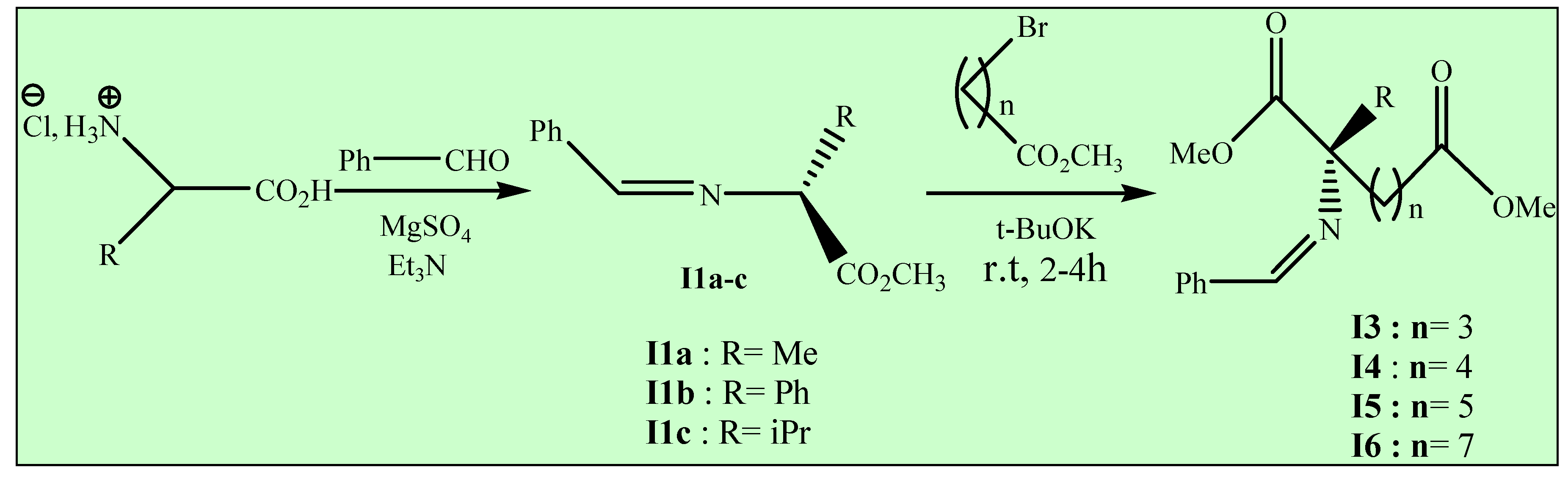
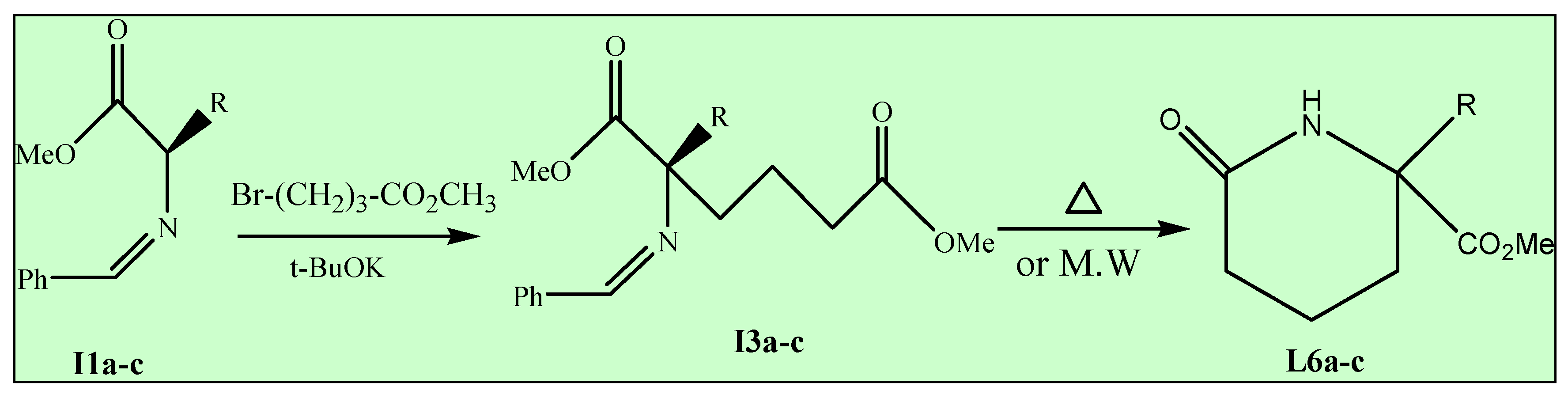
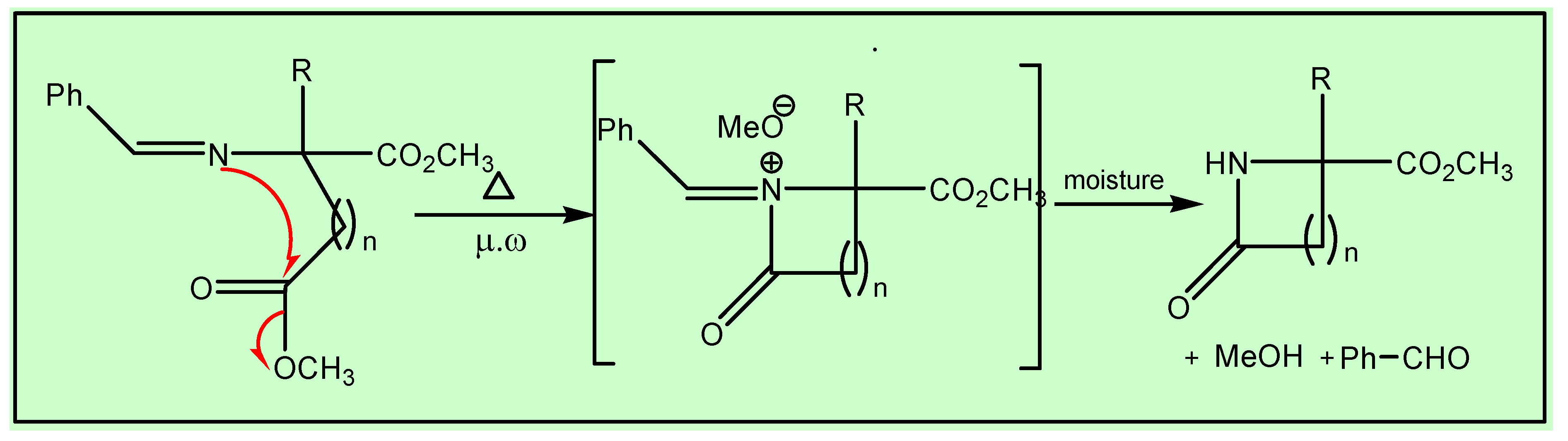
Method B (Conventional heating)
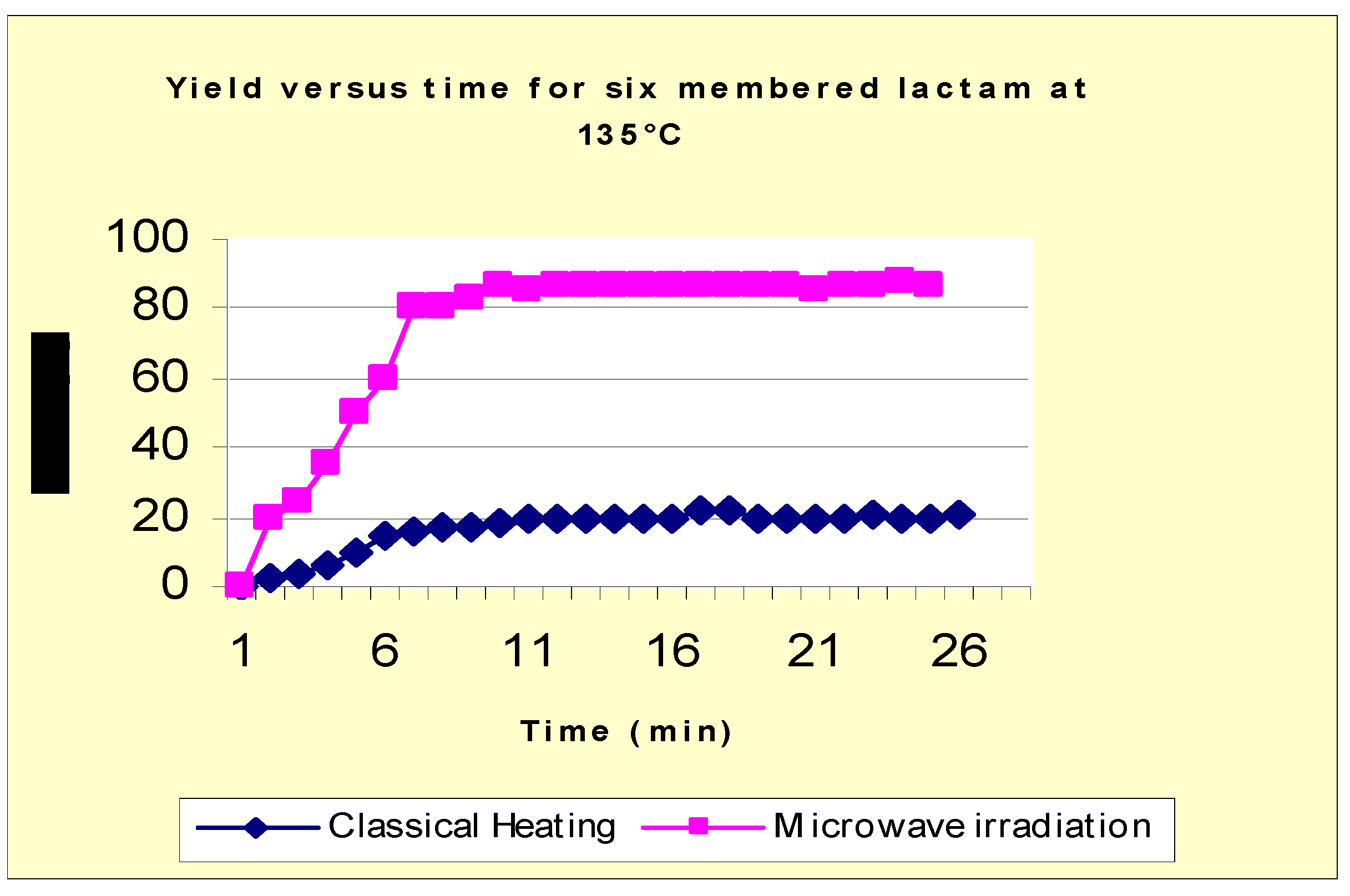
Reactions were performed under the same conditions using a previously heated oil bath, set at a temperature slightly higher (about 20°C) than the temperature measured in the microwave oven. As this study was done only for the comparison purposes and to see the influence of reaction times on the yields, the products were only analysed by 1H-NMR.
Methyl 2-(benzylideneamino)propanoate (
I1a): C
11H
13NO
2; Yield: 91%; Colorless oil, bp: 85°C/ 6.3x10
-2Torr (lit [
23] mp 105-107 °C/2 Torr);
1H-NMR δ: 1.52-1.54 (d, 3H,
J= 3 Hz, C
H3), 3.74 (s, 3H, MeCO), 4.12-4.19 (q, 1H,
J= 6.81 Hz, C
H), 7.38-7.43 (m, 5H, Ph), 8.31 (s, 1H, C
H=);
13C-NMR δ: 19.45 (
CH
3), 52.42 (O
CH
3), 67.98 (
CH), 128.35 (Ar
CH), 128.51 (Ar
CH), 131.13 (Ar
CH), 135.69 (Ar
C), 163.04 (H
C=N), 176.99 (
C=O); MS m/z: found [M-.CH
3]
+ 176.07115, requires 176.0711.
Dimethyl 2-(benzylideneamino)-2-methylpentanedioate (
I2a) [
23]: C
15H
19NO
4: Yield: 70%; yellow oil, bp: 90°C/6.3x10
-2Torr;
1H-NMR δ: 1.50 (s, 3H, C
H3), 2.12-2.16 (t, 2H,
J=7.75 Hz, C
H2), 2.43-2.54 (t, 2H,
J= 8.21 Hz, C
H2), 3.62 (s, 3H,
MeCO), 3.71 (s, 3H,
MeCO), 7.27-7.41 (m, 5H, Ph), 8.26 (s, 1H, C
H=);
13C NMR δ: 25.88 (
CH
3), 30.46 (
CH
2), 32.67 (
CH
2), 51.60 (O
CH
3), 53.09 (O
CH
3), 62.83 (
CH), 127 (Ar
CH), 128.59 (Ar
CH), 128.85 (Ar
CH), 135.69 (Ar
C), 163.23 (H
C=N), 174.92 (
C=O), 178.17 (
C=O); MS m/z: Found [M-.CH
3]
+ 262.1099, requires 262.1079; Found [M-.OCH
3]
+ 246.1153, requires 246.1130.
Dimethyl 2-(benzylideneamino)-2-methylhexanedioate (I3a): C16H21NO4; Yield: 91%; yellow oil, bp: 90°C/6.3x10-2Torr; 1H-NMR δ: 1.60 (s, 3H, CH3), 1.75-1.82 (qt, 2H, J= 7.8 Hz, CH2), 1.98-2.06 (t, 2H, J=9 Hz, CH2), 2.24-2.30 (t, 2H, J=8.2 Hz, CH2), 3.61 (s, 3H, MeCO), 3.70 (s, 3H, MeCO), 7.15-7.81 (m, 5H, Ph), 8.33 (s, 1H, CH=); 13C-NMR δ: 19.88 (CH2), 24.76 (CH3), 33.46 (CH2), 35.67 (CH2), 52.43 (OCH3), 54.70 (OCH3), 61.41 (CH), 126.68 (ArCH), 128.14 (ArCH), 130.85 (ArCH), 136.69 (ArC), 160.23 (HC=N), 174.42 (C=O), 176.27 (C=O); MS m/z: Found [M-.CO2CH3]+ 232.3061, requires 232.3070.
Dimethyl 2-(benzylideneamino)-2-methylheptanedioate (I4a): C17H23NO4; Yield: 98%; yellow oil, bp: 85°C/8x10-2Torr; 1H-NMR δ: 1.52 (s, 3H, CH3), 1.37-1.77 (m, 4H, 2CH2), 1.91-2.01 (t, 2H, J=6 Hz, CH2), 2.32-2.40 (t, 2H, J=12 Hz, CH2), 3.68 (s, 3H, MeCO), 3.78 (s, 3H, MeCO), 7.33-7.82 (m, 5H, Ph), 8.28 (s, 1H, CH=); 13C-NMR δ: 23.10 (CH2), 23.82 (CH2), 25.22 (CH3), 33.90 (CH2), 39.74 (CH2), 51.47 (OCH3), 52.15 (OCH3), 68.42 (CH),127.83 (ArCH), 128.79 (ArCH), 130.87 (ArCH), 136.36 (ArC), 159.26 (HC=N), 174.05 (C=O), 174.68 (C=O); MS m/z: Found [M-.OCH3]+ 274.1443, requires 274.1452; Found [M-.CO2CH3]+ 246.1494, requires 246.1493.
Dimethyl 2-(benzylideneamino)-2-methyloctanedioate (I5a): C18H25NO4; Yield: 97.3%; yellow oil, bp: 85°C/8x10-2Torr; 1H-NMR δ: 1.33-1.39 (qt, 2H, J= 6 Hz, CH2), 1.50 (s, 3H, CH3), 1.61-1.69 (qt, 2H, J=5.46 Hz, CH2), 1.90-1.98 (qt, 2H, J=5.2 Hz, CH2), 2.29-2.34 (t, 2H, J=8 Hz, CH2), 3.66 (s, 3H, MeCO), 3.75 (s, 3H, MeCO), 7.35-7.80 (m, 5H, Ph), 8.26 (s, 1H, CH=); 13C-NMR δ: 23.11 (CH2), 23.88 (CH2), 24.78 (CH2), 29.42 (CH3), 34.00 (CH2), 39.94 (CH2), 51.48 (OCH3), 52.14 (OCH3), 68.51 (CH), 128.28 (ArCH), 128.56 (ArCH), 130.85 (ArCH), 136.06 (ArC), 159.18 (HC=N), 174.21 (C=O), 175.22 (C=O); MS m/z: Found [M-.CO2CH3]+ 260.1650, requires 260.1646.
Dimethyl 2-(benzylideneamino)-2-methyldecanedioate (I6a): C20H29NO4; Yield: 96.7%; yellow oil, bp: 80°C/9x10-2Torr; 1H-NMR δ: 1.22-1.46 (m, 6H, 3CH2), 1.52 (s, 3H, CH3), 1.60-1.73 (t, 2H, J=7.04 Hz, CH2), 1.86-2.19 (m, 4H, 2CH2), 2.29-2.33 (t, 2H, J=7.59 Hz, CH2), 3.69 (s, 3H, MeCO), 3.78 (s, 3H, MeCO), 7.32-7.80 (m, 5H, Ph), 8.28 (s, 1H, CH=); 13C-NMR δ: 22.78 (CH2), 23.18 (CH2), 23.31 (CH2), 24.45 (CH2), 28.57 (CH2), 29.42 (CH3), 34.01 (CH2), 40.24 (CH2), 51.38 (OCH3), 51.80 (OCH3), 63.50 (CH), 128.27 (ArCH), 128.54 (ArCH), 131.06 (ArCH), 137.06 (ArC), 162.26 (HC=N), 174.12 (C=O), 176.21 (C=O); MS m/z: Found [M-.CO2CH3]+ 288.1963, requires 288.1980.
Methyl (benzylideneamino)(phenyl)acetate (I1b): C16H15NO2; Yield:78%; pale yellow solid, mp: 63°C, (from EtOH); 1H-NMR δ: 3.77 (s, 3H, MeCO), 5.23 (s, 1H, CH), 7.28-7.87 (m, 10H, 2Ph), 8.37 (s, 1H, CH=); 13C-NMR δ: 52.52 (OCH3), 69.78 (CH), 127.84 (ArCH), 128.12 (ArCH), 128.40 (ArCH), 128.68 (ArCH), 128.72 (ArCH), 131.28 (ArCH), 135.68 (ArC), 138.11 (ArC), 163.81 (HC=N); 171.58 (C=O); MS m/z: Found [M-.CO2CH3]+ 194.0969, requires 194.0980.
Dimethyl 2-(benzylideneamino)-2-phenylpentanedioate (I2b): C20H21NO4; Yield: 85%; yellow oil, bp: 85°C/8x10-2Torr; 1H-NMR δ: 2.32-2.44 (t, 2H, J=6.5 Hz, CH2), 2.48-2.64 (t, 2H, J=7 Hz, CH2), 3.63 (s, 3H, MeCO), 3.79 (s, 3H, MeCO), 7.24-7.74 (m, 10H, 2Ph), 8.39 (s, 1H, CH=); 13C-NMR δ: 30.12 (CH2), 31.05 (CH2), 50.42 (OCH3), 52.52 (OCH3), 70.18 (CH), 126.74 (ArCH), 127.12 (ArCH), 128.39 (ArCH), 128.78 (ArCH), 129.52 (ArCH), 131.33 (ArCH), 135.58 (ArC), 137.21 (ArC), 163.78 (HC=N), 173.78 (C=O), 174.65 (C=O); MS m/z: Found M+. 339.1470, requires 339.1502.
Dimethyl 2-(benzylideneamino)-2-phenylhexanedioate (I3b): C21H23NO4; Yield: 85.6%; yellow oil, bp: 92°C/10-2Torr; 1H NMR δ: 1.52-1.80 (qt, 2H, J=5.16 Hz, CH2), 2.28-2.32 (t, 2H, J=6 Hz, CH2), 2.38-2.46 (t, 2H, J=6.7 Hz, CH2), 3.63 (s, 3H, MeCO), 3.77 (s, 3H, MeCO), 7.33-7.89 (m, 10H, 2Ph), 8.22 (s, 1H, CH=); 13C-NMR δ: 19.28 (CH2), 27.05 (CH2), 29.25 (CH2), 51.32 (OCH3), 52.29 (OCH3), 69.28 (CH), 127.14 (ArCH), 127.21 (ArCH), 128.30 (ArCH), 128.88 (ArCH), 129 (ArCH), 131.13 (ArCH), 136.08 (ArC), 137.11 (ArC), 161.78 (HC=N), 174.58 (C=O), 175.15 (C=O); MS m/z: Found [M-.CO2CH3]+ 294.1494, requires 294.1489.
Dimethyl 2-(benzylideneamino)-2-phenylheptanedioate (I4b): C22H25NO4; Yield: 98%; yellow oil, bp: 90°C/10-1Torr; 1H-NMR δ: 1.24-1.46 (m, 4H, 2CH2), 1.56-1.64 (t, 2H, J=9 Hz, CH2), 2.23-2.26 (t, 2H, J=4.23 Hz, CH2), 3.60 (s, 3H, MeCO), 3.73 (s, 3H, MeCO), 7.27-7.81 (m, 10H, 2Ph), 8.17 (s, 1H, CH=); 13C-NMR δ: 23.43 (CH2), 25.26 (CH2), 33.88 (CH2), 40.29 (CH2), 51.40 (OCH3), 52.35 (OCH3), 74.89 (CH), 126.84 (ArCH), 127.40 (ArCH), 128.39 (ArCH), 128.49 (ArCH), 128.60 (ArCH), 131.11 (ArCH), 136.37 (ArC), 141.92 (ArC), 161.16 (HC=N), 173.76 (C=O), 176.15 (C=O); MS m/z: Found [M-.CO2CH3]+ 308.1650, requires 308.1657.
Dimethyl 2-(benzylideneamino)-2-phenyloctanedioate (I5b): C23H27NO4; Yield: 97.11%; yellow oil, bp: 90°C/2x10-1Torr; 1H-NMR δ: 1.27-1.62 (m, 6H, 3CH2), 1.79-1.83 (t, 2H, J=7.5 Hz, CH2), 2.18-2.26 (t, 2H, J=4.4 Hz, CH2), 3.63 (s, 3H, MeCO), 3.72 (s, 3H, MeCO), 7.33-7.89 (m, 10H, 2Ph), 8.28 (s, 1H, CH=); 13C-NMR δ: 23.46 (CH2), 24.70 (CH2), 29.43 (CH2), 33.98 (CH2), 40.49 (CH2), 51.42 (OCH3), 52.32 (OCH3), 74.97 (CH), 126.85 (ArCH), 127.35 (ArCH), 128.36 (ArCH), 128.48 (ArCH), 128.60 (ArCH), 131.07 (ArCH), 139.27 (ArC), 141.81 (ArC), 161.06 (HC=N), 174.56 (C=O), 175.75 (C=O); MS m/z: Found [M-.CO2CH3]+ 322.1807, requires 322.1805.
Dimethyl 2-(benzylideneamino)-2-phenyldecanedioate (I6b): C25H31NO4; Yield: 96.5%; yellow oil, bp: 95°C/2x10-1Torr; 1H-NMR δ: 1.28-1.47 (t, 3H, J=13 Hz, CH2), 1.58-1.80 (m, 4H, 2CH2), 1.83-1.85 (t, 2H, J=11.5 Hz, CH2), 2.22-2.35 (m, 4H, 2CH2), 2.58-2.80 (t, 2H, J=11 Hz, CH2), 3.62 (s, 3H, MeCO), 3.70 (s, 3H, MeCO), 7.14-7.87 (m, 10H, 2Ph), 8.21 (s, 1H, CH=); 13C-NMR δ: 19.86 (CH2), 23.70 (CH2), 25.43 (CH2), 29.03 (CH2), 30.19 (CH2), 34.05 (CH2), 40.58 (CH2), 51.32 (OCH3), 52.12 (OCH3), 73.67 (CH), 126.89 (ArCH), 128.34 (ArCH), 128.48 (ArCH), 128.59 (ArCH), 129.50 (ArCH), 130.06 (ArCH), 138.37 (ArC), 142.71 (ArC), 161.26 (HC=N), 173.46 (C=O), 174.75 (C=O); MS m/z: Found [M-.CO2CH3]+ 350.2120, requires 350.2117.
Methyl 2-(benzylideneamino)-3-methylbutanoate (I1c): C13H17NO2; Yield: 94.5%; yellow oil, bp: 85°C/2x10-2Torr; 1H-NMR: δ: 0.99-0.94 (dd, 6H, J= 6.88 Hz, 2CH3), 2.37-2.43 (m, 1H, J= 6 Hz, CH), 3.67-3.71 (d, 1H, J= 12 Hz, CH), 3.75 (s, 3H, MeCO), 7.39-7.84 (m, 5H, Ph), 8.26 (s, 1H, CH=); 13C- NMR δ: 18.66 (CH3), 19.51 (CH3), 31.73 (CH), 51.93 (OCH3), 80.39 (CH), 128.32 (ArCH), 128.51 (ArCH), 131.07 (ArCH), 135.69 (ArC), 163.33 (HC=N), 172.44 (C=O); MS m/z: Found [M-.CO2CH3]+ 160.2434, requires 160.2441.
Dimethyl 2-(benzylideneamino)-2-isopropylpentanedioate (I2c): C17H23NO4; Yield: 81.2%; yellow oil, bp: 90°C/10-2Torr; 1HNMR δ: 0.95-1.01 (dd, 6H, J= 9 Hz, 2CH3), 1.13-1.18 (t, 2H, J= 3 Hz, CH2), 2.34-2.43 (m, 1H, J= 9 Hz, CH), 2.70-2.96 (t, 2H, J= 9 Hz, CH2), 3.78 (s, 3H, MeCO), 3.82 (s, 3H, MeCO), 7.44-7.80 (m, 5H, Ph), 8.28 (s, 1H, CH=); 13C-NMR δ: 19.80 (CH3), 20.17 (CH3), 29.85 (CH2), 30.25 (CH), 31.13 (CH2), 50.91 (OCH3), 52.06 (OCH3), 68.20 (C), 128.59-136.8 (Ar), 162.23 (HC=N), 174.64 (C=O), 175.33 (C=O); MS m/z: Found [M-.CO2CH3]+ 246.1619, requires 246.1627.
Dimethyl 2-(benzylideneamino)-2-isopropylhexanedioate (I3c): C18H25NO4 ; Yield: 89%; yellow oil, bp: 90°C/2x10-1Torr; 1H-NMR δ: 0.93-1.05 (dd, 6H, J= 9 Hz, 2CH3), 2.04-2.21 (qt, 2H, J= 2.3 Hz, CH2), 2.37-2.44 (t, 2H, J= 10.7 Hz, CH2), 2.51-2.57 (m, 1H, J= 12 Hz, CH), 3.37-3.43 (t, 2H, J= 3 Hz, CH2), 3.69 (s, 3H, MeCO), 3.75 (s, 3H, MeCO), 7.34-7.82 (m, 5H, Ph), 8.25 (s, 1H, CH=); 13C-NMR δ: 20.15 (CH3), 20.57 (CH3), 21.11 (CH2), 29.95 (CH2), 30.23 (CH), 31.03 (CH2), 51.82 (OCH3), 52.03 (OCH3), 62.42 (C), 128.79-136.77 (Ar), 163.03 (HC=N), 175.34 (C=O), 175.69 (C=O); MS m/z: Found [M-.CO2CH3]+ 260.1778, requires 260.1784.
Dimethyl 2-(benzylideneamino)-2-isopropylheptanedioate (I4c): C19H27NO4; Yield: 86.7%; yellow oil, bp: 96°C/ 6.3.10-4Torr; 1H-NMR δ: 0.93-1.05 (dd, 6H, J= 9 Hz, 2CH3),1.60-2.00 (m, 4H, J= 6.72 Hz, 2CH2), 2.22-2.43 (qt, 1H, J= 4.57 Hz, CH), 2.44-2.47 (t, 1H, J= 3 Hz, CH2), 3.40-3.45 (t, 2H, J= 4.05 Hz, CH2), 3.70 (s, 3H, MeCO), 3.77 (s, 3H, MeCO), 7.44-7.90 (m, 5H, Ph), 8.37 (s, 1H, CH=); 13C- NMR δ: 19.76 (CH3), 19.98 (CH3), 20.15 (CH2), 20.66 (CH2), 30.17 (CH), 31.28 (CH2), 32.07 (CH2), 52.32 (OCH3), 52.63 (OCH3), 71.60 (C), 128.80-136.57 (Ar), 162.33 (HC=N), 173.84 (C=O), 174.29 (C=O); MS m/z: Found [M-.OCH3]+ 302.1932, requires 302.1940.
Dimethyl 2-(benzylideneamino)-2-isopropyloctanedioate (I5c): C20H29NO4\ Yield: 86%; yellow oil, bp: 106°C/6x0-4Torr; 1H-NMR δ: 0.95-1.01 (dd, 6H, J= 9 Hz, 2CH3),1.45-1.87 (m, 6H, J= 9.87 Hz, 3CH2), 1.90-2.04 (qt, 1H, J= 4.57 Hz, CH), 2.33-2.40 (t, 1H, J= 10.6 Hz, CH2), 3.39-3.47 (t, 2H, J= 4.71 Hz, CH2), 3.70 (s, 3H, MeCO), 3.78 (s, 3H, MeCO), 7.32-7.91 (m, 5H, Ph), 8.29 (s, 1H, CH=); 13C-NMR δ: 18.96 (CH3), 19.07 (CH3), 19.73 (CH2), 19.98 (CH2), 20.05 (CH2), 31.07 (CH), 31.76 (CH2), 32.01 (CH2), 51.62 (OCH3), 52.23 (OCH3), 78.90 (C), 128.70-136.82 (Ar), 156.53 (HC=N), 175.18 (C=O), 176.08 (C=O); MS m/z: Found [M-.CO2CH3]+ 288.2089, requires 288.2097.
Dimethyl 2-(benzylideneamino)-2-isopropyldecanedioate (I6c): C22H33NO4; Yield: 83.4%; yellow oil, bp: 110°C/10-3Torr; 1H-NMR δ: 0.93-1.00 (dd, 6H, J= 4.57 Hz, 2CH3),1.61-1.94 (m, 11H, CH, 5CH2), 2.33-2.40 (t, 1H, J= 10.44 Hz, CH2), 3.39-3.45 (t, 2H, J= 8.37 Hz, CH2), 3.69 (s, 3H, MeCO), 3.76 (s, 3H, MeCO), 7.37-7.82 (m, 5H, Ph), 8.26 (s, 1H, CH=); 13C-NMR δ: 18.63 (CH3), 19.48 (CH3), 21.11 (CH2), 23.40 (CH2), 28.08 (CH2), 31.59 (CH), 31.71 (CH2), 31.95 (CH2), 51.49 (CH2), 51.91 (CH2), 62.11 (OCH3), 64.25 (OCH3), 80.37 (C), 128.54-135.68 (Ar), 163.28 (HC=N), 172.41 (C=O), 173.52 (C=O); MS m/z: Found [M-.CO2CH3]+ 316.2406, requires 316.2410.
Methyl 2-methyl-5-oxopyrrolidine-2-carboxylate (L5a): C7H11NO3; Yield: 85%, viscous and brown aspect; 1H-NMR δ: 1.47 (s, 3H, CH3), 1.92-2.02 (t, 2H, J= 8.32 Hz, CH2), 2.32-2.48 (t, 2H, J= 5.87 Hz, CH2), 3.70 (s, 3H, MeCO), 6.10 (large s, 1H, NH); 13C-NMR δ: 25.88 (CH3), 30.46 (CH2), 32.67 (CH2), 53.09 (CH3CO), 62.83 (C), 174.91 (C=O), 178.17 (C=O); MS m/z: Found [M-.CO2CH3]+ 98.0608, requires 98.0605.
Methyl 2-methyl-6-oxopiperidine-2-carboxylate (L6a): C8H13NO3; Yield: 81%, brown pasty solid; 1H- NMR δ: 1.41 (s, 3H, CH3), 1.58-1.70 (qt, 2H, J= 7.56 Hz, CH2), 2.22-2.40 (t, 2H, J= 11.63 Hz, CH2), 3.40-3.53 (t, 2H, J= 10.43 (CH2), 3.75 (s, 3H, MeCO), 6.59 (large s, 1H, NH); 13C-NMR δ: 20.55 (CH3), 22.39 (CH2), 30.60 (CH2), 32.04 (CH2), 51.12 (CH3CO), 59.71 (C), 172.00 (C=O), 174.35 (C=O); MS m/z: Found [M-.CO2CH3]+ 112.07624, requires 112.0769.
Methyl 2-methyl-7-oxoazepane-2-carboxylate (L7a): C9H15NO3; Yield: 85%, brown paste; 1H-NMR δ: 1.01-1.74 (m, 4H, 2CH2),1.46 (s, 3H, CH3), 2.35-2.42 (t, 2H, J= 9 Hz, CH2), 3.15-3.36 (t, 2H, J= 10.03 Hz, (CH2), 3.71 (s, 3H, MeCO), 5.93 (large s, 1H, NH); 13C-NMR : δ: 23.10 (CH2), 23.74 (CH2), 23.81 (CH3), 29.69 (CH2), 33.89 (CH2), 52.12 (CH3CO), 68.41 (C), 174.03 (C=O), 174.66 (C=O); MS m/z: Found [M-.OCH3]+ 172.1047, requires 172.1052.
Methyl 2-methyl-8-oxoazocane-2-carboxylate (L8a): C10H17NO3; Yield: 85%, brown paste; 1H-NMR δ: 1.20-1.74 (m, 6H, 3CH2),1.53 (s, 3H, CH3), 2.27-2.35 (t, 2H, J= 10.13 Hz, CH2), 3.23-3.41 (t, 2H, J= 5.21 Hz, (CH2), 3.69 (s, 3H, MeCO), 5.84 (large s, 1H, NH); 13C-NMR δ: 23.15 (CH2), 23.74 (CH2), 23.81 (CH3), 29.69 (CH2), 33.89 (CH2), 52.12 (CH3CO), 68.41 (C), 174.03 (C=O), 174.66 (C=O); MS m/z: Found [M-.CO2H]+ 172.1320, requires 172.1337.
Methyl 2-methyl-10-oxoazecane-2-carboxylate (L9a): C12H21NO3; Yield: 83%, brown paste; 1H-NMR δ: 1.03-1.29 (m, 10H, 5CH2),1.49 (s, 3H, CH3), 1.58-1.72 (t, 2H, J= 12.5 Hz, CH2), 2.26-2.36 (t, 2H, J= 11 Hz, (CH2), 3.66 (s, 3H, MeCO), 5.56 (large s, 1H, NH); 13C-NMR δ: 18.85 (CH2), 19.25 (CH2), 20.35 (CH2), 24.25 (CH2), 25.37 (CH2), 29.69 (CH3), 34.57 (CH2), 35.79 (CH2), 51.46 (CH3CO), 66.31 (C), 175.00 (C=O), 175.75 (C=O); MS m/z: Found [M-.CO2H]+ 200.1642, requires 200.16505.
Methyl 5-oxo-2-phenylpyrrolidine-2-carboxylate (L5b): C12H13NO3 : Yield: 86%, brown dough, 1H NMR (300 MHz, CDCl3): δ: 2.60-2.66 (t, 2H, J= 3.95 Hz, CH2),3.01-3.08 (t, 2H, J= 9 Hz, CH2), 3.79 (s, 3H, MeCO), 5.78 (large s, 1H, NH), 7.35-7.79 (m, 5H, Ph), 13C NMR (75 MHz, CDCl3): δ: 20.24 (CH2), 21.87 (CH2), 30.36 (CH3CO), 66.51 (C), 126.74 (ArCH), 127.12 (ArCH), 128.39 (ArCH), 128.78 (ArCH), 129.52 (ArCH), 131.33 (ArCH), 135.58 (ArC), 137.21 (ArC), 176.70 (C=O), 176.88 (C=O); m/z: Found [M-.CO2Me]+ 160.0758, requires 160.07624.
Methyl 6-oxo-2-phenylpiperidine-2-carboxylate (L6b): C13H15NO3; Yield: 88%, brown paste; 1H- NMR δ: 1.55-1.68 (qt, 2H, J= 6 Hz, CH2), 2.25-2.31 (t, 2H, J= 5 Hz, CH2), 2.40-2.49 (t, 2H, J= 4.35 Hz, CH2), 3.80 (s, 3H, MeCO), 6.52 (large s, 1H, NH), 7.33-7.90 (m, 5H, Ph); 13C-NMR δ: 17.28 (CH2), 30.88 (CH2), 33.04 (CH2), 53.13 (CH3CO), 65.61 (C), 125.05 (ArCH), 128.30 (ArCH), 129.00 (ArCH), 140.71 (ArC), 171.80 (C=O), 171.99 (C=O); MS m/z: Found [M+.] 233.1061, requires 233.10519.
Methyl 7-oxo-2-phenylazepane-2-carboxylate (L7b): C14H17NO3; Yield: 79%, brown paste; 1H-NMR δ: 1.37-1.59 (qt, 2H, J= 6 Hz, CH2), 1.63-1.76 (qt, 2H, J= 3.5 Hz, CH2), 2.23-2.41 (t, 2H, J= 11 Hz, CH2), 3.41-3.47 (t, 2H, J= 9 Hz, CH2), 3.70 (s, 3H, MeCO), 6.02 (large s, 1H, NH), 7.32-7.83 (m, 5H, Ph); 13C-NMR δ: 22.94 (CH2), 24.82 (CH2), 36.14 (CH2), 37.64 (CH2), 52.81 (CH3CO), 65.19 (C), 125.01-127.92 (ArH), 141.75 (ArC), 174.85 (C=O), 176.02 (C=O); MS m/z: Found [M-.COOH]+ 220.1331, requires 220.13375.
Methyl 8-oxo-2-phenylazocane-2-carboxylate (L8b): C15H19NO3; Yield: 78%, brown paste; 1H-NMR δ: 1.10-1.84 (m, 6H, 3CH2), 2.21-2.41 (t, 2H, J= 12 Hz, CH2), 2.98-3.05 (t, 2H, J= 9 Hz, CH2), 3.73 (s, 3H, MeCO), 6.32 (large s, 1H, NH), 7.33-7.86 (m, 5H, Ph); 13C-NMR δ: 21.74 (CH2), 22.22 (CH2), 23.14 (CH2), 36.36 (CH2), 36.93 (CH2), 52.86 (CH3CO), 65.36 (C), 125.01-127.92 (ArH), 141.31 (ArC), 175.81 (C=O), 176.83 (C=O); MS m/z: Found [M-.COOH]+ 234.1488, requires 234.1494.
Methyl 10-oxo-2-phenylazecane-2-carboxylate (L9b): C17H23NO3; Yield: 79%, brown paste; 1H-NMR δ: 0.92-1.36 (m, 10H, 5CH2), 1.47-1.76 (t, 2H, J= 9.31 Hz, CH2), 2.21-2.37 (t, 2H, J= 10.63 Hz, CH2), 3.71 (s, 3H, MeCO), 6.36 (large s, 1H, NH), 7.34-8.06 (m, 5H, Ph), 13C-NMR δ: 18.84 (CH2), 19.04 (CH2), 20.43 (CH2), 22.42 (CH2), 30.61 (CH2), 36.73 (CH2), 37.43 (CH2), 52.81 (CH3CO), 65.52 (C), 125.11-128.35 (ArH), 141.00 (ArC), 176.37 (C=O), 176.76 (C=O); MS m/z: Found [M-.COOH]+ 262.1789, requires 262.1807.
Methyl 2-isopropyl-5-oxopyrrolidine-2-carboxylate (L5c): C9H15NO3; Yield: 85%, brown paste; 1H-NMR δ: 1.01-1.06 (dd, 6H, J= 9 Hz, 2CH3), 1.08-1.30 (m, 1H, J= 6 Hz, CH), 2.93-3.00 (t, 2H, J= 9 Hz, CH2), 3.58-3.65 (t, 2H, J= 9 Hz, CH2), 3.77 (s, 3H, MeCO), 6.09 (large s, 1H, NH); 13C-NMR δ: 18.56 (CH3), 19.15 (CH3), 28.25 (CH), 30.13 (CH2), 30.3 (CH2), 53.03 (OCH3), 69.77 (C), 172.92 (C=O), 174.51 (C=O); MS m/z: Found [M-.CO2CH3]+ 126.1030, requires 126.1052.
Methyl 2-isopropyl-6-oxopiperidine-2-carboxylate (L6c): C10H17NO3; Yield: 82%, brown paste; 1H-NMR δ: 1.04-1.29 (dd, 6H, J= 9 Hz, 2CH3), 1.82-1.95 (qt, 2H, J= 6.5 Hz, CH2), 2.03-2.09 (m, 1H, J= 6 Hz, CH), 2.18-2.24 (t, 2H, J= 9 Hz, CH2), 2.36-2.42 (t, 2H, J= 9 Hz, CH2), 3.71 (s, 3H, MeCO), 6.31 (large s, 1H, NH); 13C-NMR δ: 18.60 (CH3), 19.16 (CH3), 20.13 (CH2), 29.65 (CH), 31.14 (CH2), 34.13 (CH2), 53.28 (OCH3), 65.43 (C), 172.55 (C=O), 173.58 (C=O); MS m/z: Found [M]+ 199.1196, requires 199.1208.
Methyl 2-isopropyl-7-oxoazepane-2-carboxylate (L7c): C11H19NO3; Yield: 80%, brown paste; 1H-NMR δ: 1.04-1.27 (dd, 6H, J= 9 Hz, 2CH3), 1.32-1.45 (m, 4H, 2CH2), 1.66-1.84 (t, 2H, J= 6 Hz, CH2), 2.06-2.17 (m, 1H, J= 6 Hz, CH), 2.35-2.45 (t, 2H, J= 9 Hz, CH2), 3.70 (s, 3H, MeCO), 6.90 (large s, 1H, NH); 13C-NMR δ: 20.12 (CH3), 19.86 (CH3), 21.24 (CH2), 23.23 (CH2), 25.92 (CH2), 29.46 (CH), 34.67 (CH2), 52.68 (OCH3), 69.52 (C), 175.18 (C=O), 175.41 (C=O); MS m/z: Found [M-COOH]+ 186.1356, requires 186.1365.
Methyl 2-isopropyl-8-oxoazocane-2-carboxylate (L8c): C12H21NO3; Yield: 78%, black paste; 1H-NMR δ: 0.80-1.01 (m, 6H, 3CH2), 1.05-1.27 (dd, 6H, J= 9 Hz, 2CH3), 1.89-1.95 (t, 2H, J= 6 Hz, CH2), 2.01-2.07 (m, 1H, J= 6 Hz, CH), 2.38-2.44 (t, 2H, J= 9 Hz, CH2), 3.69 (s, 3H, MeCO), 7.13 (large s, 1H, NH), 13C-NMR δ: 19.43 (CH2), 21.43 (CH2), 21.89 (CH2), 24.32 (CH3), 25.46 (CH3), 28.20 (CH2), 31.27 (CH), 33.65 (CH2), 52.78 (OCH3), 68.72 (C), 174.98 (C=O), 176.38 (C=O); MS m/z: Found [M-COOH]+ 200.1513, requires 200.1521.
Methyl 2-isopropyl-10-oxoazecane-2-carboxylate (L9c): C14H25NO3; Yield: 76%, black paste; 1H-NMR δ: 0.86-1.02 (dd, 6H, J= 6 Hz, 2CH3), 1.17-1.44 (m, 10H, 5CH2), 1.65-1.96 (m, 1H, J= 6 Hz, CH), 2.21-2.34 (t, 2H, J= 9 Hz, CH2), 3.40-3.56 (t, 2H, J= 10.5 Hz, CH2), 3.70 (s, 3H, MeCO), 6.28 (large s, 1H, NH); 13C-NMR δ: 19.13 (CH3), 19.45 (CH3), 21.79 (CH2), 20.13 (CH2), 25.24 (CH2), 26.05 (CH), 28.15 (CH2), 30.63 (CH2). 35.12 (CH2), 35.67 (CH2), 52.98 (OCH3), 68.83 (C), 173.65 (C=O), 176.68 (C=O); MS m/z: Found [M-COOH]+ 228.18151, requires 228.1834.