Synthesis of New N-Quaternary-3-benzamidoquinuclidinium Salts
Abstract
:Introduction
Results and Discussion
Experimental Section
General
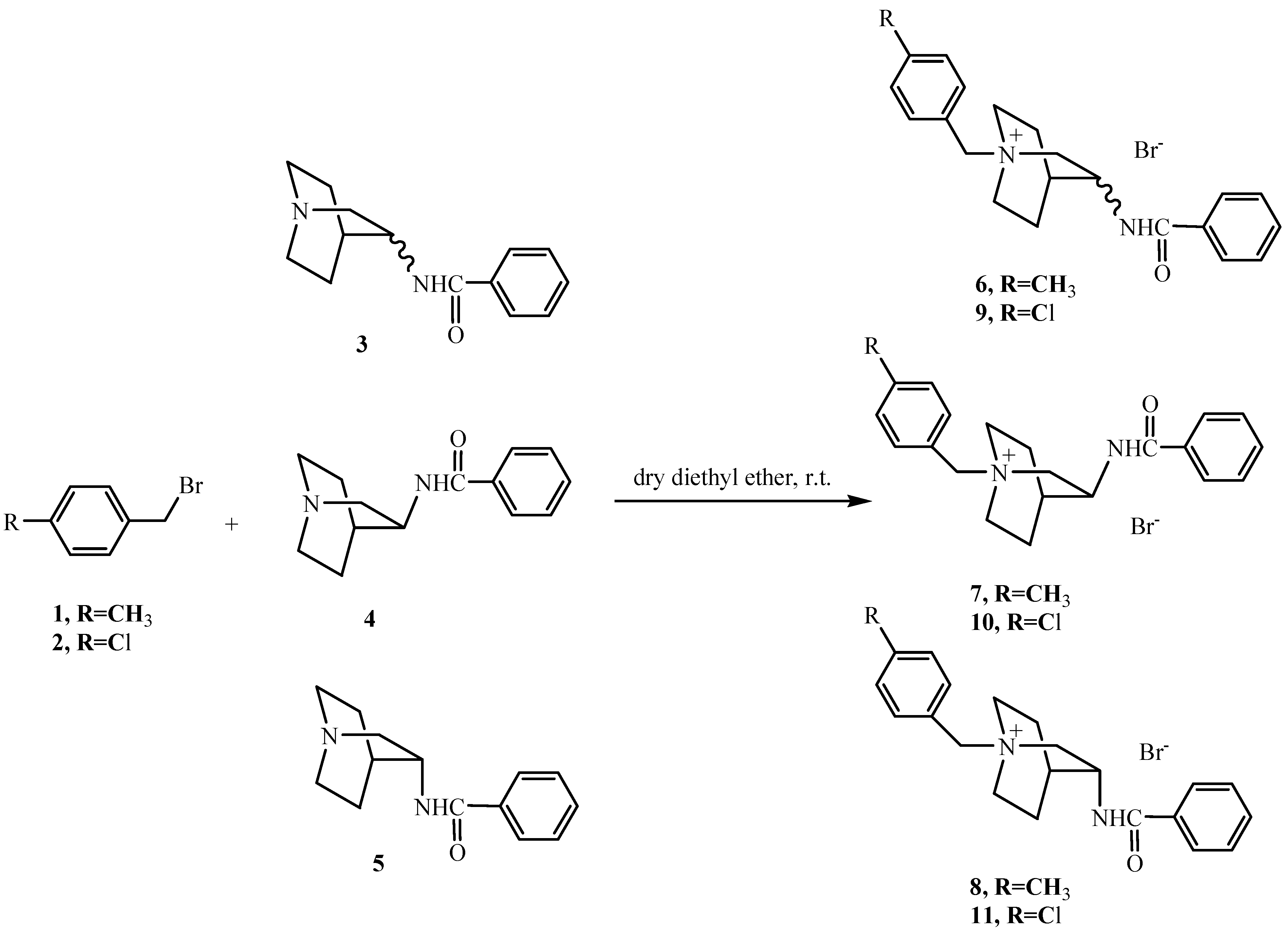
General procedure for the synthesis of N-quaternary quinuclidinium salts 6-11
 ‑74° (c=0.41, CHCl3). IR, 1H- and 13C-NMR were identical to those of 6.
‑74° (c=0.41, CHCl3). IR, 1H- and 13C-NMR were identical to those of 6. +76° (c=0.38, CHCl3). IR, 1H- and 13C-NMR were identical to those of 6.
+76° (c=0.38, CHCl3). IR, 1H- and 13C-NMR were identical to those of 6. -40° (c=0.2, CHCl3); IR, 1H- and 13C-NMR were identical to those of 9.
-40° (c=0.2, CHCl3); IR, 1H- and 13C-NMR were identical to those of 9. +40° (c=02, CHCl3); IR, 1H- and 13C-NMR were identical to those of 9.
+40° (c=02, CHCl3); IR, 1H- and 13C-NMR were identical to those of 9.Acknowledgements
References
- Mashkovsky, M.D.; Yakhontov, L.N.; Kaminka, M.E.; Mikhlina, E.E. Further developments in research on the chemistry and pharmacology of synthetic quinuclidine derivatives. Prog. Drug. Res. 1983, 27, 9–61. [Google Scholar]
- Sterling, G.H.; Doukas, P.H.; Sheldon, R.J.; O'Neill, J.J. In vivo protection against toxicity by known inhibitors of acetylcholine synthesis in vitro. Biochem. Pharmacol. 1988, 37, 379–384. [Google Scholar]
- Sterling, G.H.; Doukas, P.H.; Jackson, C.; Caccese, R.; O'Neill, K.J.; O'Neill, J.J. 3-Carbamyl-N-alylquinuclidinium bromide. Effects on cholinergic activity and protection against soman. Biochem. Pharmacol. 1993, 45, 465–472. [Google Scholar]
- Amitai, G.; Balderman, D.; Bruckstein-Davidovici, R.; Spiegelstein, M. US Patent 4,675,326, 1987.
- Amitai, G.; Rabinovitz, I.; Zomber, G.; Chen, R.; Cohen, G.; Adani, R.; Raveh, L. Proceedings of the 5th International Symposium On Protection Against Chemical and Biological Warfare Agents; Stockholm, Defence Research Establishment: Umea, Sweden, 1995; pp. 247–254.
- Simeon-Rudolf, V.; Reiner, E.; Škrinjarić-Špoljar, M.; Radić, B.; Lucić, A.; Primožič, I.; Tomić, S. Quinuclidinium-imidazolium compounds: synthesis, mode of interaction with acetylcholinesterase and effect upon Soman intoxicated mice. Arch. Toxicol. 1998, 72, 289–295. [Google Scholar]
- Langlois, M.; Soulier, J.L.; Allainmat, M.; Shen, S.; Gallais, C. Derivatives of quinuclidine as 5-HT3 receptor antagonists: Influence of an additional carbonyl group on the recognition of chirality by the receptor. Bioorg. Med. Chem. Lett. 1993, 3, 1555–1558. [Google Scholar]
- Ringdahl, B.; Jope, R.S.; Jeden, D.J. Inhibition of high affinity choline transport by stereoisomers of some 3-quinuclidinol derivatives. Biochem. Pharmacol. 1984, 33, 2819–2822. [Google Scholar] [CrossRef]
- Sternbach, L.H.; Kaiser, S. Antispasmodics. II. Esters of Basic Bicyclic Alcohols. J. Am. Chem. Soc. 1952, 74, 2219–2221. [Google Scholar] [CrossRef]
- Kalir, A.; Sali, E.; Shirin, E. Preparation of (+)-3-quinuclidinol. Isr. J. Chem. 1971, 9, 267–268. [Google Scholar]
- Rehavi, M.; Maayani, S.; Sokolovsky, M. Enzymatic resolution and cholinergic properties of (±) 3-quinuclidinol derivatives. Life Sci. 1977, 21, 1293–1302. [Google Scholar] [CrossRef]
- Odžak, R.; Tomić, S. 3-Amidoquinuclidine derivatives: Synthesis of compounds and inhibition of butyrylcholinesterase. Bioorg. Chem. 2006, 34, 90–98. [Google Scholar]
- Sample Availability: Samples of the compounds are available from authors.
© 2006 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Odzak, R.; Tomic, S. Synthesis of New N-Quaternary-3-benzamidoquinuclidinium Salts. Molecules 2006, 11, 726-730. https://doi.org/10.3390/11090726
Odzak R, Tomic S. Synthesis of New N-Quaternary-3-benzamidoquinuclidinium Salts. Molecules. 2006; 11(9):726-730. https://doi.org/10.3390/11090726
Chicago/Turabian StyleOdzak, Renata, and Srdjanka Tomic. 2006. "Synthesis of New N-Quaternary-3-benzamidoquinuclidinium Salts" Molecules 11, no. 9: 726-730. https://doi.org/10.3390/11090726



