Revisiting the Reaction Between Diaminomaleonitrile and Aromatic Aldehydes: a Green Chemistry Approach
Abstract
:Introduction
Results and Discussion
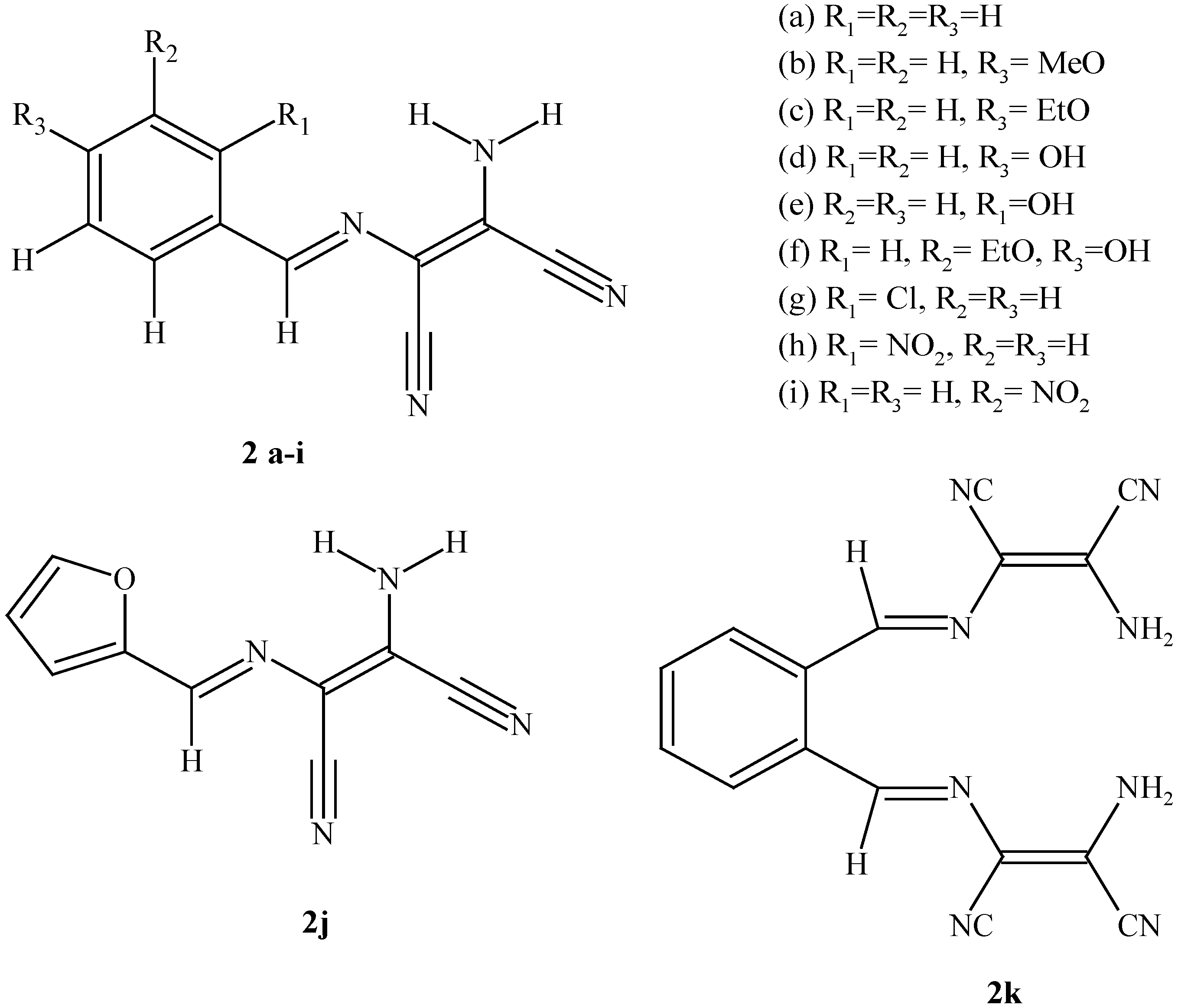
| 2a | 2b | 2c | 2d | 2e | 2f | 2g | 2h | 2i | 2j | 2k | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| NH2 | 7.94 | 7.77 | 7.76 | 7.76 | 7.86 | 7.72 | 8,19 | 8.27 | 8.27 | 7.77 | 7.77 |
| 1 | 113.7 | 115.1 | 115.1 | 115.2 | 115.0 | 115.2 | 114.6 | 114.9 | 114.6 | 114.9 | 114.8 |
| 2 | 126.9 | 126.3 | 126.3 | 125.9 | 126.5 | 125.8 | 128.4 | 129.4 | 128.6 | 126.7 | 127.7 |
| 3 | 103.9 | 103.6 | 103.6 | 103.9 | 103,9 | 103.9 | 103.0 | 103.0 | 102.0 | 103.7 | 103.7. |
| 4 | 112.9 | 114.3 | 114.3 | 114.4 | 114.4 | 114.4 | 114.0 | 114.5 | 114.1 | 114.1 | 114.3 |
| ArCHN | 156.1 | 155.2 | 155.2 | 155.6 | 153.4 | 155.8 | 150.4 | 150.9 | 153.2 | 143.5 | 153.6 |
| 8.31 | 8.21 | 8.20 | 8.16 | 8.59 | 8.13 | 8.56 | 8.61 | 8.39 | 8.08 | 8.85 | |
| 1´ | 135.7 | 128.9 | 128.7 | 127.4 | 121.7 | 127.8 | 133.3 | 130.2 | 137.7 | 151.9 | 134.8 |
| 2´ | 128.5 | 131.4 | 131.5 | 131.7 | 158.6 | 112.7 | 135.1 | 149.9 | 123.3 | --- | 134.8 |
| 7.93 | 7.98 | 7.97 | 7.87 | --- | 7.65 | --- | --- | 8.87 | --- | --- | |
| 3´ | 128.6 | 114.7 | 115.2 | 116.2 | 116.9 | 147.8 | 130.5 | 125.6 | 148.7 | 118.1 | 130.0 |
| 7.47 | 7.02 | 7.00 | 6.84 | 6.92 | --- | 7.55 | 8.08 | --- | 7.27 | 8.28 | |
| 4´ | 131.3 | 162.6 | 161.9 | 161.4 | 133.7 | 151.3 | 133.7 | 132.6 | 125.8 | 113.5 | 131.4 |
| 7.48 | 7.44 | --- | 6.97 | 7.33 | --- | 7.50 | 7.71 | 8.29 | 6.73 | 7.56 | |
| 5´ | 128.6 | 114.7 | 115.2 | 116.2 | 119.9 | 115.9 | 127.9 | 134.3 | 130.6 | 147.5 | 131.4 |
| 7.47 | 7.02 | 7.00 | 6.84 | 6.91 | 6.85 | 7.40 | 7.81 | 7.75 | 7.96 | 7.56 | |
| 6´ | 128.5 | 131.4 | 131.5 | 131.7 | 129.4 | 125.2 | 129.5 | 130.4 | 135.5 | --- | 130.0 |
| 7.93 | 7.98 | 7.97 | 7.87 | 8.03 | 7.35 | 8.44 | 8,53 | 8.44 | --- | 8.28 |
| 2a | 2b | 2d | 2e | 2f | 2g | 2h | 2i | 2j | 2k | |
|---|---|---|---|---|---|---|---|---|---|---|
| ArCHN | 8.31 | 8.21 | 8.16 | 8.59 | 8.13 | 8.56 | 8.61 | 8.39 | 8.08 | 8.85 |
| 2a | 2b | 2c | 2d | 2e | 2f | 2g | 2h | 2i | 2j | 2k | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| CN1 | 2240 | 2231 | 2237 | 2247 | 2235 | 2231 | 2245 | 2247 | 2237 | 2237 | 2237 |
| CN4 | 2205 | 2207 | 2197 | 2200 | 2206 | 2212 | 2203 | 2204 | 2200 | 2200 | 2200 |
| Aldehyde | Product | Procedure A | Procedure B |
|---|---|---|---|
| Benzaldehyde | 2a | 78 | 49 |
| p-Methoxybenzaldehyde | 2b | 93 | 62 |
| 3-Ethoxy-4-hydroxybenzaldehyde | 2f | 53 | 38 |
| o-Chlorobenzaldehyde | 2g | 79 | 14 |
Conclusions
Experimental
General
General procedures for the synthesis of imines 2a-k
Acknowledgements
References
- Al-Azmi, A.; Elassar, A. Z. A.; Booth, B. L. The chemistry of diaminomaleonitrile and its utility in heterocyclic synthesis. Tetrahedron 2003, 59, 2749–2763. [Google Scholar]
- Alves, M. J.; Booth, B. L.; Proença, M. F. Synthesis of 5-amino-4-(cyanoformimidoyl)-1H-imidazole: a reactive intermediate for the synthesis of 6-carbamoyl-1,2-dihydropurines and 6-carbamoylpurines. J. Chem. Soc., Perkin Trans. 2 1990, 1705–1712. [Google Scholar]
- Booth, B. L.; Dias, A. M.; Proença, M. F.; Zaki, M. E. A. The Reactions of Diaminomaleonitrile with Isocyanates and Either Aldehydes or Ketones Revisited. J. Org. Chem. 2001, 66, 8436–8441. [Google Scholar] [CrossRef] [PubMed]
- Ohtsuka, Y. Chemistry of diaminomaleonitrile. 3. Reaction with isocyanate: a novel pyrimidine synthesis. J. Org. Chem. 1978, 43, 3231–3234. [Google Scholar]
- Ohtsuka, Y. Chemistry of diaminomaleonitrile. 4. Nitrile hydration of the Schiff bases. J. Org. Chem. 1979, 44, 827–830. [Google Scholar]
- Shirai, K.; Matsuoka, M.; Fukunishi, K. New syntheses and solid state fluorescence of azomethine dyes derived from diaminomaleonitrile and 2,5-diamino-3,6-dicyanopyrazine. Dyes Pigm. 2000, 47, 107–115. [Google Scholar]
- Begland, R. W.; Hartter, D. R.; Jones, F. N.; Saw, D. J.; Sheppard, W. A.; Webster, O. W.; Weigert, F. J. Hydrogen cyanide chemistry. VIII. New chemistry of diaminomaleonitrile. Heterocyclic synthesis. J. Org. Chem. 1974, 39, 2341–2350. [Google Scholar]
- Barton, J. W.; Goodland, M. C.; Gould, K. J.; McOmie, J. F. W.; Mound, W. R. Biphenylenes-xxxi: Condensation of benzocyclobutene-1,2-dione with aliphatic and heterocyclic 1,2-diamines and the synthesis of cis-2-cyano-3-(2'-cyanovinyl)-1,4-diaz. Tetrahedron 1979, 35, 241–247. [Google Scholar] [CrossRef]
- Beall, L. S.; Mani, N. S.; White, A. J. P.; Williams, D. J.; Barret, A. G. M.; Hoffman, B. M. Porphyrazines and Norphthalocyanines Bearing Nitrogen Donor Pockets: Metal Sensor Properties. J. Org. Chem. 1998, 63, 5806–5817. [Google Scholar] [CrossRef] [PubMed]
- Woehrle, D.; Buttner, P. Polymeric schiff's base chelates and their precursors 8a, some cobalt chelates as catalysts for the isomerization of quadrycyclane to norbornadien. Polym. Bull. 1985, 13, 57–64. [Google Scholar]
- Robertson, P. S.; Vaughan, J. Derivatives of the Hydrogen Cyanide Tetramer: Structure and Chemistry1. J. Am. Chem. Soc. 1958, 80, 2691–2693. [Google Scholar] [CrossRef]
- Holger, H.; Benedikt, B. Eur. Pat. Appl. 88-116980, 1989.
- Johnson, D. M.; Reybuck, S. E.; Lawton, R. G.; Ramussen, P. G. Condensation of DAMN with Conjugated Aldehydes and Polymerizations of the Corresponding Imines. Macromolecules 2005, 38, 3615–3621. [Google Scholar] [CrossRef]
- Nesterov, V. V.; Antipin, M. Y.; Nesterov, V. N.; Penn, B. G.; Frazier, D. O.; Timofeeva, T. V. Thermally Stable Imines as New Potential Nonlinear Optical Materials. Crystal Growth Des. 2004, 4, 521–531. [Google Scholar] [CrossRef]
- Rivera, A.; Ríos-Motta, J. An unusual product obtained from condensation between ethylene-diamine and formaldehyde in basic medium. Tetrahedron Lett. 2005, 46, 5001–5004. [Google Scholar] [CrossRef]
- Simion, A.; Simion, C.; Kanda, T.; Nagashima, S.; Mitoma, Y.; Yamada, T.; Mimura, K.; Tashiro, M. Synthesis of imines, diimines and macrocyclic diimines as possible ligands, in aqueous solution. J. Chem. Soc. Perkin Trans I 2001, 2071–2078. [Google Scholar]
- Jurcik, V.; Woilhelm, R. Preparation of aminals in water. Tetrahedron 2004, 60, 3205–3210. [Google Scholar] [CrossRef]
- Rasshofer, W.; Voegtle, F. Condensed imidazoles via cyclization of diaminomaleodinitrile with dialdehydes. J. Chem. Res. (S) 1977, 11, 265–268. [Google Scholar]
- Hinkel, L. E.; Richards, G. O.; Thomas, O. Studies on hydrogen cyanide. Part X. The tetrapolymer. J. Chem. Soc. 1937, 1432–1437. [Google Scholar]
- Yozo, O.; Takakazu, K.; Eiko, T. Jpn Patent 53065823, 1978.
- Zuman, P. Reactions of Orthophthalaldehyde with Nucleophiles. Chem. Rev. 2004, 104, 3217–3238. [Google Scholar] Nan’ya, S.; Tange, T.; Maekawa, E. Synthesis of 1,2-Disubstituted Isoindoles from o-Phthalaldehyde and Primary Amines. J. Heterocycl. Chem. 1985, 22, 449–451. [Google Scholar]
- Rideout, D.C.; Breslow, R. Hydrophobic acceleration of Diels-Alder reactions. J. Am. Chem. Soc. 1980, 102, 7816–7817. [Google Scholar] [CrossRef]
- Popp, F. D. Condensation of glyoxal and diaminomaleonitrile. Chem. Ind. 1973, 17, 852–859. [Google Scholar]
- Laitinen, A.; Takebayashi, Y.; Kylanlahti, I.; Yli-Kauhaluoma, J.; Sugeta, T.; Otake, K. Ene reaction of allylbenzene and N-methylmaleimide in subcritical water and ethanol. Green Chem. 2004, 6, 49–52. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds 2c and 2k are available from the authors.
© 2006 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Rivera, A.; Ríos-Motta, J.R.-M.; León, F. Revisiting the Reaction Between Diaminomaleonitrile and Aromatic Aldehydes: a Green Chemistry Approach. Molecules 2006, 11, 858-866. https://doi.org/10.3390/11110858
Rivera A, Ríos-Motta JR-M, León F. Revisiting the Reaction Between Diaminomaleonitrile and Aromatic Aldehydes: a Green Chemistry Approach. Molecules. 2006; 11(11):858-866. https://doi.org/10.3390/11110858
Chicago/Turabian StyleRivera, Augusto, Jaime Ríos-Motta Ríos-Motta, and Francisco León. 2006. "Revisiting the Reaction Between Diaminomaleonitrile and Aromatic Aldehydes: a Green Chemistry Approach" Molecules 11, no. 11: 858-866. https://doi.org/10.3390/11110858




