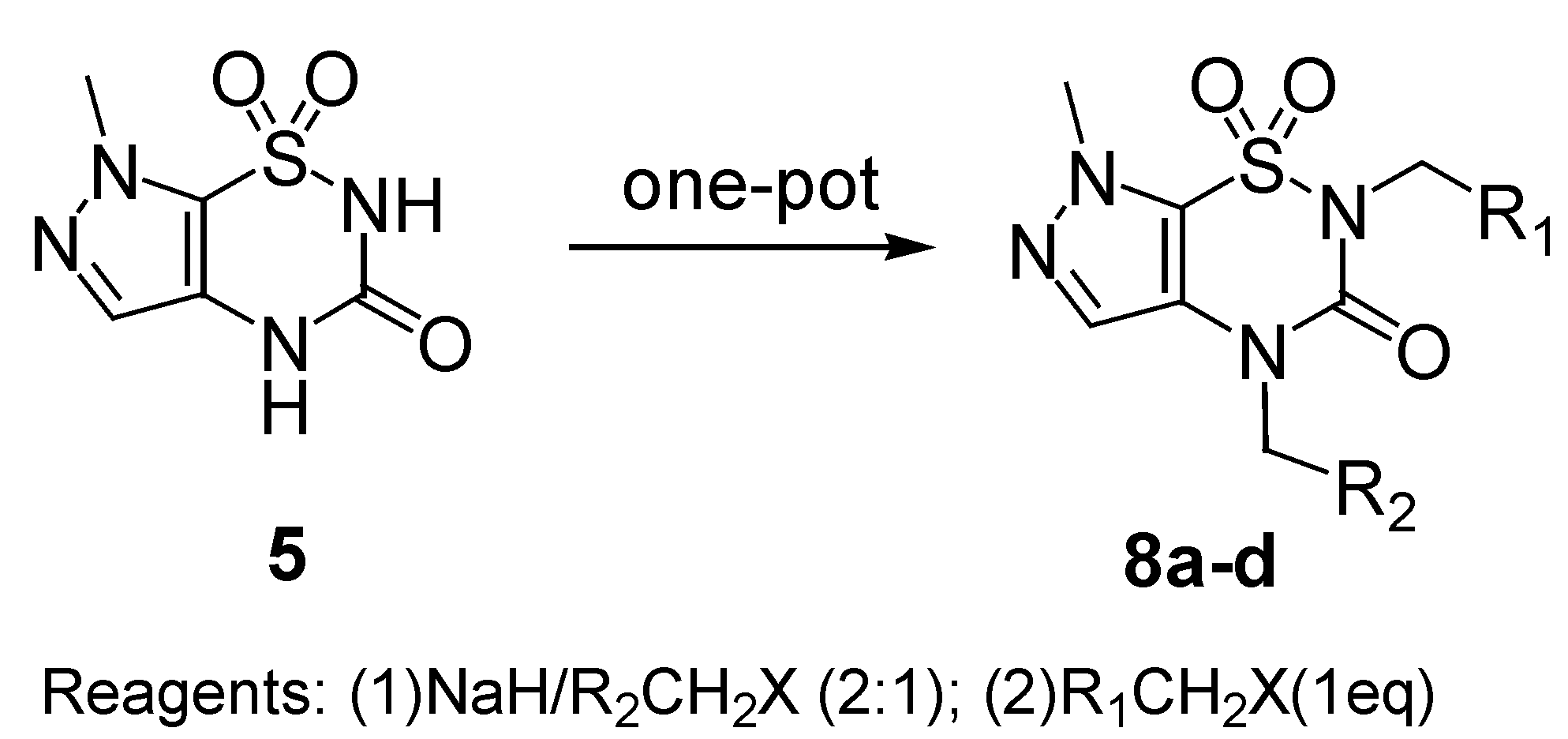Results and Discussion
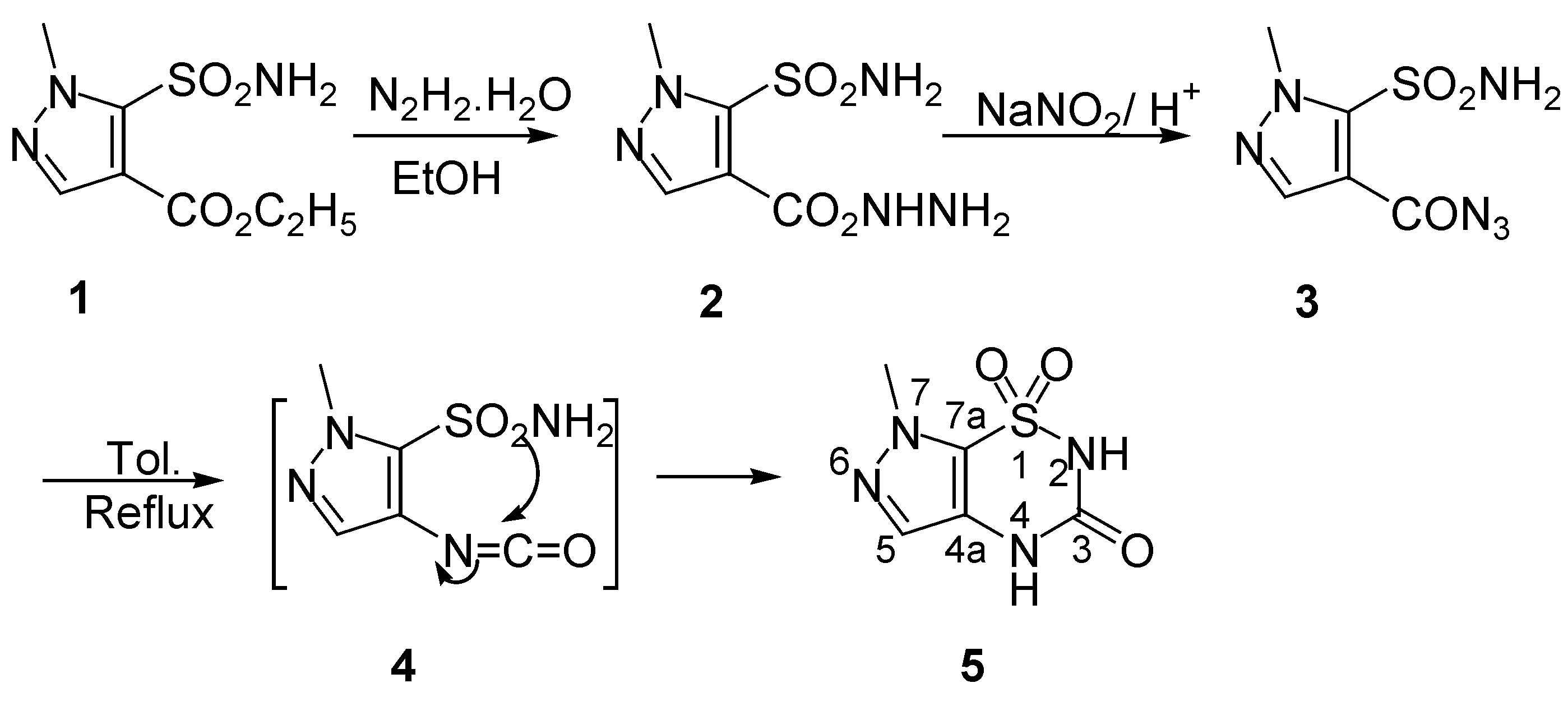
7-Methylpyrazolo[4,5-
e][1,2,4]thiadiazin-3(2
H, 4
H)-one 1,1-dioxide (
5) was synthesized in a similar manner to the thiophene and regioisomeric pyrazole series [
7], by a route which started with hydrazinolysis of ethyl 1-methyl-5-sulfamoylpyrazole-4-carboxylate (
1), a commercially available product, with hydrazine hydrate in refluxing ethanol, thus forming the hydrazide
2 in excellent yield. Carboxy azide
3, which was obtained by the reaction of compound
2 with sodium nitrite in diluted hydrochloric acid at ca. 10°C, was pure enough for use in the next ring closure step without further purification. Thus, by refluxing compound
3 in anhydrous toluene, a classical Curtius rearrangement took place through the intermediacy of isocyanate
4 to afford compound
5, a new regioisomer of 6-methylpyrazolo[4,5-
e][1,2,4]thiadiazin-3(2
H, 4
H)-one 1,1-dioxide prepared by our previous work [
7]. (
Scheme 1). Structural assignments for the ring system in
5 were based on its
1H- and
13C-NMR, IR and MS spectral analysis.
The method used for the regioselective preparation of the new
N2-substituted 7-methylpyrazolo-[4,5-e][1,2,4]thiadiazine derivatives
6 paralleled that described in ref. [
8], including deprotonation of the
5 using one equivalent of sodium hydride in DMF solvent at a temperature below 5°C, and followed by alkylation with one equivalent of alkyl halide at 80°C for 2-8 h, thus giving the mono
N2-substituted derivatives
6 (
Scheme 2). The crude products were separated by flash column chromatography and purified by recrystallization from ethanol in good yields (81-85%,
Table 1).
Table 1.
N2-substituted derivatives 6a-d and O-alkylated derivatives 6’a-d of 7-methyl-pyrazolo[4,5-e][1,2,4]thiadiazin-3(2H,4H)-one 1,1-dioxide (5).
Table 1.
N2-substituted derivatives 6a-d and O-alkylated derivatives 6’a-d of 7-methyl-pyrazolo[4,5-e][1,2,4]thiadiazin-3(2H,4H)-one 1,1-dioxide (5).
| Entry | N2-/O-CH2R1 | Yield % | mp(°C) | δCH2 |
|---|
| 5 | H | 75 | 216-218 | — |
| 6a | benzyl | 81 | 175-177 | 5.06 |
| 6b | 4-Cl-benzyl | 80 | 192-194 | 4.95 |
| 6c | 4-Br-benzyl | 83 | 220-222 | 4.94 |
| 6d | 3-Cl-benzyl | 81 | 187-188 | 4.97 |
| 6’a | benzyl | 12 | 215-216 | 5.40 |
| 6’b | 4-Cl-benzy | 14 | 238-240 | 5.34 |
| 6’c | 4-Br-benzyl | 13 | 246-248 | 5.34 |
| 6’d | 3-Cl-benzyl | 16 | 224-227 | 5.36 |
Under these conditions compounds
6 were the predominant products, mainly due to the more acidic nature of the hydrogen at the N
2 position and the resulting easier deprotonation than the hydrogen at the N
4 position, which is caused by the strong electric withdraw effect by the sulfonyl group. Meanwhile, the isomeric 3-
O-alkylated pyrazolo[4,5-e][1,2,4]thiadiazine
6’ was observed as a side product in approximately 15% yields, which is attributed to the tautomerism between the nitrogen anion and the carbonyl-oxygen anion. The O-alkylated isomers
6’a-d have not been reported so far. In the
1H-NMR spectra, the chemical shifts of the O-CH
2 groups are distinguished from that of mono
N2- or
N4-alkylated derivatives, while linking with the same substituent (
Table 1 and
Table 2,
e.g. benzyl group,
N2-CH
2, δ 5.06;
N4-CH
2, δ 4.90; 3-
O-CH
2, δ 5.40).
During our ongoing research on the alkylation reaction of the compound
5, we used 2 equivalents of base rather than only one, in order to perform double deprotonation of
5. Quenching of the disodium salt with one equivalent of the electrophile, we observed complete regioselectivity of the reaction, since only the
N4-alkylated regioisomers
7 were produced, and none of
N2-substituted compounds
6 was found. We speculate that the stronger nucleophilicity of N
4 anion allowed a preferential N
4 alkylation. We also deduced that the relatively high steric hindrance afforded by the 1-sulfonyl and 3-carbony groups further disfavored the alkylation at N
2 site.
The
N4-alkylated product was confirmed by the chemical shift of the CH
2 signal, and by means of NOE experiments and HMBC sequences to establish long-distance proton/carbon correlations. It was shown that the
N4-CH
2 correlated exclusively with the both quaternary carbon C-3 and C-4a, which is different from the
N2-CH
2, that only correlated with quaternary carbon C-3. A series of
N4-alkylated derivatives
7a-d were prepared in high yield (80-86%) and the results were shown in
Table 2.
Table 2.
N4-substituted-7-methylpyrazolo[4,5-][1,2,4]thiadiazin-3(2H,4H)-one 1,1-dioxides.
Table 2.
N4-substituted-7-methylpyrazolo[4,5-][1,2,4]thiadiazin-3(2H,4H)-one 1,1-dioxides.
| Entry | N4-CH2R2 | Yield % | Mp (°C) | δCH2 |
|---|
| 7a | benzyl | 85 | 187-189 | 4.90 |
| 7b | 4-Cl-benzyl | 84 | 218-230 | 4.86 |
| 7c | 4-Br-benzyl | 86 | 240-242 | 4.85 |
| 7d | 3-Cl-benzyl | 81 | 194-196 | 4.88 |
N2,
N4-Disubstituted hetero[1,2,4]thiadiazines with different substituents are usually prepared by stepwise alkylation, first at N
2 and then at N
4 [
7,
9]. Using to the aforementioned regioselective alkylation method,
N2,
N4-disubstituted derivatives
8 were synthesized in a one-pot reaction, by addition of two equivalents of NaH and one equivalent of R
2CH
2X first, followed by addition of one equivalent of R
1CH
2X when the synthesis of intermediate
7 (monitored by TLC) was shown to be finished. The crude products
8 were obtained and purified by recrystallization to give white solids in ca. 80% yield (
Scheme 4). A series of
N2,
N4-dialkylated derivatives
8a-d was prepared by this method and are listed in
Table 3. The structures of all synthesized compounds were confirmed by
1H- and
13C-NMR, IR and MS spectroscopic analysis.
Table 3.
N2,N4-substituted-7-methylpyrazolo[4,5-e][1,2,4]thiadiazine-3(2H,4H)-one 1,1-dioxides 8a-d.
Table 3.
N2,N4-substituted-7-methylpyrazolo[4,5-e][1,2,4]thiadiazine-3(2H,4H)-one 1,1-dioxides 8a-d.
| Entry | N2-CH2R1 | N4-CH2R2 | Yield % | mp(°C) | δN2-CH2 | δN4-CH2 |
|---|
| 8a | benzyl | 2-Br-benzyl | 82 | 107-108 | 5.17 | 5.14 |
| 8b | benzyl | 2-Cl-benzyl | 80 | 105-107 | 5.19 | 5.13 |
| 8c | 4-Cl-benzyl | 2-Br-benzyl | 81 | 129-131 | 5.15 | 5.08 |
| 8d | 4-Cl-benzyl | 2-Cl-benzyl | 79 | 117-119 | 5.15 | 5.02 |
Experimental Section
General
All melting points were determined on a micromelting point apparatus and are uncorrected. 1H-NMR (600 MHz) and 13C-NMR (150 MHz) spectra were obtained on a Bruker Avance-600 instrument in the indicated solvent. Chemical shifts are expressed in δ units and TMS as internal reference. Infrared spectra (IR) were recorded with a Nexus 470FT-IR Spectrometer. Mass spectra were taken on a LC Autosampler Device: Standard G1313A instrument. Flash column chromatography was performed on column packed with silica gel 60 (230-400 mesh). Solvents were reagent grade and when necessary, were purified and dried by standard methods. Concentration of the reaction solutions involved the use of a rotary evaporator under reduced pressure.
Synthesis of 7-Methyl-1,1,3-trioxo-2H,4H-pyrazolo[4,5-e][1,2,4]thiadiazine (5)
The synthesis was carried out in analogy to the preparation of the corresponding thieno[3,4-e][1,2,4]thiadiazine and the regioisomeric 6-methylpyrazo[3,4-e][1,2,4]thiadiazine derivatives [
7,
8]. Recrystallization from ethanol gave a white solid. IR (KBr, cm
-1): 3244, 3152 (NH); 3014 (Py-CH); 1692 (C=O); 1342, 1141 (SO
2);
1H-NMR (DMSO-
d6) δ: 11.49 (s, 1H, exchanged with deuterium by D
2O addition, NH); 7.40 (s, 1H, Py-CH); 3.94 (s, 3H, CH
3);
13C-NMR (DMSO-
d6) δ: 152.4 (C=O), 125.3 (C-5), 124.3 (C-4a), 123.4 (C-7a), 38.3 (CH
3); MS (EI) m/z: 202.2 (M
+); Anal. calcd for C
5H
6N
4O
3S: C, 29.70; H, 2.99; N, 27.71; Found: C 29.76; H 3.03; N 27.66.
General Procedure for the Preparation of 2-Substituted-7-methyl-1,1,3-trioxo-2H,4H-pyrazolo[4,5-e][1,2,4]thiadiazines 6a-d and 3-Substituted-7-methyl-1,1-dioxo-4H-pyrazolo[4,5-e][1,2,4]thiadia-zines 6’a-d
To a solution of compound 5 (1 equiv.) in dry DMF (4 mL) was added sodium hydride (60% dispersion in mineral oil, 1 equiv.) in portions, under an inert atmosphere (N2), while the temperature was kept below 10°C. After stirring for 15 min, the alkyl halide (1 equiv.) was added dropwise. The reaction mixture was stirred at room temperature for 20 min and at 30-50°C for 12-20 h (checked by TLC). After the solvent was evaporated under reduced pressure, the products were separated by flash column chromatography (1:3 ethyl acetate/cyclohexane) to give the compounds 6 and 6’ respectively, which were purified by recrystallization from ethanol.
2-Benzyl-7-methyl-1,1,3-trioxo-2H,4H-pyrazolo[4,5-e][1,2,4]thiadiazine (6a) and 3-Benzyloxy-7-methyl-1,1-dioxo-4H-pyrazolo[4,5-e][1,2,4]thiadiazine (6’a). Compound 5 was alkylated with benzyl bromide at 30°C for 12h to give 6a and 6’a, which after purification gave white solids: 6a: IR (KBr, cm-1): 3266 (NH); 1693 (C=O); 1328, 1197 (SO2); 1H-NMR (CDCl3) δ: 9.09 (s, 1H, NH), 7.20 (s, 1H, PyH), 7.49 (d, 2H, J=7.31, PhH), 7.29-7.36 (m, 3H, PhH), 5.06 (s, 2H, NCH2), 4.13 (s, 3H, CH3); 13C-NMR (CDCl3) δ: 150.3 (C=O), 135.4 (C-1’), 128.7, 128.5, 128.1, 125.0 (C-4a), 123.0 (C-5), 122.1 (C-7a), 44.0 (N2-CH2), 39.0 (CH3); MS (EI): m/z 293.3 (M+1); 6’a: IR (KBr, cm-1): 3276 (NH); 1614(C=N); 1300, 1176 (SO2); 1H-NMR (DMSO-d6) δ: 12.33 (s, 1H, NH), 7.49 (s, 1H, PyH), 7.64 (dd, 1H, J=7.34Hz, J=1.85Hz, PhH), 7.55 (dd, 1H, J=7.73, J=1.16, PhH), 7.42-7.46 (m, 3H, PhH), 5.40 (s, 2H, NCH2), 3.98 (s, 3H, CH3); 13C-NMR (DMSO-d6) 151.7(C=N), 133.4(C-1’), 132.4, 131.4, 131.0, 129.7, 127.7, 125.5(C-4a), 123.9(C-5), 123.4(C-7a), 68.0(O-CH2), 38.4(CH3); MS (EI): m/z 292.3(M+).
2-(p-Chlorobenzyl)-7-methyl-1,1,3-trioxo-2H,4H-pyrazolo[4,5-e][1,2,4]thiadiazine (6b) and 3-(p-chlorobenzyloxy)-7-methyl-1,1-dioxo-4H-pyrazolo[4,5-e][1,2,4] thiadiazine (6’b). Reaction of compound 5 and 4-chlorobenzyl chloride at 50°C for 20 h gave compounds 6b and 6’b as white solids after purification. 6b: IR (KBr, cm-1): 3225 (NH), 1682 (C=O), 1334, 1185 (SO2); 1H-NMR (DMSO-d6) δ: 11.37 (s, 1H, NH), 7.45 (s, 1H, PyH), 7.36-7.41 (m, 4H, PhH), 4.95 (s, 2H, NCH2), 4.03 (s, 3H, CH3); 13C-NMR (DMSO-d6) δ: 148.8 (C=O), 135.6 (C-1’), 132.4, 129.9, 128.6, 125.7 (C-4a), 123.8 (C-5), 121.4 (C-7a), 42.3 (N2-CH2), 38.9 (CH3); MS(EI): m/z 327.3 (M+1); 6’b: IR (KBr, cm-1): 3242 (NH); 1610 (C=N); 1290, 1176(SO2); 1H- NMR (DMSO-d6) δ: 12.32 (s, 1H, NH), 7.53 (s, 1H, PyH), 7.48-7.51 (m, 4H, PhH), 5.34 (s, 2H, NCH2), 3.96 (s, 3H, CH3); 13C-NMR (DMSO-d6): 151.9 (C=N), 134.1 (C-1’), 133.5, 130.7, 128.8, 125.6 (C-4a), 124.0 (C-5), 123.6 (C-7a), 69.7 (O-CH2), 38.4 (CH3); MS (EI): m/z 327.3 (M+1).
2-(p-Bromobenzyl)-7-methyl-1,1,3-trioxo-2H,4H-pyrazolo[4,5-e][1,2,4]thiadiazine (6c) and 3-(p-Bromobenzyloxy)-7-methyl-1,1-dioxo-4H-pyrazolo[4,5-e][1,2,4] thiadiazine (6’c). Compound 5 and 4-bromobenzyl bromide at 30°C for 12 h gave after purification compounds 6c and 6’c as white solids. 6c: IR (KBr, cm-1): 3302 (NH); 1686 (C=O); 1364, 1197(SO2); 1H-NMR (DMSO-d6,) δ: 11.28 (s, 1H, NH), 7.43 (s, 1H, PyH), 7.53 (d, 2H, J=7.91, PhH), 7.31 (d, 2H, J=7.70, PhH), 4.94 (s, 2H, NCH2), 4.03 (s, 3H, CH3); 13C-NMR (DMSO-d6) δ: 148.7 (C=O), 136.0 (C-1’), 131.4, 130.0, 125.6, 123.8 (C-4a), 121.3 (C-5), 120.1 (C-7a), 42.3 (N2-CH2), 38.8 (CH3); MS (EI): m/z 373.1 (M+2), 371.2 (M+); 6’c: IR (KBr, cm-1): 3292(NH); 1605(C=N); 1274, 1173 (SO2); 1H-NMR (DMSO-d6) δ: 12.24 (s, 1H, NH), 7.47 (s, 1H, PyH), 7.62 (d, 2H, J=7.33, PhH), 7.45 (d, 2H, J=7.44, PhH), 5.34 (s, 2H, NCH2), 3.96 (s, 3H, CH3); 13C-NMR (DMSO-d6) δ: 151.8 (C=N), 134.4 (C-1’), 131.6, 130.8, 125.5, 123.9 (C-4a), 123.5 (C-5), 122.0 (C-7a), 69.7 (O-CH2), 38.3 (CH3); MS: m/z 373.1 (M+2), 371.2 (M+).
2-(m-Chlorobenzyl)-7-methyl-1,1,3-trioxo-2H,4H-pyrazolo[4,5-e][1,2,4]thiadiazine (6d) and 3-(m-Chlorobenzyloxy)-7-methyl-1,1-dioxo-4H-pyrazolo[4,5-e][1,2,4]thiadiazine (6’d). Compound 5 and 3-chlorobenzyl chloride at 50°C for 20h gave compounds 6d and 6’d as white solids after purification. 6d: IR (KBr, cm-1): 3223 (NH); 1678 (C=O); 1334, 1181 (SO2); 1H-NMR (DMSO-d6) δ: 11.39 (s, 1H, NH), 7.45 (s, 1H, PyH), 7.31-7.40 (m, 4H, PhH), 4.97 (s, 2H, NCH2), 4.03 (s, 3H, CH3); 13C-NMR (DMSO-d6) δ: 148.8 (C=O), 139.1 (C-1’), 133.1, 130.5, 127.7, 126.6, 125.7, 123.8 (C-4a), 121.3 (C-5), 120.1 (C-7a), 42.4 (N2-CH2), 38.9 (CH3); MS: m/z 327.3 (M+1); 6’d: IR (KBr, cm-1): 3211 (NH), 1610 (C=N);1312, 1174 (SO2); 1H-NMR (DMSO-d6) δ: 12.34 (s, 1H, NH), 7.50 (s, 1H, PyH), 7.59 (s, 1H, PhH), 7.45 (s, 3H, PhH), 5.36 (s, 2H, NCH2), 3.97 (s, 3H, CH3); 13C-NMR (DMSO-d6) δ: 151.9 (C=N), 137.6 (C-1’), 133.4, 130.7, 128.7, 128.4, 127.2, 125.6 (C-4a), 124.0 (C-5), 123.6 (C-7a), 69.6 (O-CH2), 38.4 (CH3); MS (EI): m/z 327.3 (M+1).
General Procedure for the Preparation of 4-Substituted-7-methyl-1,1,3-trioxo-2H,4H-pyrazolo[4,5-e] [1,2,4]thiadiazines (7a-d)
To a solution of compound 5 (1 equiv.) in dry DMF (4 mL) was added sodium hydride (60% dispersion in mineral oil, 2 equiv.) in portions, under an inert atmosphere (N2) while the temperature was kept below 10°C. After 60 min stirring, the alkyl halide (1 equiv.) was added dropwise. The reaction mixture was stirred at room temperature for 20 min and at 40-60°C for 8-12h (checked by TLC), and acidified with dilute hydrochloric acid (pH 4-6). The crude products obtained after the solvent was evaporated under reduced pressure were then separated by flash column chromatography using the indicated solvent system and purified by recrystallization from ethanol.
4-Benzyl-7-methyl-1,1,3-trioxo-2H,4H-pyrazolo[4,5-e][1,2,4]thiadiazine (7a). Compound 5 was reacted with benzyl bromide at 30°C for 10 h to give 7a as a white solid after by flash column chromatography separation (CH2Cl2/CH3OH 4:1) of the crude product and recrystallization. IR (KBr, cm-1): 1694 (C=O); 1342, 1141 (SO2); 1H-NMR (DMSO-d6) δ: 7.18-7.29 (m, 5H, PhH), 7.09 (s, 1H, PyH), 4.90 (s, 2H, NCH2), 3.83 (s, 3H, CH3); 13C-NMR (DMSO-d6) δ: 152.3 (C=O), 138 (C-1’), 128.4, 128.2, 127, 126.1 (C-4a), 125.0 (C-5), 123.2 (C-7a), 46.2 (CH2), 37.3 (CH3); MS (EI): m/z 292.3 (M+).
4-(p-Chlorobenzyl)-7-methyl-1,1,3-trioxo-2H,4H-pyrazolo[4,5-e][1,2,4]thiadiazine (7b). Compound 5 was reacted with 4-chlorobenzyl chloride at 50-60°C for 24 h to give 7b as a white solid after flash column chromatography (CH2Cl2/CH3OH 4:1) of the crude product and recrystallization. IR (KBr, cm-1): 1692 (C=O); 1327, 1135 (SO2); 1H-NMR (DMSO-d6) δ: 7.32-7.35 (m, 4H, PhH), 7.14 (s, 1H, PyH), 4.86 (s, 2H, NCH2), 3.82 (s, 3H, CH3); 13C-NMR (DMSO-d6) δ: 152 .0 (C=O), 137.1 (C-1’), 131, 128.9, 128.3, 128.1 (C-4a), 125.0 (C-5), 123.0 (C-7a), 46.0 (CH2), 37.1 (CH3); MS (EI): m/z 327.3 (M+1).
4-(p-Bromobenzyl)-7-methyl-1,1,3-trioxo-2H,4H-pyrazolo[4,5-e][1,2,4]thiadiazine (7c). Compound 5 and 4-bromobenzyl bromide at 30°C for 10 h gave 7c as a white solid after purification of the crude reaction product by flash column chromatography (CH2Cl2/CH3OH 3:1) and recrystallization. IR (KBr, cm-1): 1693 (C=O); 1323, 1136 (SO2); 1H-NMR (DMSO-d6) δ: 7.20-7.47 (m, 4H, PhH), 7.14 (s, 1H, PyH), 4.85 (s, 2H, NCH2), 3.82 (s, 3H, CH3); 13C-NMR (DMSO-d6) δ: 152.2 (C=O), 138.3 (C-1’), 131.2, 129.1, 128.3, 125.4 (C-4a), 123.2 (C-5), 119.0 (C-7a), 47.2 (CH2), 37.3 (CH3); MS (EI): m/z 371.2 (M+1).
4-(m-Chlorobenzyl)-7-methyl-1,1,3-trioxo-2H,4H-pyrazolo[4,5-e][1,2,4]thiadiazine (7d). Compound 5 and 3-chlorobenzyl chloride at 50-60°C for 24 h gave 7d as a white solid after separation of the crude product by flash column chromatography (CH2Cl2/CH3OH 4:1) and recrystallization. IR (KBr, cm-1): 1695 (C=O); 1335, 1141 (SO2); 1H-NMR (DMSO-d6) δ: 7.22-7.33 (m, 4H, PhH), 7.18 (s, 1H, PyH), 4.88 (s, 2H, NCH2), 3.83 (s, 3H, CH3); 13C-NMR (DMSO-d6) δ: 152 (C=O), 141.2 (C-1’), 132.8, 130.4, 128.0, 126.9, 126.8, 125.8 (C-4a), 125.7 (C-5), 123.2 (C-7a), 46.4 (CH2), 37.3 (CH3); MS (EI): m/z 327.3 (M+1).
General Procedure for the Preparation of 2,4-Disubstituted 7-methyl-1,1,3-trioxo-2H,4H-pyrazolo [4,5-e][1,2,4]thiadiazine Derivatives 8a-d
To a solution of compound 5 (1 equiv.) in dry DMF (4 mL/mmol) was added sodium hydride (60% dispersion in mineral oil, 2 equiv.) in portions, under an inert atmosphere (N2) and keeping the temperature below 10°C. After stirring for 60 min, the appropriate alkyl halide (R2CH2X, 1 equiv.) was added dropwise. The reaction mixture was stirred at room temperature for 20 min and at 30-60°C for 8-12h (checked by TLC), then the second alkyl halide (R1CH2X, 1 equiv.) was added dropwise. Stirring of the mixture was continued for 12-20 h at 40-60°C, and then it was acidified (pH 4-6) with dilute hydrochloric acid. After the solvent was evaporated under reduced pressure, the crude products obtained were purified by recrystallization from ethanol to give 8a-d as white solids.
2-Benzyl-4-(o-bromobenzyl)-7-methyl-1,1,3-trioxo-2H,4H-pyrazolo[4,5-e][1,2,4]thiadiazine (8a). Compound 5 was alkylated with 2-bromobenzyl bromide (30-40°C for 12h), then with benzyl bromide (40-50°C for 12h) to give 8a. IR (KBr, cm-1): 1692 (C=O); 1324, 1192 (SO2); 1H-NMR (CDCl3) δ: 7.11 (s, 1H, PyH), 7.60 (d, 1H, J=7.87, PhH), 7.51 (d, 2H, J=7.46, PhH), 6.91 (d, 1H, J=7.57, PhH), 6.90-7.52 (m, 5H, PhH), 5.17 (s, 2H, NCH2), 5.14 (s, 2H, NCH2), 4.15 (s, 3H, CH3); 13C-NMR (CDCl3) δ: 148.3 (C=O), 133.3 (C-1’), 135.3 (C-1”), 129.2, 128.3, 132.9, 129.2, 127.9, 127.8, 126.9, 125.1, 125.3 (C-4a), 122.9 (C-5), 122.2 (C-7a), 49.1 (N4-CH2), 44.6 (N2-CH2), 38.9 (CH3); MS (EI): m/z 463.3 (M+2), 461.3 (M+).
2-Benzyl-4-(o-chlorobenzyl)-7-methyl-1,1,3-trioxo-2H,4H-pyrazolo[4,5-e][1,2,4]thiadiazine (8b). Compound 5 was alkylated with 2-chlorobenzyl chloride (50-60°C for 12 h), then with benzyl bromide (40-50°C for 12 h) to give 8b. IR (KBr, cm-1): 1691 (C=O), 1326, 1193 (SO2); 1H-NMR (CDCl3) δ: 6.95 (s, 1H, PyH), 7.13-7.51 (m, 9H, PhH), 5.19 (s, 2H, NCH2), 5.14 (s, 2H, NCH2), 4.15 (s, 3H, CH3); 13C-NMR (CDCl3) δ: 149.3 (C=O), 132.5 (C-1’), 135.3 (C-1”), 128.6, 128.3, 131.8, 129.7, 129.0, 127.9, 127.2, 170.0, 125.3 (C-4a), 124.9 (C-5), 120.0 (C-7a), 46.6 (N4-CH2), 44.5 (N2-CH2), 38.9 (CH3); MS (EI): m/z 417.4 (M+1).
2-(p-Chlorobenzyl)-4-(o-bromobenzyl)-7-methyl-1,1,3-trioxo-2H,4H-pyrazolo[4,5-e][1,2,4]thiadia-zine (8c). Compound 5 was alkylated with 2-bromobenzyl bromide (30-40°C for 12 h), then with 4-chlorobenzyl chloride (50-60°C for 12 h) to give 8c. IR (KBr, cm-1): 1695 (C=O); 1331, 1192 (SO2); 1H-NMR (CDCl3) δ: 7.12 (s, 1H, PyH), 7.60 (dd, 1H, J=7.83, J=1.15, PhH), 6.88-7.47 (m, 7H, PhH), 5.15 (s, 2H, NCH2), 5.08 (s, 2H, NCH2), 4.14 (s, 3H, CH3); 13C-NMR (CDCl3) δ: 149.2 (C=O), 134.1 (C-1’), 137.3 (C-1”), 132.5, 131.6, 129.6, 129.5, 129.0 128.4, 128.1, 127.2, 126.9, 126.7, 125.2(C-4a), 125.0(C-5), 122.6 (C-7a), 46.6 (N4-CH2), 43.7 (N2-CH2), 38.9 (CH3); MS: m/z 497.3 (M+2), 495.2 (M+).
2-(p-Chlorobenzyl)-4-(o-chlorobenzyl)-7-methyl-1,1,3-trioxo-2H,4H-pyrazolo[4,5-e][1,2,4]thiadia-zine (8d). Compound 5 was alkylated with 2-chlorobenzyl chloride (40-50°C for 20 h), then with 4-chlorobenzyl chloride (50-60°C for 12 h) to give 8d. IR (KBr, cm-1): 1693 (C=O); 1331, 1193 (SO2); 1H-NMR (DMSO-d6) δ: 7.75(s, 1H, PyH), 7.50 (dd, 1H, J=7.86, J=1.23, PhH), 7.01 (dd, 1H, J=7.63, J=1.25, PhH), 7.26-7.41 (m, 6H, PhH), 5.15 (s, 2H, NCH2), 5.02 (s, 2H, NCH2), 4.08 (s, 3H, CH3); 13C-NMR (DMSO-d6) δ: 148.8 (C=O), 133.0 (C-1’), 135.2 (C-1”), 132.1, 132.4, 129.9, 129.8, 129.5, 128.5, 127.7, 127.4, 126.3 (C-4a), 125.8 (C-5), 122.2 (C-7a), 47.2 (N4-CH2), 43.5 (N2-CH2), 39.1 (CH3); MS (EI): m/z 453.4 (M+2), 451.4 (M+).