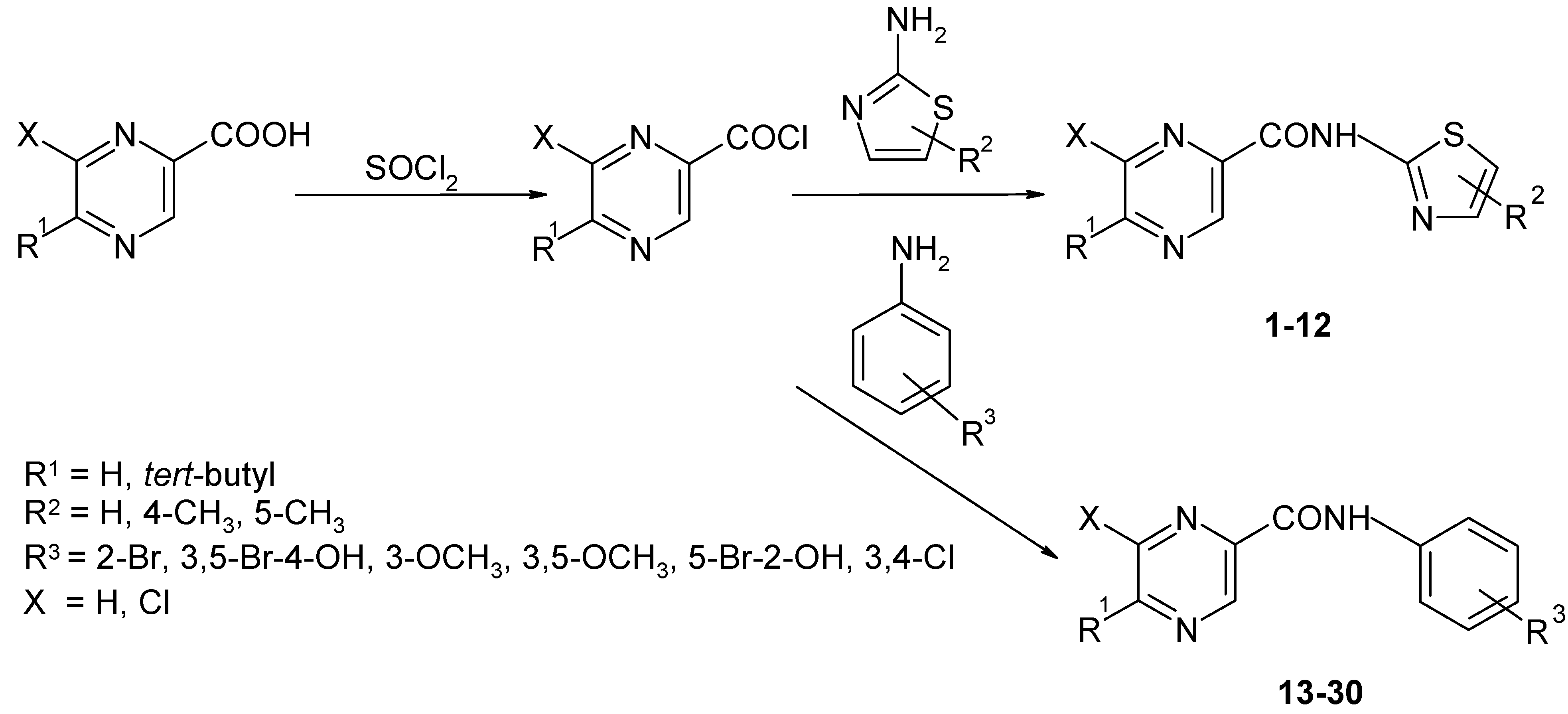
Synthesis of amides 2a-r
A mixture of acid,
i.e. pyrazine-2-carboxylic [
20], 6-chloropyrazine-2-carboxylic [
21], 5-
tert-butylpyrazine-2-carboxylic [
7] or 5-
tert-butyl-6-chloropyrazine-2-carboxylic [
7] acids, respectively, (50.0 mmol) and thionyl chloride (5.5 mL, 75.0 mmol) was refluxed in dry toluene (20 mL) for about 1 h. Excess thionyl chloride was removed by repeated evaporation
in vacuo with fresh dry toluene. The crude acyl chloride dissolved in dry acetone (50 mL) was added dropwise to a stirred solution of the corresponding substituted amine (50.0 mmol) in dry pyridine (50 mL) kept at room temperature. After the addition was complete, stirring continued for another 30 min. The reaction mixture was then poured into cold water (100 mL) and the crude amide was collected and recrystallized from aqueous ethanol.
Pyrazine-2-carboxylic acid thiazol-2-ylamide (
1). Yield: 88%; m.p. 187-188 °C (Ref. [
16]: m.p. 187-189 °C); For C
8H
6N
4OS (206.2) calculated: 46.59% C, 2.93% H, 27.17% N; found: 46.55% C, 2.91% H, 26.98% N;
RF = 0.43; IR cm
-1: 3432 (N-H), 1668 (C=O);
1H-NMR (CDCl
3), δ: 11.14 (bs, 1H, NH), 9.52 (d, 1H,
J=1.79 Hz, H3), 8.86 (d, 1H,
J=1.79 Hz, H6), 8.65-8.63 (m, 1H, H5), 7.55 (d, 1H,
J=3.57 Hz, H4´), and 7.09 (d, 1H,
J=3.57 Hz, H5´);
13C-NMR (CDCl
3), δ: 160.7, 157.3, 148.3, 144.9, 143.0, 142.7, 138.2, and 114.3.
6-Chloropyrazine-2-carboxylic acid thiazol-2-ylamide (2). Yield: 98%; m.p. 153-155 °C; For C8H5ClN4OS (240.7) calculated: 39.92% C, 2.09% H, 23.28% N; found: 40.03% C, 1.92% H, 23.33% N; RF = 0.65; IR cm-1: 3435 (N-H), 1675 (C=O); 1H-NMR (DMSO-d6), δ: 10.91 (bs, 1H, NH), 9.17 (s, 1H, H3), 7.59 (d, 1H, J=3.57 Hz, H4´), 7.36 (d, 1H, J=3.57 Hz, H5´), and 1.50 (s, 9H, CH3); 13C-NMR (DMSO-d6), δ: 163.6, 161.5, 158.2, 145.8, 141.7, 140.9, 137.7, 114.8, 38.8, and 28.2.
5-tert-Butylpyrazine-2-carboxylic acid thiazol-2-ylamide (3). Yield: 45%; m.p. 131-132 °C; For C12H14N4OS (262.3) calculated: 54.94% C, 5.38% H, 21.36% N; found: 55.06% C, 5.43% H, 21.38% N. RF = 0.63; IR cm-1: 3432 (N-H), 1676 (C=O); 1H-NMR (CDCl3), δ: 11.02 (bs, 1H, NH), 9.39 (d, 1H, J=1.37 Hz, H3), 8.66 (d, 1H, J=1.38 Hz, H6), 7.54 (d, 1H, J=3.58 Hz, H4´), 7.07 (d, 1H, J=3.57 Hz, H5´), and 1.45 (s, 9H, CH3); 13C-NMR (CDCl3), δ: 168.8, 161.0, 157.4, 143.2, 139.7, 139.7, 138.1, 114.2, 37.2, and 29.7.
5-tert-Butyl-6-chloropyrazine-2-carboxylic acid thiazol-2-ylamide (4). Yield: 97%; m.p. 148-150 °C; For C12H13ClN4OS (296.8) calculated: 48.56% C, 4.42% H, 18.88% N; found: 48.46% C, 4.65% H, 18.80% N; RF = 0.88; IR cm-1: 3448 (N-H), 1675 (C=O); 1H-NMR (DMSO-d6), δ: 12.49 (bs, 1H, NH), 9.17 (s, 1H, H3), 7.59 (d, 1H, J=3.6 Hz, H4´), 7.36 (d, 1H, J=3.6 Hz, H5´), and 1.50 (s, 9H, CH3); 13C-NMR (DMSO-d6), δ: 163.6, 161.5, 158.2, 145.8, 141.7, 140.9, 137.7, 114.8, 38.8, and 28.2.
Pyrazine-2-carboxylic acid (4-methylthiazol-2-yl)amide (5). Yield: 67%; m.p. 144-145 °C; For C9H8N4OS (220.3) calculated: 49.08% C, 3.66% H, 25.44% N; found: 48.93% C, 3.78% H, 25.63% N; RF = 0.50; IR cm-1: 3433 (N-H), 1670 (C=O); 1H-NMR (DMSO-d6), δ: 12.27 (bs, 1H, NH), 9.20 (d, 1H, J=1.0 Hz, H3), 8.95 (d, 1H, J=1.0 Hz, H6), 7.88 (m, 1H, H5), 6.90 (d, 1H, J=1.0 Hz, H5´), and 2.40 (s, 3H, J=1.0 Hz, CH3); 13C-NMR (DMSO-d6), δ: 164.0, 163.6, 148.0, 147.8, 147.5, 145.6, 139.7, 111.2, and 17.7.
6-Chloropyrazine-2-carboxylic acid (4-methylthiazol-2-yl)amide (6). Yield: 97%; m.p. 192-194 °C; For C9H7ClN4OS (254.7) calculated: 42.44% C, 2.77% H, 22.00% N; found: 42.37% C, 2.70% H, 22.13% N; RF = 0.74; IR cm-1: 3434 (N-H), 1675 (C=O); 1H-NMR (DMSO-d6), δ: 12.54 (bs, 1H, NH), 9.23 (s, 1H, H3), 9.04 (s, 1H, H5), 6.90 (d, 1H, J=1.0 Hz, H5´), and 2.30 (d, 3H, J=1.0 Hz, CH3); 13C-NMR (DMSO-d6), δ: 162.0, 158.3, 147.9, 147.5, 145.9, 144.5, 142.8, 109.0, and 16.7.
5-tert-Butylpyrazine-2-carboxylic acid (4-methylthiazol-2-yl)amide (7). Yield: 33%; m.p. 84-85 °C; For C13H16N4OS (276.4) calculated: 56.50% C, 5.84% H, 20.27% N; found: 56.44% C, 5.96% H, 20.18% N; RF = 0.69; IR cm-1: 3434 (N-H), 1676 (C=O); 1H-NMR (DMSO-d6), δ: 12.22 (bs, 1H, NH), 9.20 (d, 1H, J=1.5 Hz, H3), 8.89 (d, 1H, J=1.5 Hz, H6), 6.90 (d, 1H, J=1.0 Hz, H5´), 2.30 (d, 3H, J=1.0 Hz, CH3), and 1.39 (s, 9H, CH3); 13C-NMR (DMSO-d6), δ: 167.5, 162.3, 157.0, 147.1, 142.9, 141.4, 140.7, 109.0, 37.1, 29.6, and 17.0.
5-tert-Butyl-6-chloropyrazine-2-carboxylic acid (4-methylthiazol-2-yl)amide (8). Yield: 97%; m.p. 118-120 °C; For C13H15ClN4OS (310.8) calculated: 50.24% C, 4.86% H, 18.03% N; found: 50.17% C, 4.99% H, 18.09% N; RF = 0.91; IR cm-1: 3451 (N-H), 1675 (C=O); 1H-NMR (DMSO-d6), δ: 12.47 (bs, 1H, NH), 9.15 (s, 1H, H3), 6.89 (d, 1H, J=1.0 Hz, H5´), 2.30 (d, 3H, J=1.0 Hz, CH3), and 1.49 (s, 9H, CH3); 13C-NMR (DMSO-d6), δ: 163.5, 161.7, 146.2, 145.8, 142.1, 141.8, 140.8, 108.9, 38.7, 28.2, and 16.8.
Pyrazine-2-carboxylic acid (5-methylthiazol-2-yl)amide (9). Yield: 66%; m.p. 247-248 °C; For C9H8N4OS (220.3) calculated: 49.08% C, 3.66% H, 25.44% N; found: 49.03% C, 3.51% H, 25.32% N; RF = 0.42; IR cm-1: 3435 (N-H), 1672 (C=O); 1H-NMR (DMSO-d6), δ: 12.46 (bs, 1H, NH), 9.20 (d, 1H, J=1.65 Hz, H3), 8.91 (d, 1H, J=1.65 Hz, H6), 8.73-8.70 (m, 1H, H5), 7.28 (s, 1H, H4´), and 2.38 (s, 3H, CH3); 13C-NMR (DMSO-d6), δ: 163.0, 158.3, 148.0, 147.8, 147.5, 143.9, 136.8, 127.4, and 11.5.
6-Chloropyrazine-2-carboxylic acid (5-methylthiazol-2-yl)amide (10). Yield: 98%; m.p. 214-215 °C; For C9H7ClN4OS (254.7) calculated: 42.44% C, 2.77% H, 22.00% N; found: 42.53% C, 2.70% H, 21.95% N; RF = 0.79; IR cm-1: 3436 (N-H), 1672 (C=O); 1H-NMR (DMSO-d6), δ: 12.56 (bs, 1H, NH), 9.24 (s, 1H, H3), 9.05 (s, 1H, H5), 7.27 (s, 1H, H4´), and 2.39 (s, 3H, CH3); 13C-NMR (DMSO-d6), δ: 161.7, 157.1, 147.9, 147.5, 144.5, 142.8, 134.1, 127.4, and 11.5.
5-tert-Butylpyrazine-2-carboxylic acid (5-methylthiazol-2-yl)amide (11). Yield: 33%; m.p. 114-116 °C; For C13H16N4OS (276.4) calculated: 56.50% C, 5.84% H, 20.27% N; found: 56.59% C, 5.80% H, 20.36% N; RF = 0.80; IR cm-1: 3435 (N-H), 1677 (C=O); 1H-NMR (DMSO-d6), δ: 12.12 (bs, 1H, NH), 9.20 (s, 1H, H3), 8.88 (s, 1H, H6), 7.24 (s, 1H, H4´), 2.38 (s, 3H, J=1.0 Hz, CH3), and 1.39 (s, 9H, CH3); 13C-NMR (DMSO-d6), δ: 167.5, 162.0, 155.8, 142.9, 141.4, 140.6, 135.2, 127.6, 37.1, 29.6, and 11.4.
5-tert-Butyl-6-chloropyrazine-2-carboxylic acid (5-methylthiazol-2-yl)amide (12). Yield: 98%; m.p. 152-153 °C; For C13H15ClN4OS (310.8) calculated: 50.24% C, 4.86% H, 18.03% N; found: 50.37% C, 4.69% H, 17.79% N; RF = 0.85; IR cm-1: 3453 (N-H), 1678 (C=O); 1H-NMR (DMSO-d6), δ: 12.45 (bs, 1H, NH), 9.15 (d, 1H, J=0.5 Hz, H3), 7.27-7.24 (m, 1H, H4´), and 2.38 (d, 3H, J=0.5 Hz, CH3), 1.49 (s, 9H, CH3); 13C-NMR (DMSO-d6), δ: 163.4, 161.4, 156.7, 145.8, 141.8, 140.8, 134.4, 127.4, 38.7, 28.2, and 11.4.
6-Chloropyrazine-2-carboxylic acid (2-bromophenyl)amide (13). Yield: 24%; m.p. 118-119 °C; For C11H7BrClN3O (312.6) calculated: 42.27% C, 2.26% H, 13.44% N; found: 42.31% C, 2.17% H, 13.28% N; RF = 0.86; IR cm-1: 3436 (N-H), 1701 (C=O); 1H-NMR (CDCl3), δ: 10.11 (bs, 1H, NH), 9.39 (d, 1H, J=0.55 Hz, H3), 8.83 (d, 1H, J=0.55 Hz, H5), 8.55 (dd, 1H, J=1.65 Hz, H6´), 7.61 (dd, 1H, J=7.97 Hz, J=1.37 Hz, H3´), 7.43-7.35 (m,1H, H5´); 13C-NMR (CDCl3), δ: 159.5, 147.8, 147.6, 143.8, 142.1, 135.0, 132.6, 128.5, 126.0, 121.6, and 114.1.
5-tert-Butylpyrazine-2-carboxylic acid (2-bromophenyl)amide (14). Yield: 20%; m.p. 83-84 °C. For C15H16BrN3O (334.2) calculated: 53.91% C, 4.83% H, 12.57% N; found: 54.13% C, 4.91% H, 12.68% N; RF = 0.94; IR cm-1: 3439 (N-H), 1693 (C=O); 1H-NMR (CDCl3), δ: 10.35 (bs, 1H, NH), 9.39 (d, 1H, J=1.37 Hz, H3), 8.71 (d, 1H, J=1.65 Hz, H6), 8.62 (dd, 1H, J=8.24 Hz, J=1.65 Hz, H6´), 7.59 (dd, 1H, J=8.25 Hz, J=1.65 Hz, H3´), 7.42-7.34 (m, 1H, H4´), 7.03 (dd, 1H, J=7.42 Hz, J=1.65 Hz, H5´), and 1.45 (s, 9H, CH3); 13C-NMR (CDCl3), δ: 168.0, 161.3, 143.0, 141.3, 139.4, 135.5, 132.5, 128.4, 125.4, 121.4, 113.8, 37.1, and 29.7.
5-tert-Butyl-6-chloropyrazine-2-carboxylic acid (2-bromophenyl)amide (15). Yield: 17%; m.p. 116-117 °C; For C15H15BrClN3O (368.7) calculated: 48.87% C, 4.10% H, 11.40% N; found: 48.56% C, 4.21% H, 11.28% N; RF = 0.92; IR cm-1: 3435 (N-H), 1701 (C=O); 1H-NMR (CDCl3), δ: 10.11 (bs, 1H, NH), 9.39 (s, 1H, H3), 8.83 (s, 1H, H5), 8.55 (dd, 1H, J=8.24 Hz, J=1.37 Hz, H6´), 7.61 (dd, 1H, J=8.24 Hz, J=1.37 Hz, H3´), 7.43-7.35 (m, 1H, H4´), 7.09-7.03 (m, 1H, H5´); 13C-NMR (CDCl3), δ: 159.8, 148.0, 147.9, 144.1, 142.3, 135.2, 132.8, 128.7, 126.2, 121.8, 114.4, 37.0, and 29.7.
6-Chloropyrazine-2-carboxylic acid (3,5-dibromo-4-hydroxyphenyl)amide (16). Yield: 14%; m.p. 191-193 °C; For C11H6Br2ClN3O2 (407.5) calculated: 32.43% C, 1.48% H, 10.31% N; found: 32.33% C, 1.41% H, 10.27% N; RF = 0.89; IR cm-1: 3432 (N-H), 1685 (C=O); 1H-NMR (CDCl3), δ: 10.74 (bs, 1H, NH), 9.86 (bs, 1H, OH), 9.20 (d, 1H, J=0.55 Hz, H3), 9.05 (d, 1H, J=0.5 Hz, H5), and 8.13 (s, 2H, H2´, H6´); 13C-NMR (CDCl3), δ: 160.8, 147.8, 147.8, 147.1, 144.8, 142.5, 132.4, 124.7, and 111.8.
5-tert-Butylpyrazine-2-carboxylic acid (3,5-dibromo-4-hydroxyphenyl)amide (17). Yield: 24%; m.p. 206-208 °C; For C15H15Br2N3O2 (429.1) calculated: 41.99% C, 3.52% H, 9.79% N; found: 42.11% C, 3.41% H, 10.02% N; RF = 0.95; IR cm-1: 3432 (N-H), 1695 (C=O); 1H-NMR (CDCl3), δ: 9.38 (d, 1H, J=0.55 Hz, H3), 9.29 (bs, 1H, NH), 8.83 (d, 1H, J=0.55 Hz, H5), 7.96 (s, 2H, H2´, H6´), 5.84 (s, 1H, OH), and 1.48 (s, 9H, CH3); 13C-NMR (CDCl3), δ: 168.7, 161.5, 161.1, 143.3, 139.3, 139.1, 137.5, 123.4, 117.8, 37.4, and 29.9.
5-tert-Butyl-6-chloropyrazine-2-carboxylic acid (3,5-dibromo-4-hydroxyphenyl)amide (18). Yield: 20%; m.p. 216-217 °C; For C15H14Br2ClN3O2 (463.6) calculated: 38.87% C, 3.04% H, 9.06% N; found: 38.63% C, 2.97% H, 9.28% N; RF = 0.89; IR cm-1: 3432 (N-H), 1685 (C=O); 1H-NMR (CDCl3), δ: 9.31 (bs, 1H, NH), 9.24 (d, 1H, J=0.55 Hz, H3), 8.03 (s, 2H, H2´, H6´), 5.71 (s, 1H, OH), and 1.49 (s, 9H, CH3); 13C-NMR (CDCl3), δ: 161.2, 159.0, 147.8, 144.8, 142.7, 139.8, 132.2, 124.7, 112.1, 31.7, and 29.7.
6-Chloropyrazine-2-carboxylic acid (3-methoxyphenyl)amide (19). Yield: 74%; m.p. 139-140 °C; For C12H10ClN3O2 (263.7) calculated: 54.66% C, 3.62% H, 15.94% N; found: 54.72% C, 3.59% H, 16.09% N; RF = 0.88; IR cm-1: 3355 (NH), 2838 (OCH3), 1681 (CO); 1H-NMR (CDCl3) δ 9.39-9.37 (m, 2H, H3, NH), 8.80 (d, 1H, J=0.55 Hz, H5), 7.50 (t, 1H, J=2.20 Hz, H2´), 7.30 (d, 1H, J=7.96 Hz, H4´), 7.24-7.19 (m, 1H, H5´), 6.74 (ddd, 1H, J=7.96 Hz, J=2.47 Hz, J=1.10 Hz, H6´), and 3.84 (s, 3H, OCH3); 13C-NMR (75 MHz, CDCl3) δ 160.2, 159.3, 147.5, 147.4, 143.9, 142.2, 138.0, 129.9, 112.2, 111.1, 105.5, and 55.4.
5-tert-Butylpyrazine-2-carboxylic acid (3-methoxyphenyl)amide (20). Yield: 81%; m.p. 79-80 °C; For C16H19N3O2 (285.3) calculated: 67.35% C, 6.71% H, 14.73% N; found: 67.48% C, 6.69% H, 14.95% N; RF = 0.90; IR cm-1: 3360 (NH), 2841 (OCH3), 1677 (CO); 1H-NMR (CDCl3) δ 9.65 (bs, 1H, NH), 9.39 (d, 1H, J=1.37 Hz, H3), 8.62 (d, 1H, J=1.37 Hz, H6), 7.55 (t, 1H, J=2.20 Hz, H2´), 7.28 (t, 1H, J=7.97 Hz, H5´), 7.22-7.17 (m, 1H, H4´), 6.72 (ddd, 1H, J=7.97 Hz, J=2.47 Hz, J=1.10 Hz, H6´), 3.85 (s, 3H, OCH3), and 1.45 (s, 9H, CH3); 13C-NMR (CDCl3) δ 167.8, 161.1, 160.2, 142.9, 141.3, 1389.0, 138.6, 129.8, 111.9, 110.7, 105.2, 55.3, 37.0, and 29.7.
5-tert-Butyl-6-chloropyrazine-2-carboxylic acid (3-methoxyphenyl)amide (21). Yield: 78%; m.p. 128-129 °C; For C16H18ClN3O2 (319.8) calculated: 60.09% C, 5.67% H, 13.14% N; found: 59.88% C, 5.62% H, 13.18% N; RF = 0.86; IR cm-1: 3380 (NH), 2840 (OCH3), 1686 (CO); 1H-NMR (CDCl3) δ 9.65 (bs, 1H, NH), 9.04 (d, 1H, H3), 7.50 (t, 1H, J=2.20 Hz, H2´), 7.30 (d, 1H, J=7.96 Hz, H4´), 7.24-7.19 (m, 1H, H5´), 6.71 (ddd, 1H, J=7.96 Hz, J=2.47 Hz, J=1.10 Hz, H6´), 3.74 (s, 3H, OCH3 , and 1.34 (s, 9H, CH3); 13C-NMR (CDCl3) δ 165.2, 162.2, 160.0, 144.0, 142.7, 141.8, 139.0, 129.8, 112.7, 109.1, 105.5, 31.2, and 25.4.
6-Chloropyrazine-2-carboxylic acid (3,5-dimethoxyphenyl)amide (22). Yield: 64%; m.p. 211-212 °C; For C13H12ClN3O3 (293.7) calculated: 53.16% C, 4.12% H, 14.31% N; found: 52.81% C, 4.29% H, 14.02% N; RF = 0.88; IR cm-1: 3370 (NH), 2964, 2838 (OCH3), 1685 (CO); 1H-NMR (CDCl3) δ 9.38 (s, 1H, H3), 9.34 (bs, 1H, NH), 8.81 (s, 1H, H5), 6.98 (d, 2H, J=2.19 Hz, H2´, H6´), 6.33-6.30 (m, 1H, H4´), and 3.82 (s, 6H, OCH3); 13C-NMR (CDCl3) δ 161.2, 159.3, 147.6, 147.4, 143.9, 142.2, 138.5, 98.2, 97.7, and 55.5.
5-tert-Butylpyrazine-2-carboxylic acid (3,5-dimethoxyphenyl)amide (23). Yield: 82%; m.p. 135-136 °C; For C17H21N3O3 (315.4) calculated: 64.74% C, 6.71% H, 13.32% N; found: 63.85% C, 6.71% H, 13.23% N; RF = 0.90; IR cm-1: 3360 (NH), 2961, 2838 (OCH3), 1690 (CO); 1H-NMR (CDCl3) δ 9.62 (bs, 1H, NH), 9.38 (d, 1H, J=1.37 Hz, H3), 8.62 (d, 1H, J=1.38 Hz, H6), 7.00 (d, 2H, J=2.20 Hz, H2´, H6´), 6.29 (t, 1H, J=2.20 Hz, H4´), 3.82 (s, 6H, OCH3), and 1.44 (s, 9H, CH3); 13C-NMR (CDCl3) δ 167.8, 161.1, 161.1, 142.9, 141.3, 139.1, 139.0, 97.9, 97.2, 55.4, 37.1, and 29.7.
5-tert-Butyl-6-chloropyrazine-2-carboxylic acid (3,5-dimethoxyphenyl)amide (24). Yield: 49%; m.p. 123-124 °C; For C17H20ClN3O3 (349.8) calculated: 58.37% C, 5.76% H, 12.01% N; found: 58.57% C, 5.91% H, 12.05% N; RF = 0.92; IR cm-1: 3376 (NH), 2960, 2839 (OCH3), 1698 (CO); 1H-NMR (CDCl3) δ 9.31 (bs, 1H, NH), 9.25 (s, 1H, H3), 6.99 (d, 2H, J=2.20 Hz, H2´, H6´), 6.30 (t, 1H, J=2.20 Hz, H4´), 3.82 (s, 6H, OCH3), and 1.55 (s, 9H, CH3); 13C-NMR (CDCl3) δ 164.6, 161.1, 159.8, 145.7, 141.0, 140.2, 138.7, 98.1, 97.5, 55.4, 39.0, and 28.3.
6-Chloropyrazine-2-carboxylic acid (5-bromo-2-hydroxyphenyl)-amide (25). Yield: 71%; m.p. 154-155 °C; For C11H7BrClN3O2 (328.6) calculated: 40.21% C, 2.15% H, 12.79% N; found: 40.51% C, 1.93% H, 13.05% N; RF = 0.85; IR cm-1: 3370 (NH), 1682 (CO); 1H-NMR (CDCl3) δ 9.27 (bs, 1H, NH), 9.22 (d, 1H, J=1.1 Hz, H3), 8.98 (d, 1H, J=1.1 Hz, H5), 7.74 (d, 1H, J=2.47 Hz, H2´), 7.02 (dd, 1H, H4´), 6.62 (d, 1H, H5´), and 5.06 (bs, 1H, OH); 13C-NMR (CDCl3) δ 165.2, 149.4, 142.9, 141.1, 139.0, 131.2, 128.5, 123.6, 120.9, 116.1, and 110.1.
5-tert-Butylpyrazine-2-carboxylic acid (5-bromo-2-hydroxyphenyl)amide (26). Yield: 86%; m.p. 184-185 °C; For C15H16BrN3O2 (350.2) calculated: 51.44% C, 4.60% H, 12.00% N; found: 51.39% C, 5.61% H, 11.94% N; RF = 0.82; IR cm-1: 3368 (NH), 1685 (CO); 1H NMR (CDCl3) δ 9.55 (bs, 1H, NH), 9.37 (d, 1H, J=1.1 Hz, H3), 8.60 (d, 1H, J=1.1 Hz, H6), 8.08 (d, 1H, J=2.47 Hz, H3´), 7.47 (dd, 1H, J=8.79 Hz, J=2.47 Hz, H5´), 7.02 (d, 1H, J=8.79 Hz, H6´), 5.66 (bs, 1H, OH), and 1.44 (s, 9H, CH3); 13C-NMR (CDCl3) δ 167.9, 161.0, 149.4, 142.9, 141.1, 139.0, 131.2, 123.6, 120.9, 116.1, 110.1, 37.1, and 29.7.
5-tert-Butyl-6-chloropyrazine-2-carboxylic acid (5-bromo-2-hydroxyphenyl)amide (27). Yield: 77%; m.p. 160-161 °C; For C15H15BrClN3O2 (384.7) calculated: 46.84% C, 3.93% H, 10.92% N; found: 47.09% C, 4.12% H, 11.13% N; RF = 0.86; IR cm-1: 3373 (NH), 1691 (CO); 1H-NMR (CDCl3) δ 9.28 (bs, 1H, NH), 9.25 (s, 1H, H3), 8.06 (d, 1H, J=2.47 Hz, H3´), 7.49 (dd, 1H, J=8.79 Hz, J=2.47 Hz, H5´), 7.03 (d, 1H, J=8.79 Hz, H6´), 5.65 (bs, 1H, OH), and 1.55 (s, 9H, CH3); 13C-NMR (CDCl3) δ 164.7, 159.7, 149.7, 145.8, 140.8, 140.2, 130.8, 123.8, 121.2, 116.1, 110.1, 39.0, and 28.3.
6-Chloropyrazine-2-carboxylic acid (3,4-dichlorophenyl)amide (28). Yield: 83%; m.p. 132-133 °C; For C11H6Cl2N3O (302.5) calculated: 43.67% C, 2.00% H, 13.89% N; found: 43.51% C, 1.78% H, 14.11% N; RF = 0.88; IR cm-1: 3370 (NH), 1690 (CO); 1H-NMR (CDCl3) δ 9.41 (bs, 1H, NH), 9.38 (s, 1H, H3), 8.83 (s, 1H, H5), 8.00 (d, 1H, J=2.47 Hz, H2´), 7.59 (dd, 1H, J=8.79 Hz, J=2.47 Hz, H6´), and 7.45 (d, 1H, J=8.79 Hz, H5´); 13C-NMR (CDCl3) δ 159.3, 147.8, 147.4, 143.2, 142.1, 136.1, 132.9, 130.7, 130.6, 128.3, 121.5, and 119.0.
5-tert-Butylpyrazine-2-carboxylic acid (3,4-dichlorophenyl)amide (29). Yield: 76%; m.p. 143-144 °C; For C15H15Cl2N3O (324.2) calculated: 55.57% C, 4.66% H, 12.96% N; found: 55.63% C, 4.71% H, 13.08% N; RF = 0.92; IR cm-1: 3365 (NH), 1685 (CO); 1H-NMR (CDCl3) δ 9.67 (bs, 1H, NH), 9.37 (d, 1H, J=1.37 Hz, H3), 8.61 (d, 1H, J=1.37 Hz, H6), 8.01 (d, 1H, J=2.48 Hz, H2´), 7.58 (dd, 1H, J=8.79 Hz, J=2.47 Hz, H6´), 7.43 (d, 1H, J=8.79 Hz, H5´), and 1.45 (s, 9H, CH3); 13C-NMR (CDCl3) δ 168.2, 161.2, 143.0, 140.7, 139.0, 136.9, 133.0, 130.6, 127.7, 121.3, 118.9, 37.1, and 29.7.
5-tert-Butyl-6-Chloropyrazine-2-carboxylic acid (3,4-dichlorophenyl)amide (30). Yield: 83%, m.p. 113-114 °C For C15H14Cl3N3O (358.7) calculated: 50.23% C, 3.93% H, 11.72% N; found: 55.63% C, 4.71% H, 13.08% N; RF = 0.95; IR cm-1: 3390 (NH), 1685 (CO); 1H-NMR (CDCl3) δ 9.38 (bs, 1H, NH), 9.25 (s, 1H, H3), 8.01 (d, 1H, J=2.47 Hz, H2´), 7.59 (dd, 1H, J=8.79 Hz, J=2.48 Hz, H6´), and 7.44 (d, 1H, J=8.79 Hz, H5´), 1.55 (s, 9H, CH3); 13C-NMR (CDCl3) δ 165.1, 159.9, 145.8, 140.5, 140.3, 136.5, 133.0, 130.7, 128.2, 121.6, 119.1, 39.1, and 28.2.