractions 5 to 7 were combined and named APS-I and APS-II, respectively. Confirmation of the purity of APS-I and APS-II was done using a Sephacryl S-300HR column (1 × 30 cm) and a Sephadex G-200 column (1 × 30 cm).
Determination of molecular weight
Dextran standards, APS-I and APS-II were dissolved in distilled water at a concentration of 2.0 mg/mL and analyzed on an Agilent 1100 Series HPLC system equipped with a RID and a TSK G5000PW
XL gel column (7.8 × 300 mm) and a TSK PW
XL (6.0 mm × 40 mm) guard column (TOSOH Corporation, Japan), to determine the retention time of standards and samples [
9]. The column and detector compartment were maintained at 30°C and 35°C, respectively. Distilled water was used as mobile phase at a flow rate of 1.0 mL/min and injection volume was 10 μL. The molecular weight of APS-I and APS-II were calculated by constructing a calibration curve, in which the logarithm of the molecular weight of the Dextran standards ranging from 12,000 to 2,000,000 Da was plotted as a function of the retention time using Agilent ChemStation GPC Data Analysis Software (Rev. A.02.01).
Monosaccharide composition analysis
APS-I and APS-II were hydrolyzed in 1 mol/L trifluoroacetic acid for 10 h at 100 °C in a sealed glass tube. The residual acid was removed under a stream of N2 in a 60°C water-bath after adding methanol and taking to dryness three times, then distilled water (1 mL) was added to dissolve the residue.
TLC method [
10]: TLC glass plates (5 × 10 cm glass plates with 0.2 mm thick silica gel, QingDao Ocean Chemical Plant, P.R. China) were incubated overnight with 0.3 mol/L aqueous NaH
2PO
4 solution. The plates were then activated at 115°C for 1 h before use. Five standard monosaccharides and the hydrolyates of APS-I and APS-II (2 μL per spot) were applied on the TLC plates which were developed in TLC chambers. The first eluant [4:3:1 (v:v:v)
n-butanol-acetone-water] was run to a height of 9 cm from the origin. After drying, the plates were developed again with the second eluent [8:4:7:3 (v:v:v:v)
n-butanol-acetic ether-isopropanol-water] to a height of 9 cm from the origin. After drying, the plates were sprayed with 2% phenylamine - 2% diphenylamine - 85% phosphoric acid [dissolved in acetone in a ratio of 5:5:1 (v:v:v)] and then heated in oven at 105 °C for 1 h.
HPLC-RID method [
11]: The hydrolyzates of APS-I and APS-II were analyzed by HPLC on an Agilent 1100 series HPLC (Agilent Technologies Palo, Alto, CA, USA) equipped with a RID, using an Hypersil NH
2 column (4.6 × 250 mm, Dalian Elite Analytical Instrument Co. Ltd., P.R. China). The flow rate was 1 mL/min and the mobile phase was acetonitrile-water (82:18). The injection volume of mixed monosaccharide standards and APS-I and APS-II hydrolyzates was 10 μL. The temperature of column and optical unit were set at 30 °C and 35 °C, respectively. Identification of the monosaccharides in the hydrolyzates of APS-I and APS-II was carried out by comparing their retention times with those of standards under the same HPLC conditions. Quantitative determination was performed using the external standard method.