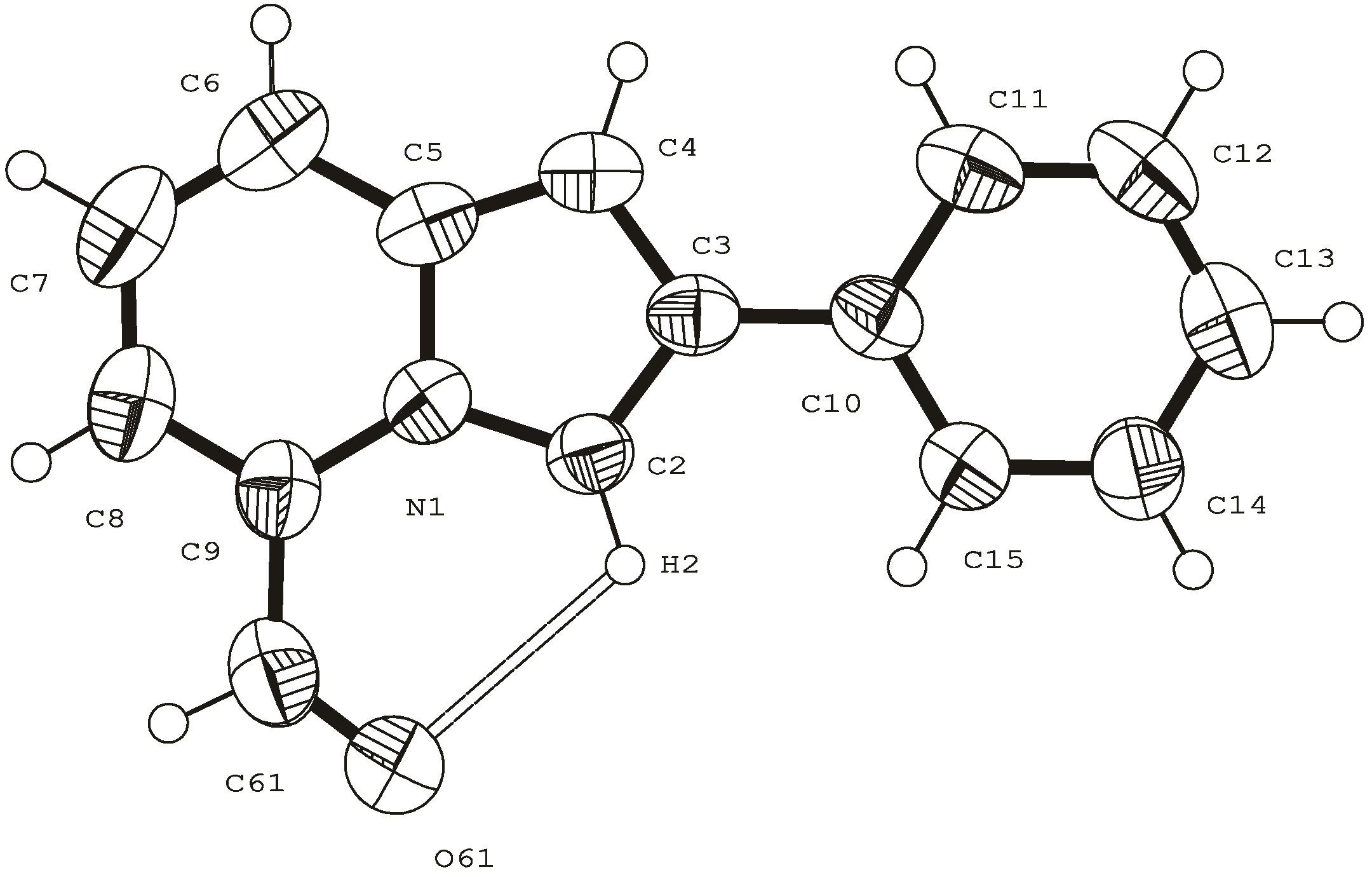
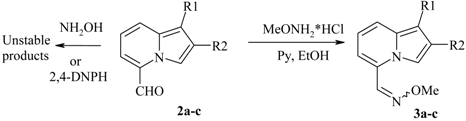
Typical procedure for preparation of O-Me oximes 3.
To a stirred solution of
2a (0.5g, 2.3 mmol) in EtOH (5 mL) pyridine (0.18 mL, 2.3 mmol) and methoxylamine hydrochloride (0.19g 2.3 mmol) were added, and the mixture was stirred at RT for 5 h. Then the reaction mixture was filtered and the precipitate (a canary-yellow solid) was identified as pure oxime
3a. The mother liquors were evaporated and purified by recrystallization from
i-PrOH. Overall yield of
3a was 0.5g (89%). For the yields and characteristics of OMe-oximes
3a-
c see
Table 3; for
1H-NMR data see
Table 4. In the reaction between 5-formylindolizine
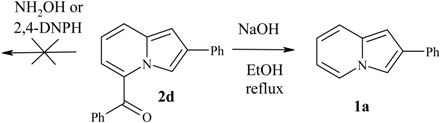
2a and hydroxylamine hydrochloride (in the presence or absence of organic bases) an intensely green new solid appeared. This precipitate was insoluble in most solvents and according to
1H-NMR, was a complex mixture. In the reaction of
2a with 2,4-DNPH the formation of a new substance can be detected (orange-yellow spot on TLC), however it decomposed during isolation. In reactions of
2a or
2d with sodium hydroxide the deacylation to
1a was observed.
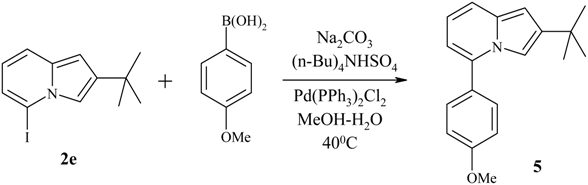
Preparation of 5-iodo-2-tert-butylindolizine 2e via direct lithiation.
The lithiation protocol was exactly the same as described above for preparation of indolizines 2a-c. The starting materials were 2-tert-butylindolizine 1b (1.0 g, 5.78 mmol), anhydrous THF (60 mL), freshly distilled TMEDA (1.75 mL, 11.6 mmol) and n-butyllithium (5.12 mL, 6.35 mmol, 1.24 M solution in n-hexane). The stirring time at -20°C (to complete the lithiation) was reduced to 2 hours. To the obtained solution of 5-lithium-2-tert-butylindolizine a solution of iodine (1.62 g, 6.35 mmol) in anhydrous THF was added, the cooling bath was removed, and the reaction mixture was allowed to warm up to RT. The obtained dark solution was poured into saturated aqueous NH4Cl and extracted with ethyl acetate. The organic phase was washed with aqueous Na2SO3 (until the organic layer became almost colorless) and with brine, dried (Na2SO4), and evaporated in vacuo giving 1.67 g (96%) of green crystals of 5-iodo-2-tert-butylindolizine (2e). Mp 57-59°C; 1H-NMR in DMSO-D6, δ (ppm): 7.32 (1H, s, H3), 7.28 (1H, d, H6, J67 = 8.9 Hz), 6.96 (1H, d, H8, J78 = 6.8 Hz), 6.53 (1H, s, H1), 6.36 (1H, m, H7), 1.35 (9H, s, tBu)
Cross-coupling reaction between 5-iodo-2-tert-butylindolizine 2e and p-methoxybenzeneboronic acid.
5-Iodo-2-tert-butylindolizine (2e, 0.299 g, 1 mmol), p-methoxybenzeneboronic acid (0.160 g, 1.05 mmol), sodium carbonate (0.265 g, 2.5 mmol) and tetra-n-butylammonium hydrosulfate (0.034 g, 0.1 mmol) were dissolved in the mixture of methanol (5 mL) and water (3 mL). The resulting mixture was purged with argon for 10 min. Then the reaction mixture was heated to 40°C and bis(triphenylphosphine)palladium dichoride Pd(PPh3)2Cl2 (0.035g, 0.05 mmol, 5 mol %) was added. The reaction mixture was stirred at 40°C for an additional 5 hours (until the TLC confirmed that the reaction was complete). Methanol was removed under reduced pressure, and water (10 mL) was added to the residue. The mixture was extracted with CHCl3, the organic layer was separated and dried (Na2SO4). Silica gel (3 g) was added to the organic solution, and the mixture was evaporated in vacuo. The obtained dry silica gel containing the crude product was placed onto the column for HPLC, and the product was purified using hexane as eluent. 5-(p-Methoxyphenyl)-2-tert-butylindolizine (5, 0.262g, 94%) was obtained as colorless crystals, Mp 113-115°C; 1H-NMR in DMSO-D6, δ (ppm): 7.53 (2H, m, Ar), 7.20 (1H, d, H6, J67=9.1 Hz), 7.09 (1H, s, H3), 7.04 (2H, m, Ar), 6.65 (1H, m, H7), 6.32 (1H, s, H1) 6.27 (1H, d, H8, J78=6.2 Hz), 3.87 (3H, s, OCH3), 1.28 (9H, s, tBu).