An Efficient Synthesis of Pyrazolo[3,4-b]quinolin-3-amine and Benzo[b][1,8]naphthyridine Derivatives
Abstract
:Introduction
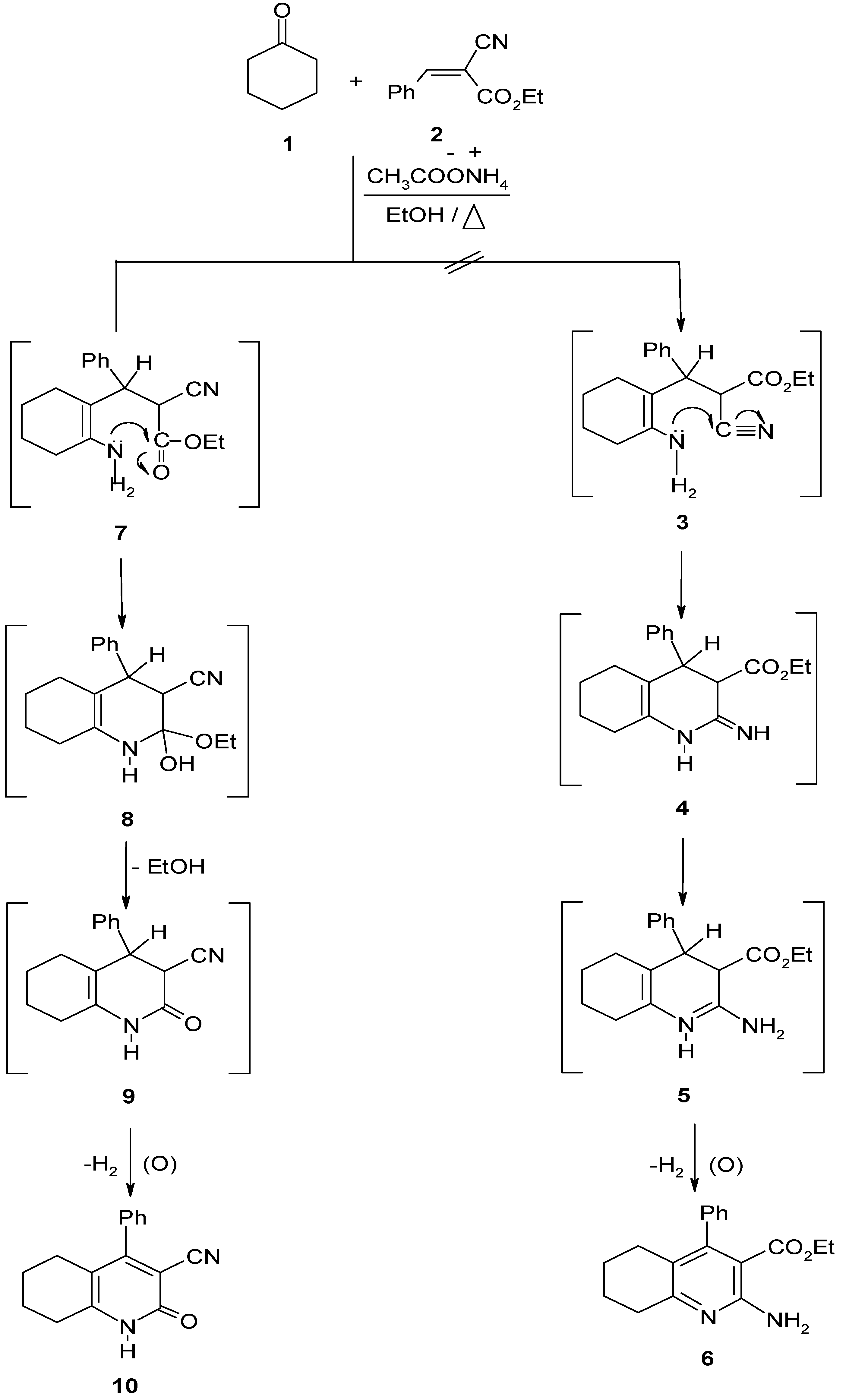
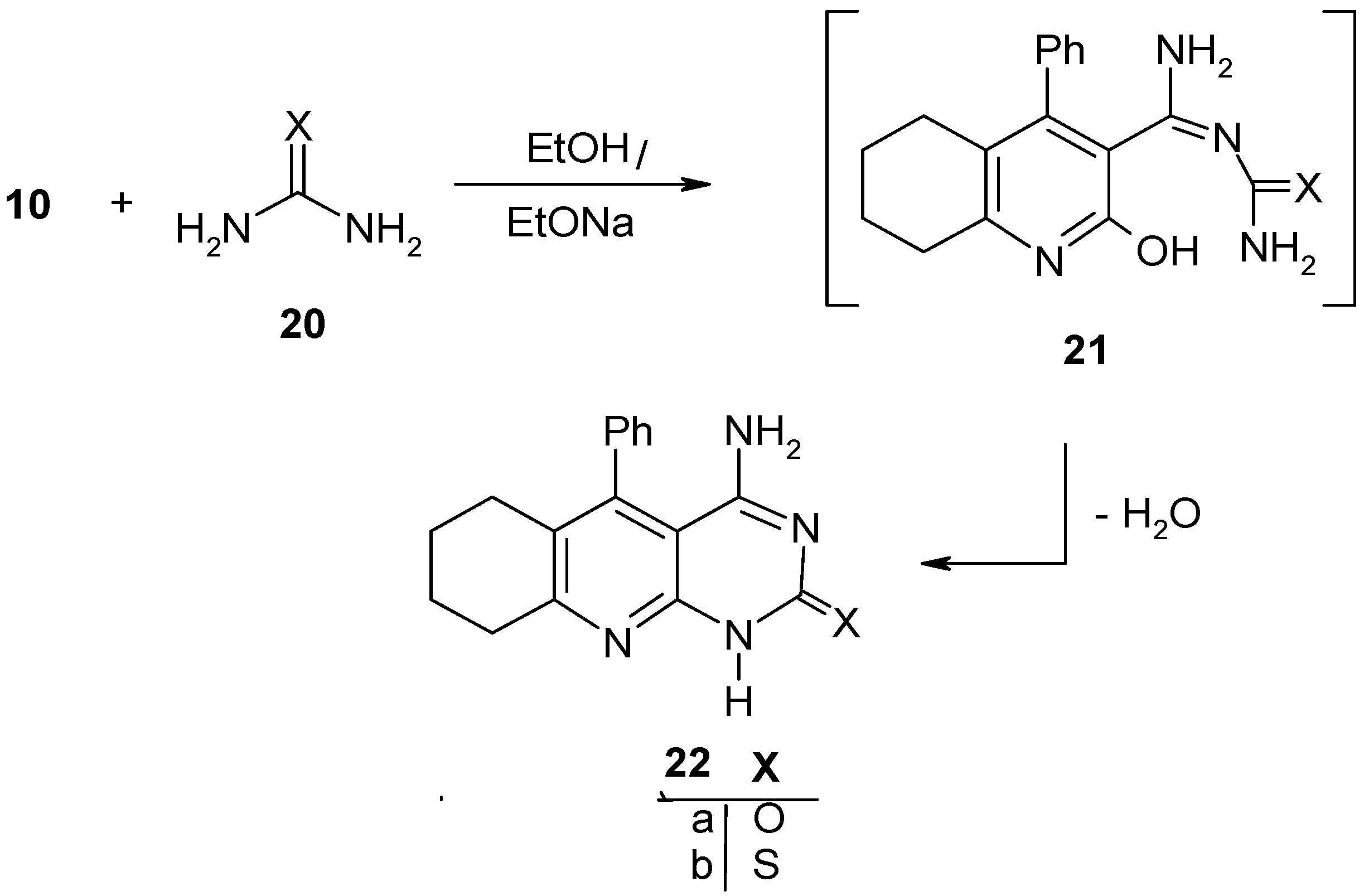
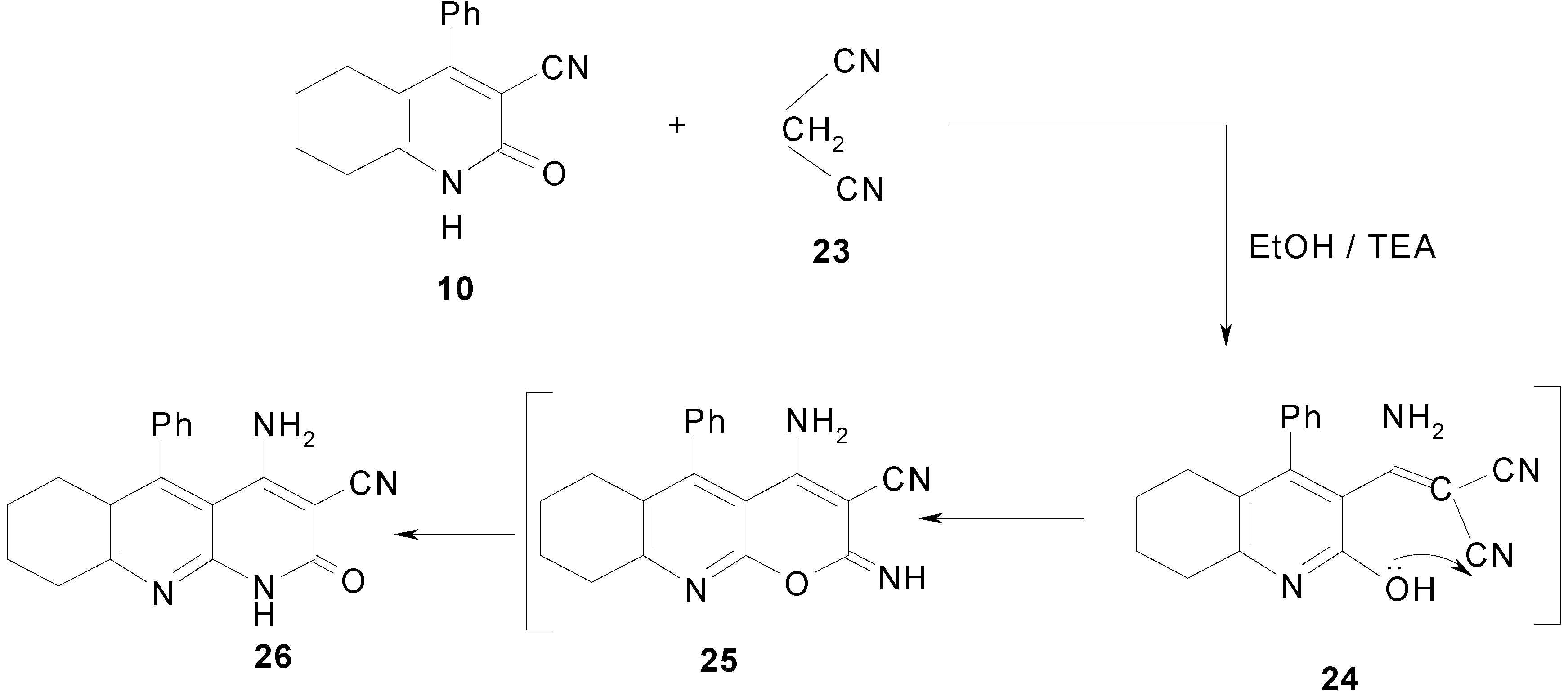
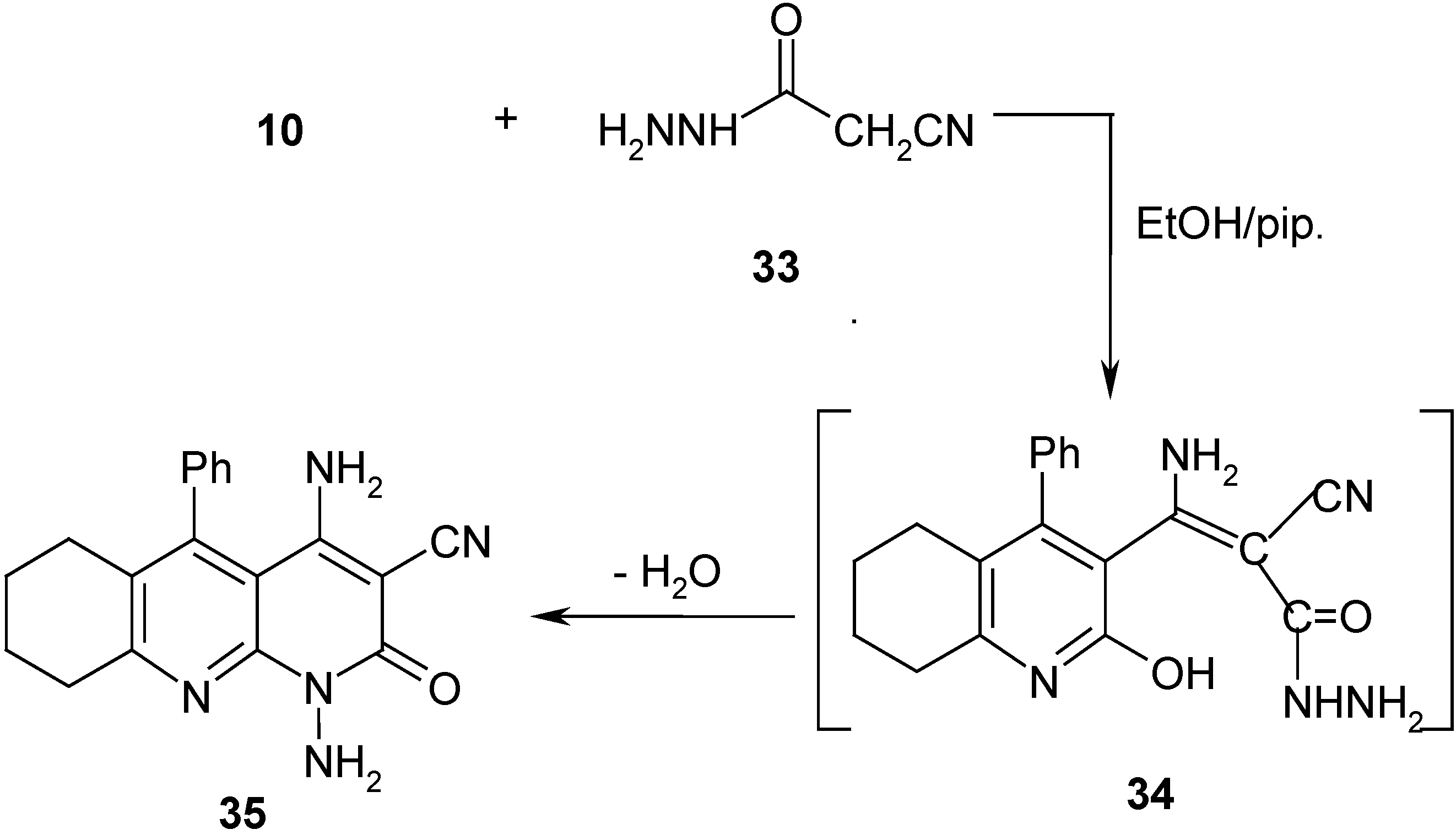
Results and Discussion
| Empirical formula | C16H14N2O |


Experimental
General
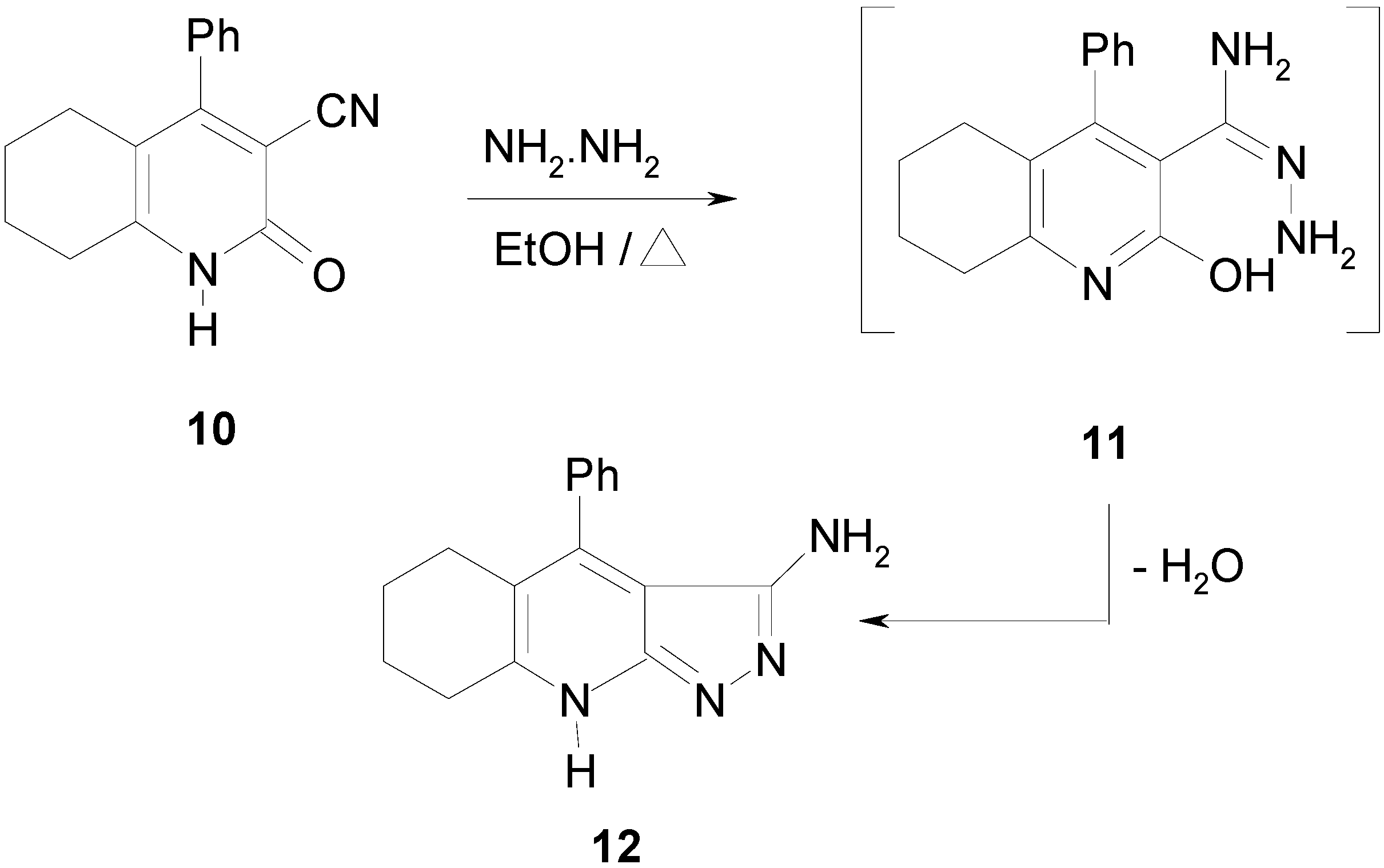
2-Oxo-4-phenyl-1,2 ,5,6,7,8-hexarahydroquinoline–3–carbonitrile (10).
5,6,7,8-Tetrahydro-4-phenyl-1H-pyrazolo[3,4-b]quinolin-3-amine (12)
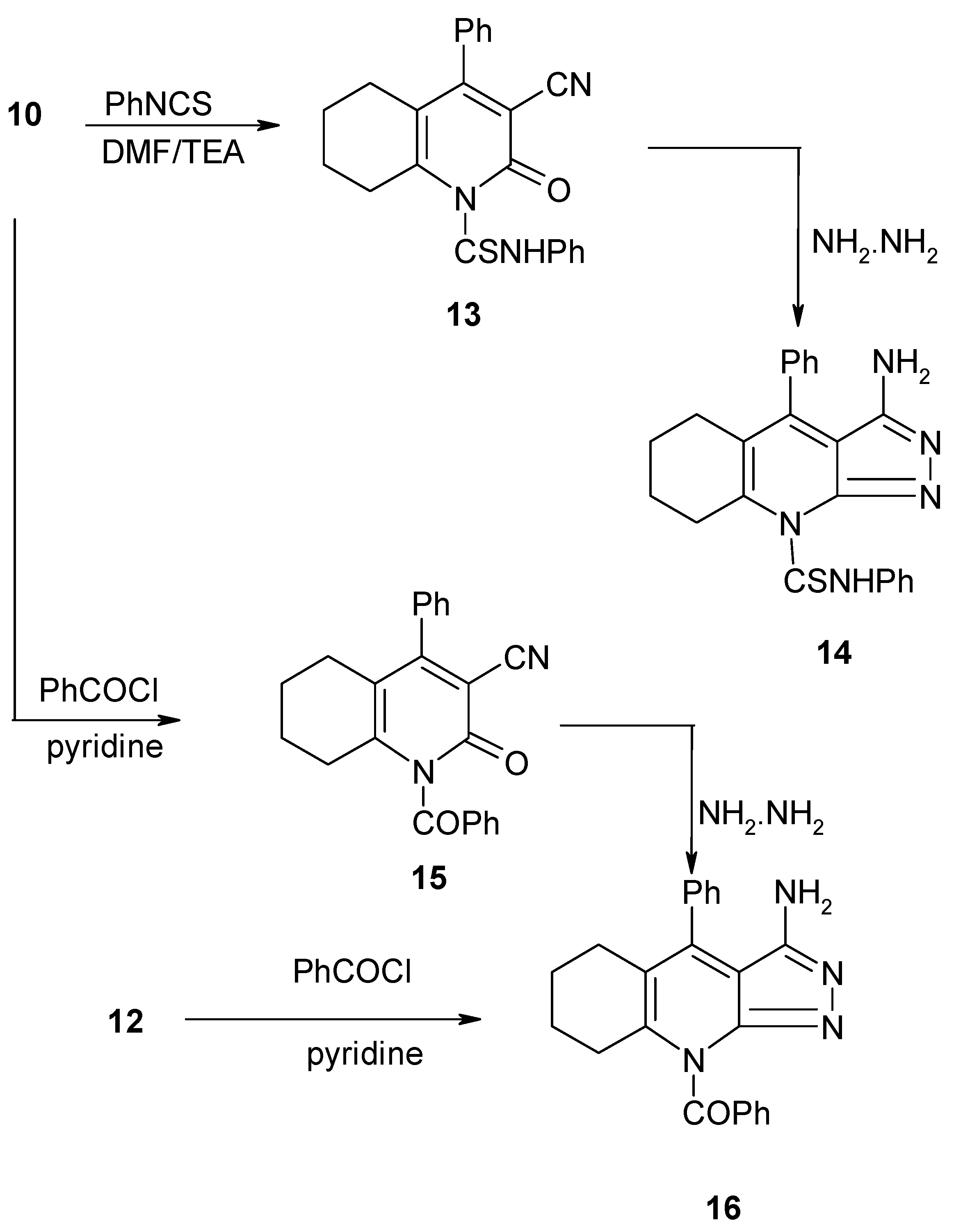
3-Cyano-5,6,7,8-tetrahydro-2-oxo-N,4-diphenylquinoline-1(2H)-carbothioamide (13)
3-Amino-N,4-diphenyl-5,6,7,8-tetrahydro-pyrazolo[3,4-b]quinolin-9-carbothioamide (14)
(3-Cyano-5,6,7,8-tetrahydro-2-oxo-4-phenylquinoline)(phenyl)methanone (15)
(3-Amino-5,6,7,8-tetrahydro-4-phenylpyrazolo[3,4-b]quinoline-9-yl)(phenyl)methanone (16)
General procedure for the synthesis of 3–amino–5,6,7,8-tetrahydro-N,4-diphenylpyrazolo[3,4-b]-quinolin-1-carbothioamide (19), 4-amino-6,7,8,9-tetrahydro-5-phenylpyrimido[4,5-b]quinolin-2(1H)-one (22a) and 4-amino-6,7,8,9-tetrahydro-5-phenylpyrimido[4,5-b]quinolin-2(1H)thione (22b).
4-Amino-1,2,6,7,8,9-hexahydro-2-oxo-5-phenyl-benzo[b][1,8]naphthyridin-3-carbonitrile (26).
General procedure for the synthesis of 4-amino-6,7,8,9-tetrahydro-2-methyl-5-phenylbenzo[b][1,8]- naphthyridin-3-carbonitrile (29) and 4-amino-2-(dicyanomethylene)-1,2,6,7,8,9-hexahydro-5-phenyl-benzo[b][1,8] naphthyridin-3-carbonitrile (32).
1,4-Diamino-1,2,6,7,8,9-hexahydro-2-oxo-5-phenylbenzo[b][1,8] naphthyridin-3-carbonitrile (35)
| Compd. | M.P.°C | Formula(mw) | Analysis % Calcd. (Found) | ||||
|---|---|---|---|---|---|---|---|
| C | H | N | S | Cl | |||
| 10 | 275 | C16H14N2O(250.30) | 76.78(77.02) | 5.64(5.82) | 11.19(11.46) | – | – |
| 12 | 225 | C16H16N4 (264.33) | 72.70(73.00) | 6.10(6.46) | 21.20(21.49) | – | |
| 13 | 240 | C23H19N3SO(385.47) | 71.66(71.89) | 4.97(5.04) | 10.90(11.15) | 8.32(8.45) | – |
| 14 | 192 | C23H21N5S (399.50) | 69.14(69.39) | 5.29 (5.56) | 17.52 (17.70) | 8.02(8.34) | – |
| 15 | 216 | C23H18N2O2(354.41) | 77.95(78.24) | 5.12(5.47) | 7.90(8.04) | – | – |
| 16 | 290 | C23H20N4O(368.44) | 74.98(75.27) | 5.47(5.79) | 15.21(15.77) | – | – |
| 19 | 223 | C23H21N5S(399.50) | 69.15(69.44) | 5.30(5.43) | 17.53(17.81) | 8.03(8.32) | – |
| Compd. | IR (cm-1) | 1H and 13C-NMR (δ, ppm) | |
|---|---|---|---|
| 10 | 3226 (NH), 2215 (CN), 1717 (CO). | 1.64-1.98 (m, 8H, 4CH2); 7.13-7.35 (m, 5H, Ar-H). 8.10 (s, 1H, NH). | |
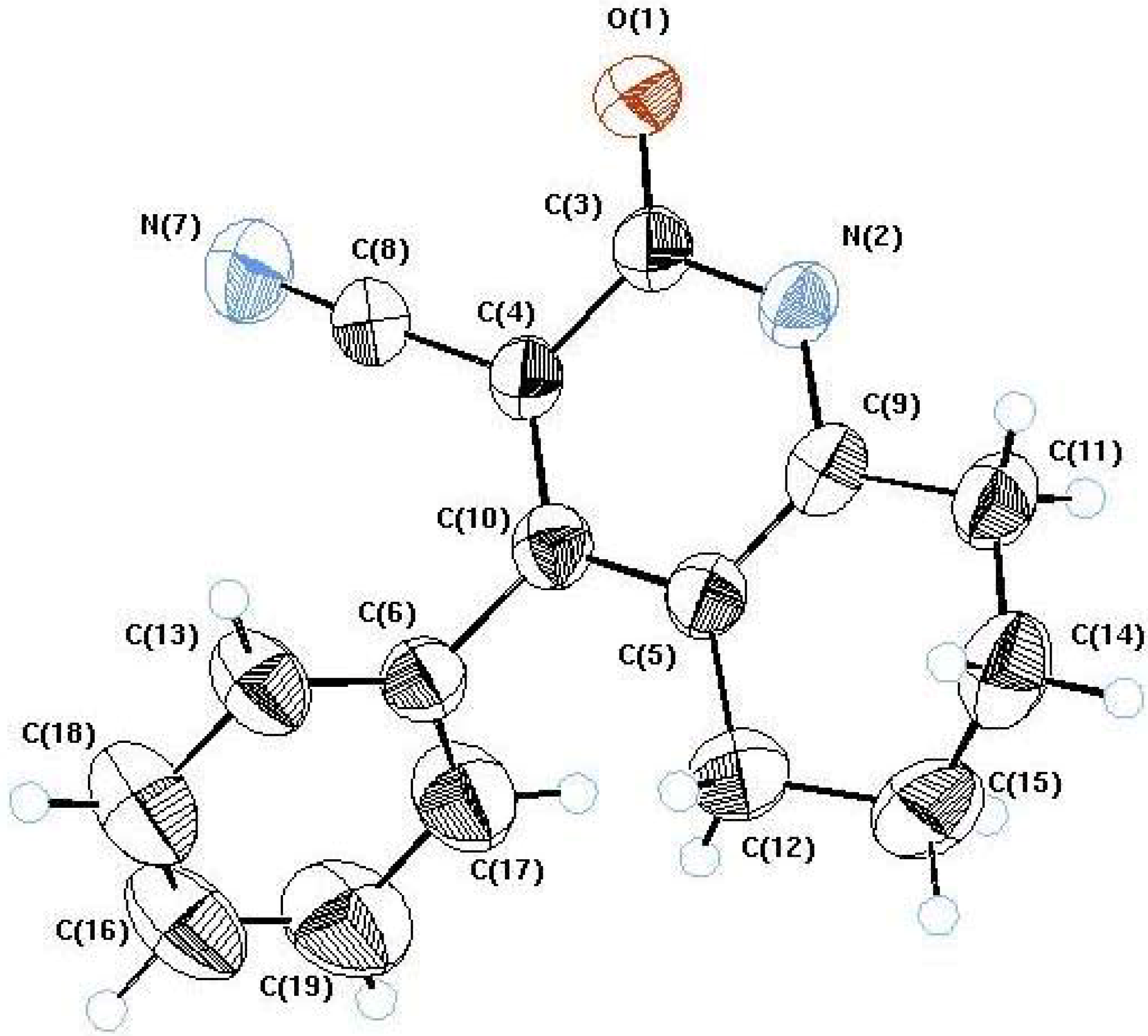
X-ray crystallography [13]
References
- Lichitsky, B. V.; Dudinov, A. A.; Krayushkin, M. M. Reaction of 3-aminocyclohex-2-en-1-ones with arylidenemalononitriles. Arkivoc 2001, 73–79. [Google Scholar]
- Chaczatrian, K.; Chaczatrian, G.; Danel, A.; Tomasik, P. The synthesis of 4-aryl-1H-pyrazolo[3,4-b]quinolines. Arkivoc 2001, 63–69. [Google Scholar]
- Nithyadevi, V.; Rajendran, S. P. An efficient synthesis of benzo[b][1,8]naphthyridine-3-carboxylic acid. J. Heterocycl. Chem. 2006, 43, 755–758. [Google Scholar] [CrossRef]
- Stadlbauer, W.; Hojas, G. Synthesis of 4-Azido-3-diazo-3H-pyrazolo[3, 4-b]quinolines. J. Chem. Soc. Perkin. Trans 1 2000, 3085–3087. [Google Scholar] [CrossRef]
- Elkholy, Y. M.; Morsy, M. A. Facial synthesis of 5,6,7,8-tetrahydropyrimido[4,5-b]quinoline derivatives. Molecules 2006, 11, 890–903. [Google Scholar] [CrossRef]
- Vijayalakshmi, S.; Ragunath, L.; Rajendran, S. P. A new approach to the synthesis of benzo[b][1,8]-naphthyridine-4(1H)ones. Heterocycl. Commun. 2001, 7, 177–182. [Google Scholar]
- El-Sayed, O. A.; Aboul-Enein, H. Y. Synthesis and antimicrobial activity of novel pyrazolo[3,4-b]quinoline derivatives. Arch. Pharm. (Weinheim) 2001, 334, 117–120. [Google Scholar]
- Wolin, R.; Wang, D.; Kelly, J.; Afonso, A.; James, L.; Kirschmeier, P.; Mcphail, A. T. Synthesis and evaluation of pyrazolo[3,4-b]quinoline ribofuranosides and their derivatives as inhibitors of Oncogenic Ras. Bioorg. Med. Chem. Lett. 1996, 6, 195–200. [Google Scholar] [CrossRef]
- Tsuzuki, Y.; Yomita, K.; Sato, Y. Synthesis and structure activity relationships of 3-substituted-1,4-dihydro-1,8-naphthyridine as novel antitumor agents. Bioorg. Med. Chem. Lett. 2004, 14, 12–16. [Google Scholar]
- Mefetah, H.; Brouant, P.; Charbit, J.; Galy, A.M.; Barbe, J. Synthesis, trypanocidal activity and DNA binding of new benzo[b][1,8]naphthyridine derivatives. Heterocycl. Commun. 1994, 1, 27–35. [Google Scholar]
- Antoine, M.; Barreau, M.; Descon, C.; Philippe, G.; Guy, P. Benzo[b][1,8]naphthyridine derivatives, their preparation and pharmaceutical compositions as antimicrobial agents. US Pat. 6548506 2003. [Google Scholar]
- Shuka, H. K.; Desia, N. C.; Astik, R. R.; Thaker, K. A. The uses of phenylthiosemecarbazide in heterocyclic syntheses. J. Indian Chem. Soc. 1984, 61, 168–175. [Google Scholar]
- .
- Mackay, S.; Gilmore, C. J.; Edwards, C.; Stewart, N.; Shankland, K. maXus Computer Program for the Solution and Refinement of Crystal Structures; Bruker Nonius: Delft, The Netherlands; Mac Science: Japan & The University of Glasgow, 1999. [Google Scholar]
- Johnson, C. K. ORTEP--II. A FORTRAN Thermal--Ellipsoid Plot Program.Report ORNL-5138; Oak Ridge National Laboratory: Oak Ridge, Tennessee, USA, 1976. [Google Scholar]
- Otwinowski, Z.; Minor, W. Methods in Enzymology; Carter, C. W., Jr., Sweet, R. M., Eds.; Academic Press: New York, 1997; Vol. 276; pp. 307–326. [Google Scholar]
- Altomare, A.; Cascarano, G.; Giacovazzo, C.; Guagliardi, A.; Burla, M. C.; Polidori, G.; Camalli, M. Pi-halogen dimer interactions and the inclusion chemistry of new tetrahaloaryl host. J. Appl. Cryst. 1994, 27, 435. [Google Scholar]
- Waasmaier, D.; Kirfel, A. Data activities in crystallography, factor function for free atoms and ions. Acta Cryst. 1995, A51, 416–431. [Google Scholar] [CrossRef]
- Sample availability: Available from the author.
© 2007 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Elkholy, Y.M. An Efficient Synthesis of Pyrazolo[3,4-b]quinolin-3-amine and Benzo[b][1,8]naphthyridine Derivatives. Molecules 2007, 12, 361-372. https://doi.org/10.3390/12030361
Elkholy YM. An Efficient Synthesis of Pyrazolo[3,4-b]quinolin-3-amine and Benzo[b][1,8]naphthyridine Derivatives. Molecules. 2007; 12(3):361-372. https://doi.org/10.3390/12030361
Chicago/Turabian StyleElkholy, Yehya M. 2007. "An Efficient Synthesis of Pyrazolo[3,4-b]quinolin-3-amine and Benzo[b][1,8]naphthyridine Derivatives" Molecules 12, no. 3: 361-372. https://doi.org/10.3390/12030361




