Evaluation of the Antimicrobial and Antioxidant Activities of Origanum dictamnus Extracts before and after Encapsulation in Liposomes
Abstract
:Introduction
Results and Discussion
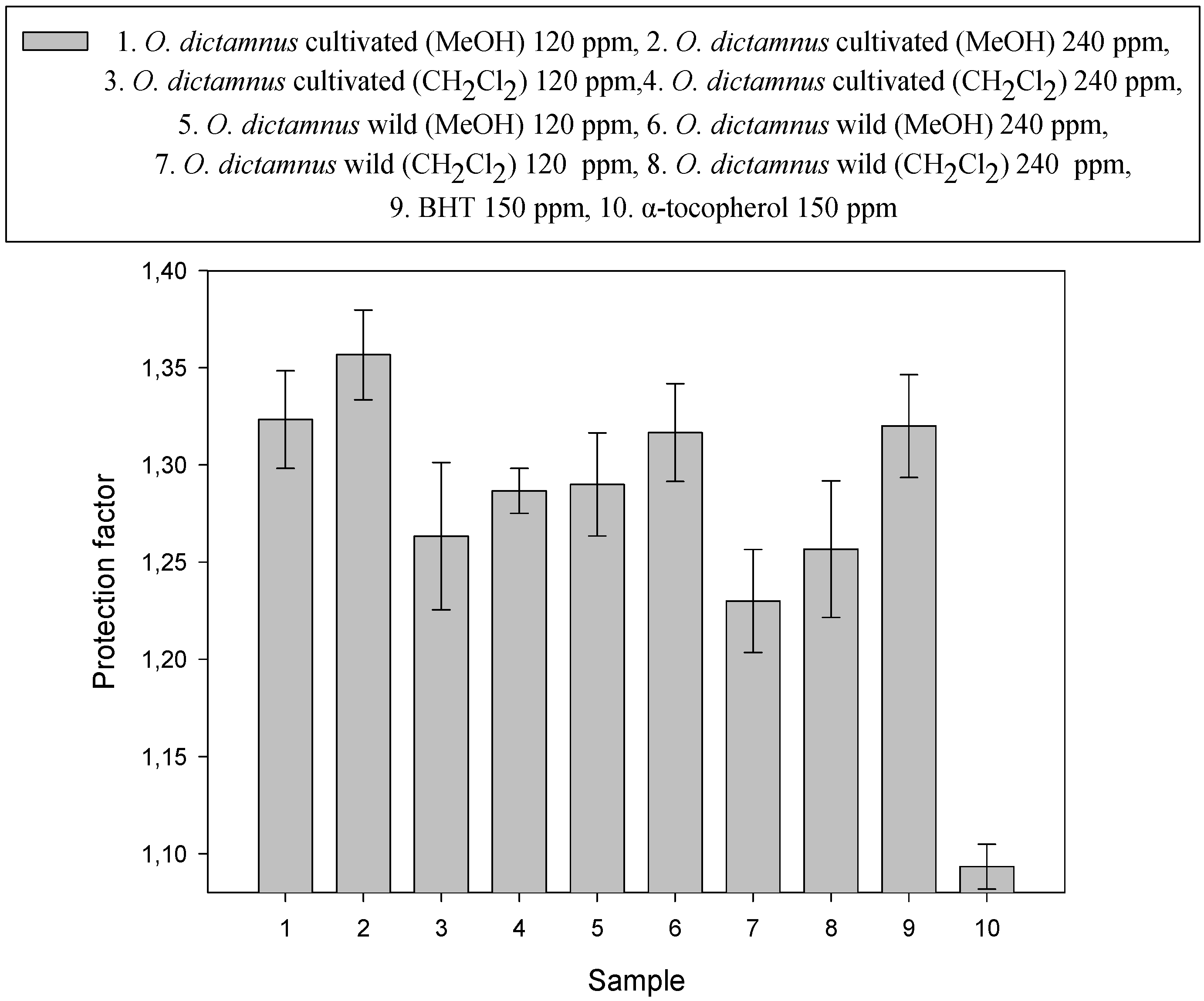
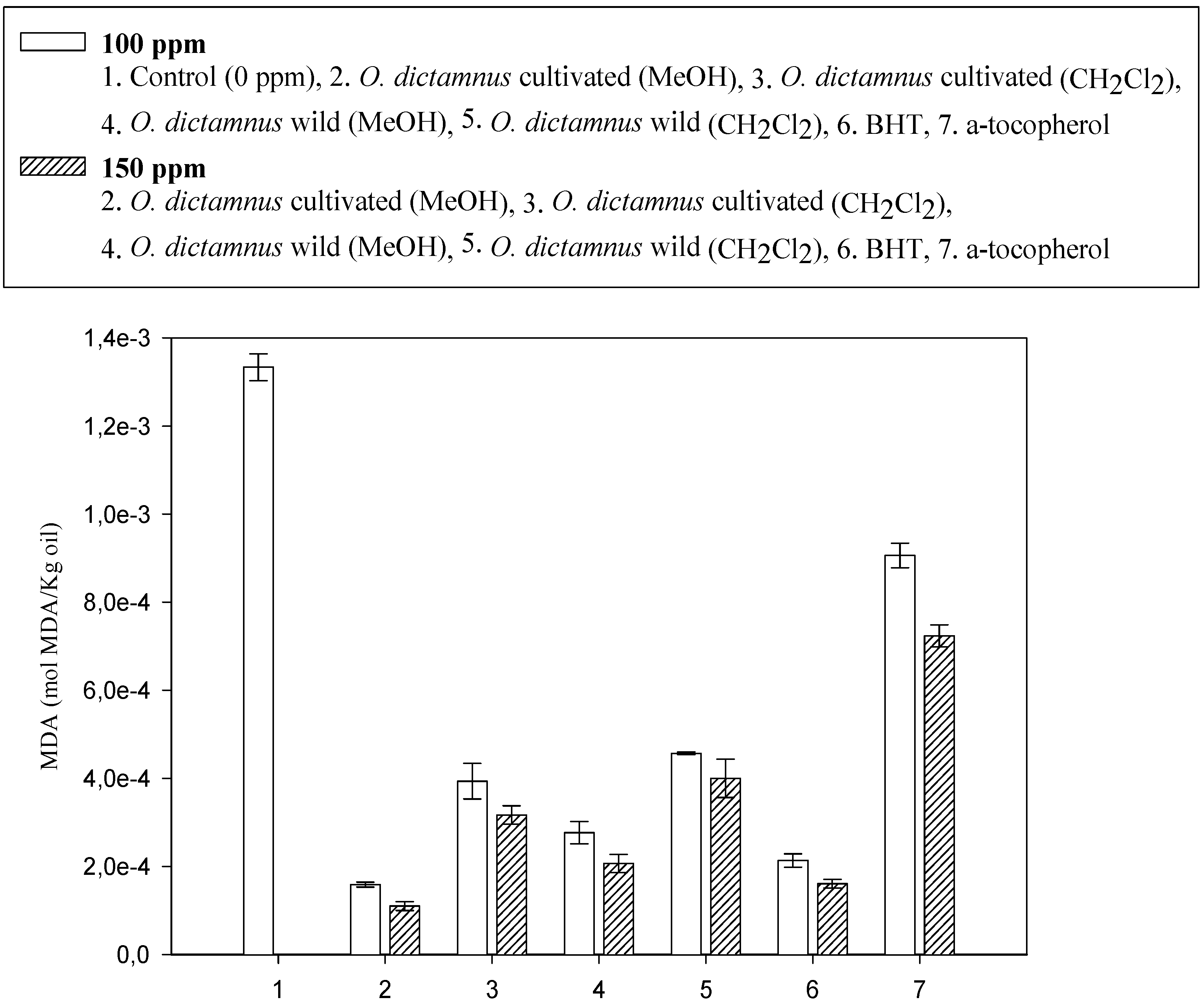
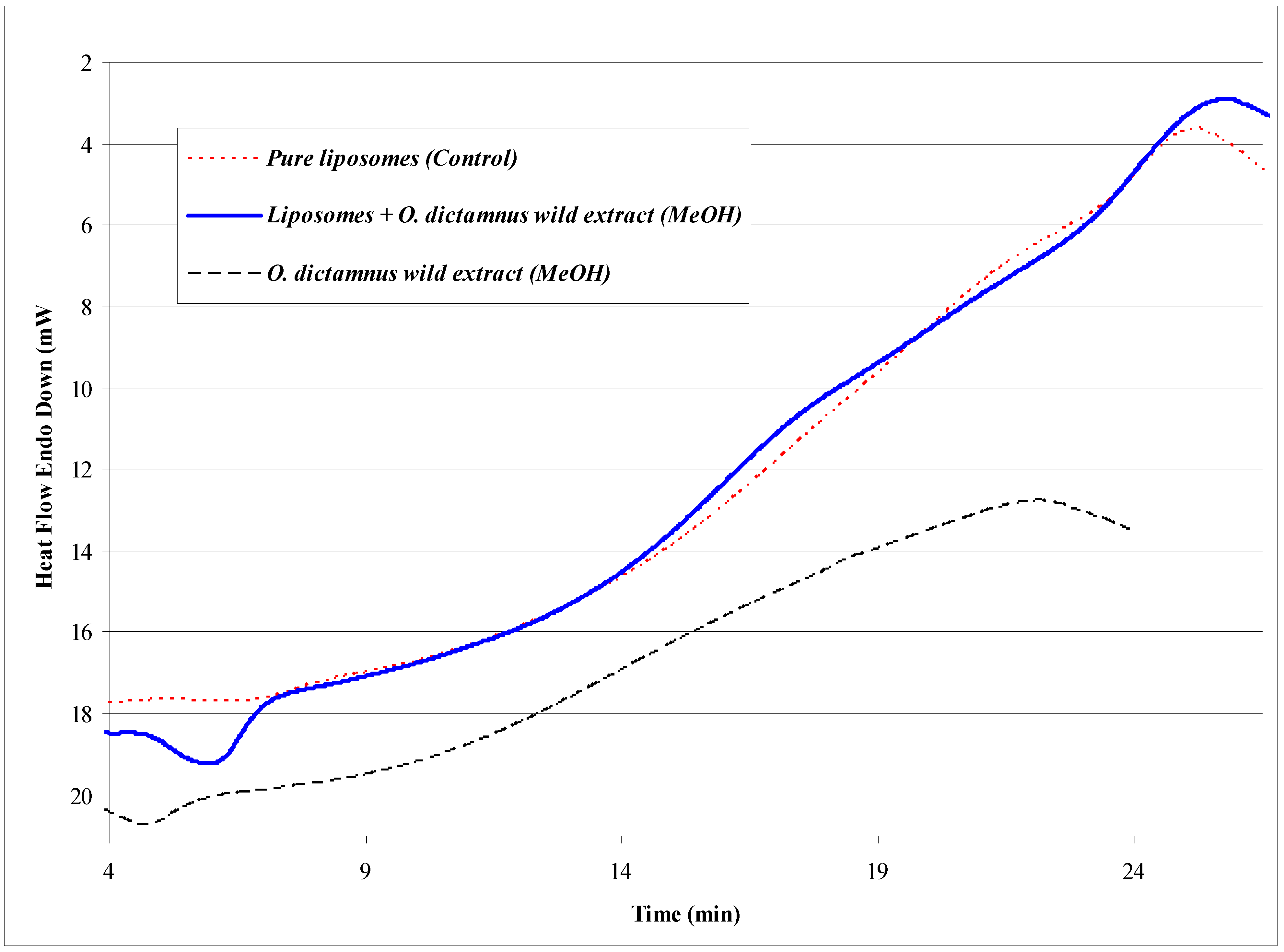
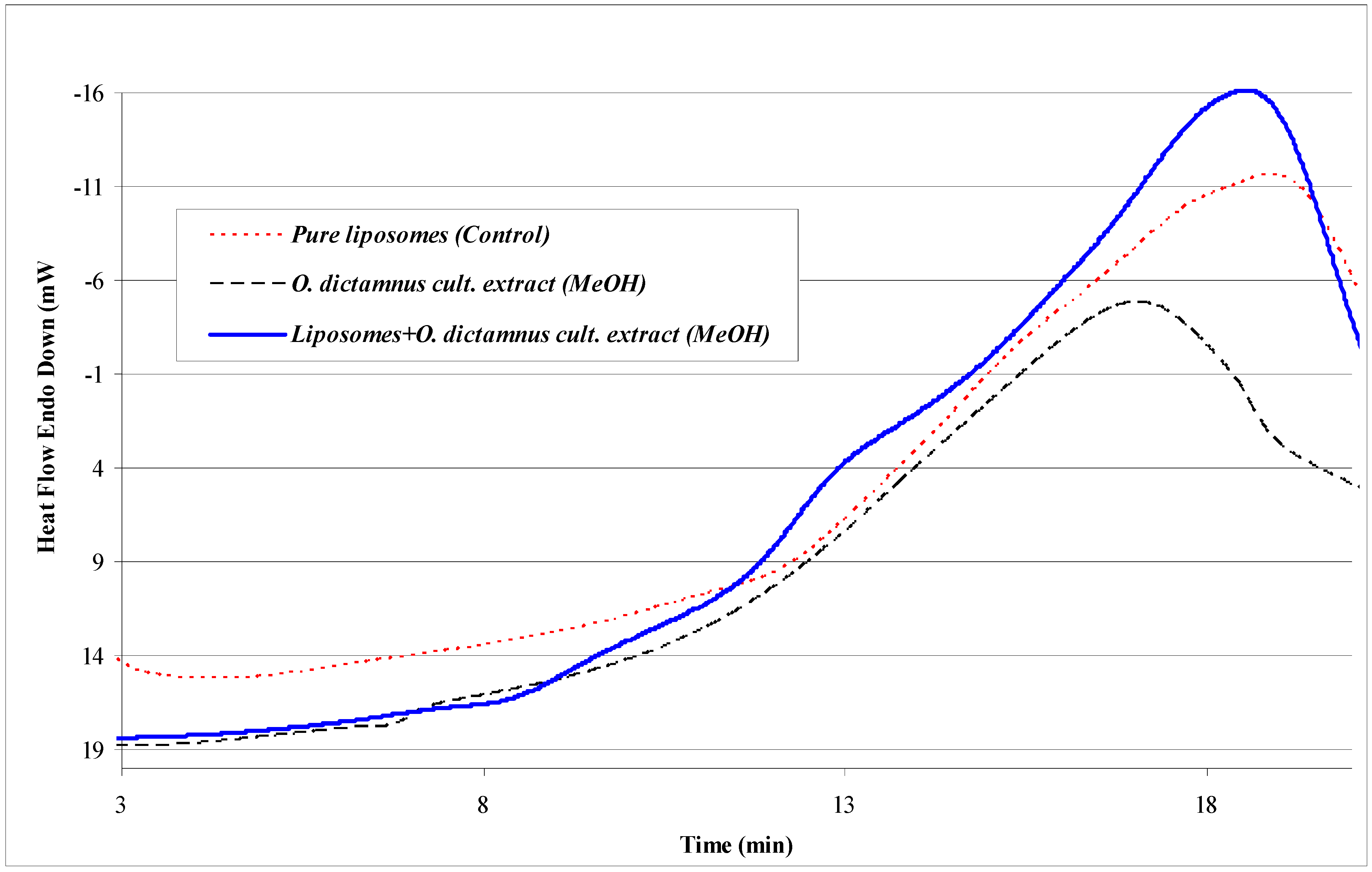
| Sample composition | Size (nm) | z-potential (mV) |
|---|---|---|
| PC:C (no extract) | 250 (15) | -10.2 (1.2) |
| PC:C + O. dictamnus | 275 (12) | -10.5 (0.4) |
| Microbial spp. | O. dictamnus wild | Liposomes + O. dictamnus wild | O. dictamnus cultivated | Liposomes + O. dictamnus cultivated | Netilmicin | Intraconazole |
|---|---|---|---|---|---|---|
| S. aureus | 12a (0.1) | 14 (0.3) | 13 (0.2) | 15 (0.2) | 21 (0.4) | - |
| S. epidermidis | 12 (0.2) | 15 (0.3) | 12 (0.1) | 15 (0.4) | 25 (0.4) | - |
| S. mutans | 12 (0.1) | 15 (0.1) | 12 (0.2) | 15 (0.2) | 24 (0.3) | - |
| S. viridans | 12 (0.1) | 15 (0.2) | 12 (0.1) | 16 (0.1) | 25 (0.1) | - |
| P. aeruginosa | 11 (0.1) | 12 (0.2) | 11 (0.3) | 12 (0.2) | 20 (0.1) | - |
| E. cloacae | 10 (0.1) | 11 (0.2) | 11 (0.1) | 12 (0.1) | 23 (0.1) | - |
| K. pneumoniae | 9 (0.1) | 11 (0.1) | 10 (0.1) | 12 (0.1) | 22 (0.3) | - |
| E. coli | 9 (0.2) | 9 (0.1) | 9 (0.2) | 10 (0.2) | 24 (0.2) | - |
| C. albicans | 9 (0.1) | 10 (0.1) | 9 (0.1) | 10 (0.2) | - | 20 (0.3) |
| C. tropicalis | 10 (0.1) | 13 (0.2) | 11 (0.2) | 14 (0.2) | - | 22 (0.3) |
| C. glabrata | 10 (0.2) | 12 (0.2) | 12 (0.1) | 14 (0.2) | - | 23 (0.3) |
| L. monocytogenes | 9 (0.2) | 12 (0.2) | 10 (0.1) | 13 (0.1) | 22 (0.3) | - |
Conclusions
Experimental
General
Evaluation of the antioxidant activity by the Rancimat method
Determination of malondialdehyde (MDA) by HPLC
Preparation of liposomes
Liposome size determination
Liposomal surface charge
Differential Scanning Calorimetry (DSC)
Antimicrobial bioassay
Statistical analysis
Acknowledgments
References and Notes
- Abdalla, A. E.; Roozen, J. P. The effects of stabilised extracts of sage and oregano on the oxidation of salad dressings. Europ. Food Res. Technol. 2001, 212, 551–560. [Google Scholar] [CrossRef]
- Azaizeh, H.; Ljubuncic, P.; Portnaya, I.; Said, O.; Cogan, U.; Bomzon, A. Fertilization-induced changes in growth parameters and antioxidant activity of medicinal plants used in traditional Arab medicine. Evid. Based Complement. Alternat. Med. 2005, 2, 549–556. [Google Scholar] [CrossRef]
- Bauer, A. W.; Kirby, W. M. M.; Sherris, J. C.; Turcu, M. Antibiotic Susceptibility Testing by a Standardized Single Disk Method. Am. J. Clin. Pathol. 1966, 45, 493–496. [Google Scholar]
- Baeumner, A.; Caroline, J.; Ching, W.; Andrew, P. A generic sandwich-type biosensor with nanomolar detection limits. Anal. Bioanal. Chem. 2004, 378, 1587–1593. [Google Scholar] [CrossRef]
- Chang, S. S.; Ostric-Matijasevic, B.; Hsieh, O. A. L.; Huang, C. L. Natural antioxidants from rosemary and sage. J. Food Sci. 1977, 42, 1102–1106. [Google Scholar] [CrossRef]
- Conforti, F.; Statti, G.; Uzunov, D.; Menichini, F. Comparative chemical composition and antioxidant activities of wild and cultivated Laurus nobilis L. leaves and Foeniculum vulgare subsp. piperitum (Ucria) Coutinho seeds. Biol. Pharm. Bull. 2006, 29, 2056–2064. [Google Scholar] [CrossRef]
- Couladis, M.; Tzakou, O.; Verykokidou, E.; Harvala, C. Screening of some Greek aromatic plants for antioxidant activity. Phytother. Res. 2003, 17, 194–195. [Google Scholar] [CrossRef]
- Economou, K. D.; Oreopoulon, V.; Thomopoulus, C. D. Antioxidative activity of some plants of the family Labiatae. J. Am. Oil Chem. Soc. 1991, 68, 109–113. [Google Scholar] [CrossRef]
- El Jastimi, R.; Edwards, K.; Lafleur, M. Characterization of permeability and morphological perturbations induced by nisin on phosphatidylcholine membranes. Biophys. J. 1999, 77, 842–852. [Google Scholar] [CrossRef]
- Fuster, M. D.; Lampi, A. M.; Hopia, A.; Kamal-Eldin, A. Effects of α- and γ-tocopherols on the autoxidation of purified sunflower triacylglycerols. Lipids 1998, 33, 715–722. [Google Scholar] [CrossRef]
- Gergis, V.; Spiliotis, V.; Poulos, C. Antimicrobial activity of essential oils from Greek Sideritis species. Pharmazie 1990, 45, 70–71. [Google Scholar]
- Gibbs, B. F.; Kermasha, S.; Alli, I.; Mulligan, C. N. Encapsulation in Food industry: a review. Int. J. Food Sci. Nutr. 1999, 50, 213–24. [Google Scholar] [CrossRef]
- Gortzi, O.; Lalas, S.; Chinou, I.; Tsaknis, J. Reevaluation of antimicrobial and antioxidant activity of Thymus spp. extracts before and after encapsulation in liposomes. J. Food Protect. 2006, 69, 2998–3005. [Google Scholar]
- Gortzi, O.; Lalas, S.; Chinou, I.; Tsaknis, J. Reevaluation of bioactivity and antioxidant activity of Myrtus communis extract before and after encapsulation in liposomes. Eur. Food Res. Technol. 2007. accepted for publication. [Google Scholar]
- Lagouri, V.; Boskou, D. Nutrient antioxidants in Oregano. Int. J. Food Sci. Nutr. 1996, 47, 493–497. [Google Scholar] [CrossRef]
- Lalas, S.; Dourtoglou, V. Use of rosemary extract in preventing oxidation during deep fat frying of potato chips. J. Am. Oil Chem. Soc. 2003, 80, 579–583. [Google Scholar] [CrossRef]
- Lawrence, S. M.; Alpar, H. O.; McAllister, S. M.; Brown, M. R. W. Liposomal (MLV) polymyxin B, physiochemical characterization and effect of surface charge on drug association. J. Drug Targ. 1993, 1, 303–310. [Google Scholar] [CrossRef]
- Litwinienko, G.; Kasprzycka-Guttman, T.; Studzinski, M. Effects of selected phenol derivatives on the autoxidation of linolenic acid investigated by DSC non-isothermal methods. Thermochim. Acta 1997, 331, 97–106. [Google Scholar]
- Moller, J. K. S.; Madsen, H. L.; Aaltonen, T.; Skibsted, L. H. Dittany (Origanum dictamnus) as a source of water-extractable antioxidants. Food Chem. 1999, 64, 215–219. [Google Scholar] [CrossRef]
- New, R.R.C. Liposomes-a practical approach; Oxford University Press: New York, 1990; pp. 109–111. [Google Scholar]
- zer, Y.; Talsma, H.; Crommelin, D. J. A.; Hical, A. A. Influence of freezing and freeze-drying on the stability of liposomes dispersed in aqueous media. Acta Pharm. Technol. 1988, 34, 129–139. [Google Scholar]
- Panizzi, L.; Flamini, G.; Cioni, P. L.; Morelli, I. Composition and antimicrobial properties of essential oils of four Mediterranean Lamiaceae. J. Ethnopharmacol. 1993, 39, 67–170. [Google Scholar]
- Pellecuer, J.; Jacob, M.; Simeon de Buechberg, M.; Allegrini, J. Therapeutic value of the cultivated mountain savory (Satureia montana L.: Labiatae). Acta Hort. 1980, 96, 35–39. [Google Scholar]
- Sachetelli, S.; Khalil, H.; Chen, T.; Beaulac, C.; Senechal, S.; Lagace, J. Demonstration of a fusion mechanism between a fluid bactericidal liposomal formulation and bacterial cells. Biochim. Biophys. Acta 2000, 1463, 254–266. [Google Scholar] [CrossRef]
- Shoji, Y.; Nakashima, H. Nutraceutics and delivery Systems. J. Drug Target. 2004, 12, 385–391. [Google Scholar]
- Sivropoulou, A.; Kokkini, S.; Lanaras, T.; Arsenakis, M. Antimicrobial activity of mint essential oils. J. Agr. Food Chem. 1995, 43, 2384–2388. [Google Scholar] [CrossRef]
- Skaltsa, H.; Harvala, C. Contribution a l’etude Chimique d’ Origanum dictamnus L. – 1re Communication (Glucosides des Feuilles). Plant. Méd. Phytothér. 1986, 4, 300–304. [Google Scholar]
- Skaltsa, H.; Harvala, C. Contribution a l’etude Chimique d’ Origanum dictamnus L. – 2nd Communication (Glucosides des Feuilles). Plant. Méd. Phytothér. 1987, 1, 56–62. [Google Scholar]
- Stamatis, G.; Kyriazopoulos, P.; Golegou, S.; Basayiannis, A.; Skaltsas, S.; Skaltsa, H. In vitro anti-Helicobacter pylori activity of Greek herbal medicines. J. Ethnopharmacol. 2003, 88, 175–179. [Google Scholar] [CrossRef]
- Sułkowski, W. W.; Pentak, D.; Nowak, K.; Sułkowska, A. The influence of temperature, cholesterol content and pH on liposome stability. J. Mol. Struct. 2005, 744-747, 737–747. [Google Scholar] [CrossRef]
- Taylor, M.; Davidson, M.; Bruce, B.; Weiss, J. Liposomal Nanocapsules in food Science and agriculture. Crit. Rev. Food Sci. 2005, 45, 587–605. [Google Scholar] [CrossRef]
- Tepe, B.; Daferera, D.; Sökmen, M.; Polissiou, M.; Sökmen, A. In Vitro Antimicrobial and Antioxidant Activities of the Essential Oils and Various Extracts of Thymus eigii M. Zohary et P.H. Davis. J. Agr. Food Chem. 2004, 52, 1132–1137. [Google Scholar] [CrossRef]
- Thanos, C. A. Plant-animal interactions in Mediterranean-Type ecosystems; Arianoutsou, M., Groves, R. H., Eds.; Kluwer Academic Publishers: Dordrect, The Netherlands, 1994; pp. 3–11. [Google Scholar]
- Tsaknis, J; Lalas, S. Extraction and identification of natural antioxidant from Sideritis euboea (mountain tea). J. Agric. Food Chem. 2005, 53, 6375–6381. [Google Scholar] [CrossRef]
- Tsaknis, J.; Hole, M.; Smith, G.; Lalas, S.; Tychopoulos, V. An HPLC rapid method of determining malondialdehyde (MDA) for evaluation of rancidity in edible oils. Analyst 1998, 123, 325–327. [Google Scholar] [CrossRef]
- Velasco, J.; Andersen, M. L.; Skibsted, L. H. Evaluation of oxidative stability of vegetable oils by monitoring the tendency to radical formation. A comparison of electron spin resonance spectroscopy with the Rancimat method and differential scanning calorimetry. Food Chem. 2004, 85, 623–632. [Google Scholar] [CrossRef]
- Sample Availability: Available from the authors.
© 2007 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Gortzi, O.; Lala, S.; Chinou, I.; Tsaknis, J. Evaluation of the Antimicrobial and Antioxidant Activities of Origanum dictamnus Extracts before and after Encapsulation in Liposomes. Molecules 2007, 12, 932-945. https://doi.org/10.3390/12050932
Gortzi O, Lala S, Chinou I, Tsaknis J. Evaluation of the Antimicrobial and Antioxidant Activities of Origanum dictamnus Extracts before and after Encapsulation in Liposomes. Molecules. 2007; 12(5):932-945. https://doi.org/10.3390/12050932
Chicago/Turabian StyleGortzi, Olga, Stavros Lala, Ioanna Chinou, and John Tsaknis. 2007. "Evaluation of the Antimicrobial and Antioxidant Activities of Origanum dictamnus Extracts before and after Encapsulation in Liposomes" Molecules 12, no. 5: 932-945. https://doi.org/10.3390/12050932







