Synthesis and Anti-tumor Activities of Novel [1,2,4]triazolo[1,5-a]pyrimidines
Abstract
:Introduction
Results and Discussion
Chemistry
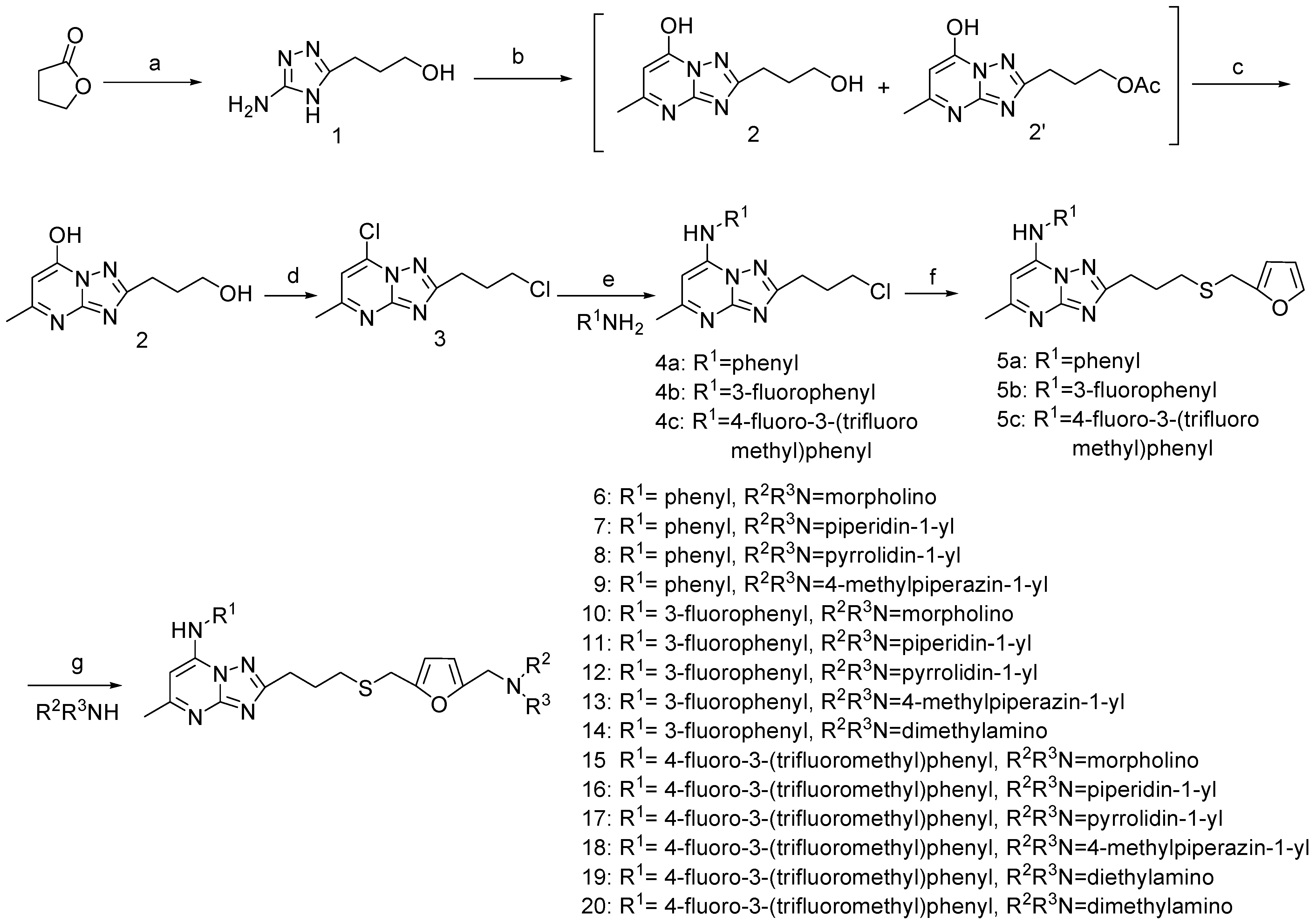
Anticancer activities
| Compd. | IC50 (μM) | Compd. | IC50 (μM) | ||
|---|---|---|---|---|---|
| Bel-7402 | HT-1080 | Bel-7402 | HT-1080 | ||
| 6 | >300 | >300 | 14 | 54.1 | 30.8 |
| 7 | 72.5 | 52.1 | 15 | 44.6 | 36.3 |
| 8 | 67.3 | 57.4 | 16 | 42.1 | 32.2 |
| 9 | >300 | 237 | 17 | 13.9 | 28.4 |
| 10 | >300 | 280 | 18 | 22.1 | 29.3 |
| 11 | 45.8 | 35.1 | 19 | 12.3 | 6.1 |
| 12 | 77.6 | 41.4 | 20 | 22.2 | 14.8 |
| 13 | >300 | 261.9 | cisplatin | 35.5 | 22.7 |
Conclusions
Experimental Section
General
5-Amino-3-(3-hydroxypropyl)-4H-[1,2,4]triazole (1).
7-Hydroxyl-2-(3-hydroxypropyl)-5-methyl-[1,2,4]triazolo[1,5-a]pyrimidine (2).
7-Chloro-2-(3-chloropropyl)-5-methyl-[1, 2, 4]triazolo[1,5-a]pyrimidine (3).
General procedure for the synthesis of 7-anilino-5-methyl-2-(3-chloroproyl)-[1,2,4]triazolo[1,5-a]pyrimidines 4a-4c.
General procedure for the synthesis of 7-anilino-5-methyl-2-(3-(furan-2-ylmethylthio)propyl)- [1,2,4]triazolo [1,5-a]pyrimidines 5a-5c.
General procedure for the synthesis of 6-20.
7-Phenylamino-5-methyl-2-(3-((5-(morpholinomethyl)furan-2-yl)methylthio)propyl)-[1,2,4]triazolo [1,5-a]pyrimidine (6).
7-Phenylamino-5-methyl-2-(3-((5-(piperidin-1-ylmethyl)furan-2-yl)methylthio)propyl)-[1,2,4]triazolo [1,5-a]pyrimidine (7).
7-Phenylamino-5-methyl-2-(3-((5-(pyrrolidin-1-ylmethyl)furan-2-yl)methylthio)propyl)-[1,2,4]triazolo [1,5-a]pyrimidine (8).
7-Phenylamino-5-methyl-2-(3-((5-((4-methylpiperazin-1-yl)methyl)furan-2-yl)methylthio)propyl)-[1,2,4] triazolo[1,5-a]pyrimidine (9).
7-(3-Fluorophenylamino)-5-methyl-2-(3-((5-(morpholinomethyl)furan-2-yl)methylthio)propyl)-[1,2,4] triazolo[1,5-a]pyrimidine (10).
7-(3-Fluorophenylamino)-5-methyl-2-(3-((5-(piperidin-1-ylmethyl)furan-2-yl)methylthio)propyl) [1,2,4] triazolo[1,5-a]pyrimidine (11).
7-(3-Fluorophenylamino)-5-methyl-2-(3-((5-(pyrrolidin-1-ylmethyl)furan-2-yl)methylthio)propyl)-[1,2,4] triazolo[1,5-a]pyrimidine (12).
7-(3-Fluorophenylamino)-5-methyl-2-(3-((5-((4-methylpiperazin-1-yl)methyl)furan-2-yl)methylthio)pro pyl)-[1,2,4]triazolo[1,5-a]pyrimidine (13).
7-(3-Fluorophenylamino)-5-methyl-2-(3-((5-((dimethylamino)methyl)furan-2-yl)methylthio)propyl)-[1,2,4]triazolo[1,5-a]pyrimidine (14).
7-(4-Fluoro-3-(trifluoromethyl)phenylamino)-5-methyl-2-(3-((5-(morpholinomethyl)furan-2-yl)methyl lthio)propyl)-[1,2,4]triazolo[1,5-a]pyrimidine (15).
7-(4-Fluoro-3-(trifluoromethyl)phenylamino)-5-methyl-2-(3-((5-(piperidin-1-ylmethyl)furan-2-yl)meth ylthio)propyl)-[1,2,4]triazolo[1,5-a]pyrimidine (16).
7-(4-Fluoro-3-(trifluoromethyl)phenylamino)-5-methyl-2-(3-((5-(pyrrolidin-1-ylmethyl)furan-2-yl) methylthio)propyl)-[1,2,4]triazolo[1,5-a]pyrimidine (17).
7-(4-Fluoro-3-(trifluoromethyl)phenylamino)-5-methyl-2-(3-((5-((4-methylpiperazin-1-yl)methyl)furan-2-yl)methylthio)propyl)-[1,2,4]triazolo[1,5-a]pyrimidine (18).
7-(4-Fluoro-3-(trifluoromethyl)phenylamino)-5-methyl-2-(3-((5-((diethylamino)methyl)furan-2-yl)-methylthio)propyl)-[1,2,4]triazolo[1,5-a]pyrimidine (19).
7-(4-Fluoro-3-(trifluoromethyl)phenylamino)-5-methyl-2-(3-((5-((dimethylamino)methyl)furan-2-yl)-methylthio)propyl)-[1,2,4]triazolo[1,5-a]pyrimidine (20).
Pharmacology
References and Notes
- Ordentlich, P.; Yan, Y.; Zhou, S.; Heyman, R. A. Identification of the Antineoplastic Agent 6-Mercaptopurine as an Activator of the Orphan Nuclear Hormone Receptor Nurr1. J. Bio. Chem. 2003, 278, 24791–24799. [Google Scholar] [CrossRef]
- Hoffmann, M.; Chrzanowska, M.; Hermann, T.; Rychlewski, J. Modeling of purine derivatives transport across cell membrance based on their partition coefficient determination and quantum chemical calculations. J. Med. Chem. 2005, 48, 4482–4486. [Google Scholar] [CrossRef]
- Monaco III, E. A.; Beaman-Hall, C. M.; Mathur, A.; Vallano, M. L. Roscovitine, olomoucine, purvalanol: inducers of apoptosis in maturing cerebellar granule neurons. Biochem Pharm. 2004, 67, 1947–1964. [Google Scholar] [CrossRef]
- Knockaert, M.; Gray, N.; Damiens, E.; Chang, Y. T.; Grellier, P.; Grant, K.; Fergusson, D.; Mottram, J.; Soete, M.; Dubremetz, J. F.; Roch, K. L.; Doerig, C.; Schultz, PG.; Meijer, L. Intracellular targets of cyclin-dependent kinase inhibitors: identification by affinity chromatography using immobilized inhibitors. Chem. Biol. 2000, 7, 411–422. [Google Scholar] [CrossRef]
- Bach, S.; Knockaert, M.; Reinhardt, J.; Lozach, O.; Schmitt, S.; Baratte, B.; Koken, M.; Coburn, S. P.; Tang, L.; Jiang, T.; Liang, D. C.; Galons, H.; Dierick, J. F.; Pinna, L. A.; Meggio, F.; Totzke, F.; Schachtele, C.; Lerman, A. S.; Carnero, A.; Wan, Y. Q.; Gray, N.; Meijer, L. Roscovitine Targets, Protein Kinases and Pyridoxal Kinase. J. Bio. Chem 2005, 280, 31208–31219. [Google Scholar] [CrossRef]
- Gaagjee, A.; Lin, X.; Kisliuk, R. L.; McGuire, J. J. Synthesis of N-{4-[(2,4-diamino-5-methyl -4,7-dihydro-3H-pyrro[2,3-d]pyrimidin-6-yl)thio]benzoyl}-l-glutamic acid and N-{4-[(2-amino-4-oxo-5-methyl-4,7-dihydro-3H-pyrro[2,3-d]pyrimidin-6-yl)thio]benzoyl}-l-glutamic acid as dual inhibitors of dihydro folate reductase and thymidylate synthase and as potential antitumor agents. J. Med. Chem. 2005, 48, 7215–7222. [Google Scholar] [CrossRef]
- Schenone, S.; Bruno, O.; Ranise, A.; Bondavalli, F.; Brullo, C.; Fossa, P.; Mosti, L.; Menozzi, G.; Carraro, F.; Naldini, A.; Bernini, C.; Manetti, F.; Botta, M. New pyrazolo[3,4-d]pyrimidines endowed with A431: antiproliferative activity and inhibitory properties of Src phosphorylation. Bioorg. Med. Chem. Lett. 2004, 14, 2511–2517. [Google Scholar] [CrossRef]
- Markwalder, J. A.; Arnone, M. R.; Benfield, P. A.; Biosdir, M.; Boisclair, M.; Burton, C. R.; Chang, C. H.; Cox, S. S.; Czerniak, P. M.; Dean, C. L.; Doleniak, D.; Grafstrom, R.; Harrison, B. A.; Kaltenbach, R. F.; Nugiel, D. A.; Rossi, K. A.; Sherk, S. R.; Sisk, L. M.; Stouten, P.; Trainor, G. L.; Worland, P.; Seitz, S. P. Synthesis and biological evaluation of 1-aryl-4,5-dihydro-1H-pyrazolo[3,4-d]pyrimidin-4-one inhibitors of cyclin-dependent kinases. J. Med. Chem. 2004, 47, 5894–5911. [Google Scholar]
- Jaramillo, C.; Diego, J. E.; Hamdouchi, C.; Collins, E.; Keyser, H.; Sanchez-Martınez, C.; Prado, M.; Norman, B.; Brooks, H. B.; Watkins, S. A.; Spencer, C. D.; Dempsey, J. A.; Anderson, B. D.; Campbell, R. M.; Leggett, T.; Patel, B.; Schultz, R. M.; Espinosa, J.; Vieth, M.; Zhang, F. M.; Timm, D. E. Aminoimidazo[1,2-a]pyridines as a new structural class of cyclin-dependent kinase inhibitors. Part 1: Design, synthesis, and biological evaluation. Bioorg. Med. Chem. Lett. 2004, 14, 6095–6099. [Google Scholar] [CrossRef]
- Havlicek, L.; Fuksova, K.; Krystof, V.; Orsag, M.; Vojtesek, B.; Strnad, M. 8-Azapurines as new inhibitors of cyclin-dependent kinases. Bioorg. Med. Chem. 2005, 13, 5399–5407. [Google Scholar]
- Brana, M. F.; Cacho, M.; Garcıa, M. L.; Mayoral, E. P.; Lopez, B.; de Pascual-Teresa, B.; Ramos, A.; Acero, N.; Llinares, F.; Munoz-Mingarro, D.; Lozach, O.; Meijer, L. Pyrazolo[3,4-c]pyridazines as Novel and Selective Inhibitors of Cyclin-Dependent Kinases. J. Med. Chem. 2005, 48, 6843–6854. [Google Scholar]
- Zurbonsen, K.; Michel, A.; Bonnet, P. A.; Gannoun-Zaki, L.; Mathieu, M. N.; Chevillard, C. Apoptotic effects of imidazow1,2-axpyrazine derivatives in the human Dami cell line. Eur. J. Pharmacol. 1997, 320, 215–221. [Google Scholar]
- Bower, J. F.; Cansfield, A.; Jordan, Allan.; Parratt, Martin.; Walmsley, L.; Willianson, D. [1,2,4]Triazolo[1,5-a]pyrimidines and their use in medicine. WO 2004108136, 2004. [Chem. Abstr. 2005, 142, P56337h]. [Google Scholar]
- Schmitt, M. R.; Kirsch, D. R.; Harris, J. E.; Beyer, C. F.; Pees, K. J.; Carter, P.; Pfrengle, W.; Albert, G. Substituted triazolopyrimidines as anticancer agents. WO 0202563, 2002. [Chem. Abstr. 2002, 136, P96032n]. [Google Scholar]
- Zhang, N.; Ayral-Kaloustian, S.; Nguyen, T.; Afragola, J.; Hernandez, R.; Lucas, J. Synthesis and SAR of [1,2,4]Triazolo[1,5-a]pyrimidines, a Class of Anticancer Agents with a Unique Mechanism of Tubulin Inhibition. J. Med. Chem. 2007, 50, 319–327. [Google Scholar]
- Schiemann, K.; Hoelzemann, G.; Rautenberg, W. Amine derivatives. WO 2005054246, 2005. [Chem. Abstr. 2005, 143, P60006n]. [Google Scholar]
- Kazuhiro, T.; Hiroshi, S.; Eriko, T. New medical application of [1,2,4]Triazolo[1,5-a]pyrimidine derivative. JP 2005154335, 2005. [Chem. Abstr. 2005, 143, P38379q]. [Google Scholar]
- Okabe, T.; Bhooshan, B.; Novinson, T.; Hillyard, I. W.; Garner, G. E.; Robins, R. K. Dialkyl bicyclic heterocycles with a bridgehead nitrogen as purine analoges possing significant cardiac inotropic activity. J. Heterocycl. Chem. 1983, 20, 735–751. [Google Scholar] [CrossRef]
- Walter, R.; Joachim, V. Umsetzung von Aminoguanidin rnit Lactonen und Carbonsaureanhyd riden. Chem. Ber. 1968, 101, 2117–2123. [Google Scholar]
- Sample Availability: Samples of the compounds mentioned above are available from authors
© 2007 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Zhao, X.-L.; Zhao, Y.-F.; Guo, S.-C.; Song, H.-S.; Wang, D.; Gong, P. Synthesis and Anti-tumor Activities of Novel [1,2,4]triazolo[1,5-a]pyrimidines. Molecules 2007, 12, 1136-1146. https://doi.org/10.3390/12051136
Zhao X-L, Zhao Y-F, Guo S-C, Song H-S, Wang D, Gong P. Synthesis and Anti-tumor Activities of Novel [1,2,4]triazolo[1,5-a]pyrimidines. Molecules. 2007; 12(5):1136-1146. https://doi.org/10.3390/12051136
Chicago/Turabian StyleZhao, Xiang-Lin, Yan-Fang Zhao, Shu-Chun Guo, Hai-Sheng Song, Ding Wang, and Ping Gong. 2007. "Synthesis and Anti-tumor Activities of Novel [1,2,4]triazolo[1,5-a]pyrimidines" Molecules 12, no. 5: 1136-1146. https://doi.org/10.3390/12051136
APA StyleZhao, X.-L., Zhao, Y.-F., Guo, S.-C., Song, H.-S., Wang, D., & Gong, P. (2007). Synthesis and Anti-tumor Activities of Novel [1,2,4]triazolo[1,5-a]pyrimidines. Molecules, 12(5), 1136-1146. https://doi.org/10.3390/12051136




