Pyrimidine Acyclo-C-Nucleosides by Ring Transformations of 2-Formyl-L-arabinal
Abstract
:Introduction
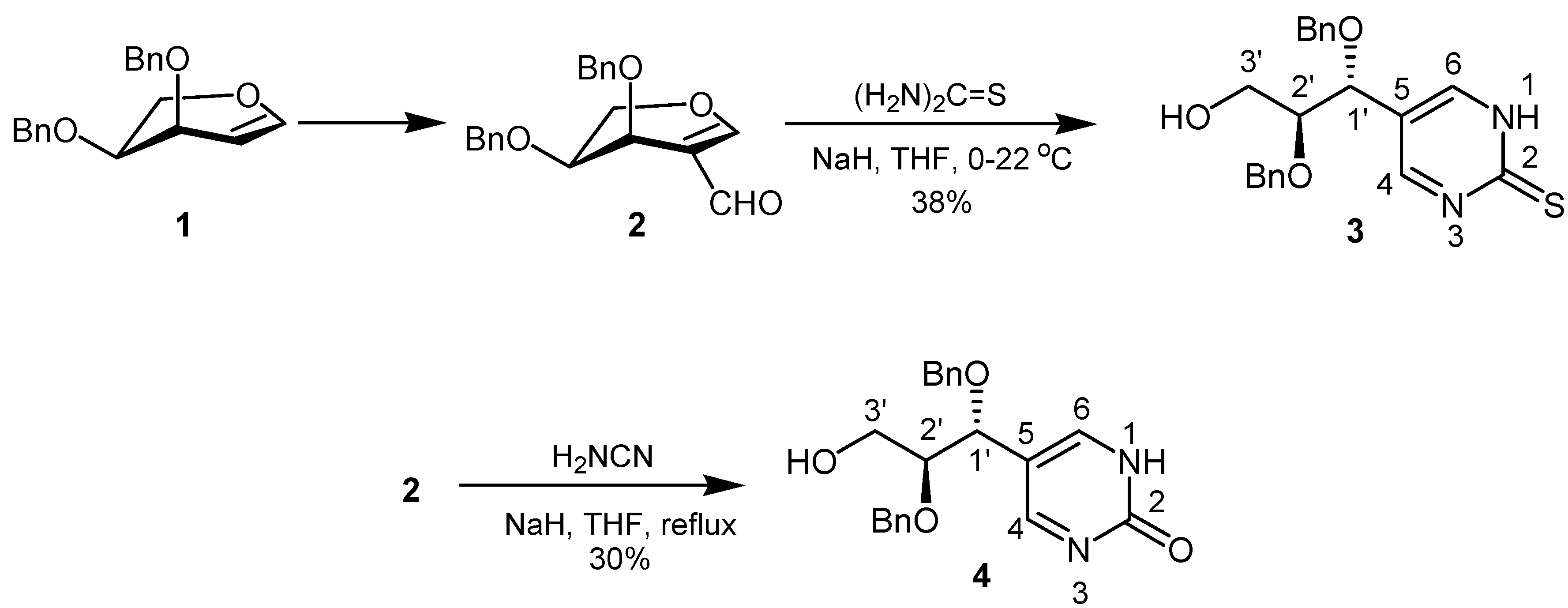
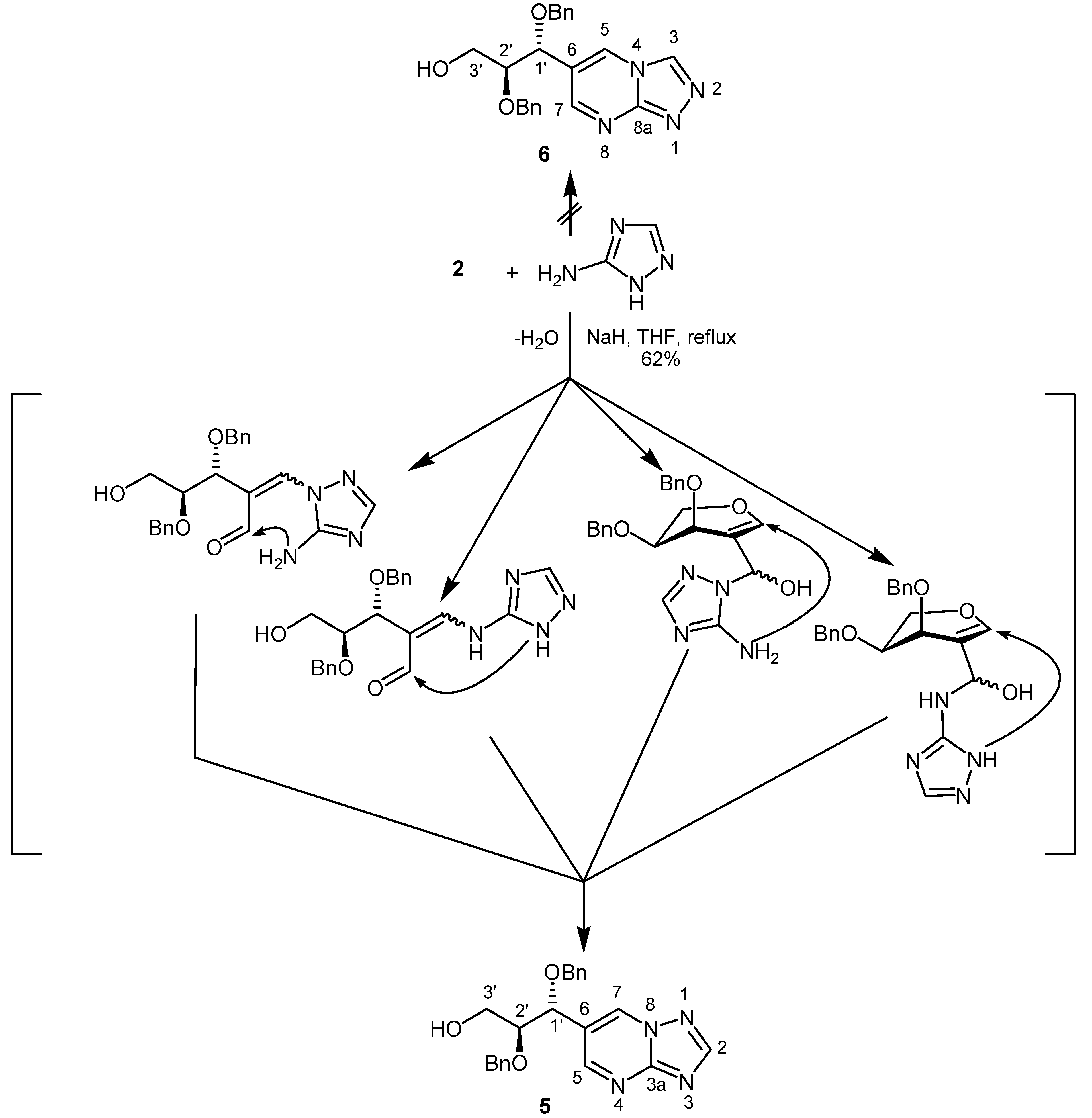
Results and Discussion
Experimental
General
5-[1R,2S-1,2-Bis(benzyloxy)-3-hydroxy-propyl]-1,2-dihydro-pyrimidine-2-thione (3)
 : – 41.1 (c = 1.0, CHCl3); IR (capillary, cm–1): 3418 (OH); 1H-NMR (250.13 MHz, CDCl3): δ = 2.07 (br, 1Η, ΟΗ), 3.56 (dt, 1H, J1´,2´ = 8.0 Hz, J2´,3´ = 4.0 Hz, H-2´), 3.83 (d, 2H, H-3´), 4.31 (d, 1H, J = 11.5 Hz, CHHPh), 4.35 (d, 1H, J = 11.5 Hz, CHHPh), 4.42 (d, 1H, H-1´), 4.50 (d, 1H, J = 11.5 Hz, CHHPh ), 4.51 (d, 1H, J = 11.5 Hz, CHHPh), 5.00 (s, 1H, NH), 7.01–7.06 (m, 2H, Ph), 7.23–7.38 (m, 6H, Ph), 8.50 (s, 2H, H-4, H-6); 13C-NMR (62.9 MHz, CDCl3): δ = 61.2 (C-3´), 71.6, 72.7 (CH2Ph), 76.7 (C-1´), 80.9 (C-2´), 125.3 (C-5), 128.0, 128.1, 128.1, 128.2, 128.5, 128.6 (Ph), 136.8, 136.9 (i-Ph), 157.0 (C-4, C-6), 170.7 (C-2); MS (CI): m/z (%): 383 (62) [M+H]+, 91 (100); Anal. Calcd. for C21H22N2O3S: C, 60.95; H, 5.80; N, 7.32; S, 8.38. Found: C, 65.86; H, 6.12; N, 7.09; S, 7.95.
: – 41.1 (c = 1.0, CHCl3); IR (capillary, cm–1): 3418 (OH); 1H-NMR (250.13 MHz, CDCl3): δ = 2.07 (br, 1Η, ΟΗ), 3.56 (dt, 1H, J1´,2´ = 8.0 Hz, J2´,3´ = 4.0 Hz, H-2´), 3.83 (d, 2H, H-3´), 4.31 (d, 1H, J = 11.5 Hz, CHHPh), 4.35 (d, 1H, J = 11.5 Hz, CHHPh), 4.42 (d, 1H, H-1´), 4.50 (d, 1H, J = 11.5 Hz, CHHPh ), 4.51 (d, 1H, J = 11.5 Hz, CHHPh), 5.00 (s, 1H, NH), 7.01–7.06 (m, 2H, Ph), 7.23–7.38 (m, 6H, Ph), 8.50 (s, 2H, H-4, H-6); 13C-NMR (62.9 MHz, CDCl3): δ = 61.2 (C-3´), 71.6, 72.7 (CH2Ph), 76.7 (C-1´), 80.9 (C-2´), 125.3 (C-5), 128.0, 128.1, 128.1, 128.2, 128.5, 128.6 (Ph), 136.8, 136.9 (i-Ph), 157.0 (C-4, C-6), 170.7 (C-2); MS (CI): m/z (%): 383 (62) [M+H]+, 91 (100); Anal. Calcd. for C21H22N2O3S: C, 60.95; H, 5.80; N, 7.32; S, 8.38. Found: C, 65.86; H, 6.12; N, 7.09; S, 7.95.5-[1R,2S-1,2-Bis(benzyloxy)-3-hydroxy-propyl]-1,2-dihydro-pyrimidin-2-one (4)
 : – 46.1 (c = 1.0, CHCl3); IR (capillary, cm–1): 3448 (OH, NH); 1H-NMR (250.13 MHz, CDCl3): δ = 2.75 (br, 1H, OH), 3.58 (dt, 1H, J1´,2´ = 7.8 Hz, J2´,3´ = 4.5 Hz, H-2´), 3.80 (d, 2H, H-3´), 4.32 (d, 1H, H-1´), 4.32 (d, 1H, J = 11.6 Hz, CHHPh), 4.36 (d, 1H, J = 11.6 Hz, CHHPh), 4.49 (d, 1H, J = 11.6 Hz, CHHPh), 4.50 (d, 1H, J = 11.6 Hz, CHHPh), 5.43 (s, 1H, NH), 7.07–7.11 (m, 2H, Ph), 7.24–7.36 (m, 8H, Ph), 8.25 (s, 2H, H-4, H-6); 13C-NMR (62.9 MHz, CDCl3): δ = 61.6 (C-3´), 71.0, 72.9 (CH2Ph), 77.2 (C-1´), 81.4 (C-2´), 121.8 (C-5), 127.9, 127.9, 128.0, 128.0, 128.4, 128.6 (Ph), 137.2, 137.3 (i-Ph), 158.0 (C-4, C-6), 162.8 (C=O); MS (EI): m/z (%): 366 (62) [M]+, 215 (85) ([M]–HOCH2CHOBn) +, 91 (89); Anal. Calcd. for C21H22N2O4: C, 68.84; H, 6.05; N, 7.65. Found: C, 68.58; H, 6.59; N, 7.69.
: – 46.1 (c = 1.0, CHCl3); IR (capillary, cm–1): 3448 (OH, NH); 1H-NMR (250.13 MHz, CDCl3): δ = 2.75 (br, 1H, OH), 3.58 (dt, 1H, J1´,2´ = 7.8 Hz, J2´,3´ = 4.5 Hz, H-2´), 3.80 (d, 2H, H-3´), 4.32 (d, 1H, H-1´), 4.32 (d, 1H, J = 11.6 Hz, CHHPh), 4.36 (d, 1H, J = 11.6 Hz, CHHPh), 4.49 (d, 1H, J = 11.6 Hz, CHHPh), 4.50 (d, 1H, J = 11.6 Hz, CHHPh), 5.43 (s, 1H, NH), 7.07–7.11 (m, 2H, Ph), 7.24–7.36 (m, 8H, Ph), 8.25 (s, 2H, H-4, H-6); 13C-NMR (62.9 MHz, CDCl3): δ = 61.6 (C-3´), 71.0, 72.9 (CH2Ph), 77.2 (C-1´), 81.4 (C-2´), 121.8 (C-5), 127.9, 127.9, 128.0, 128.0, 128.4, 128.6 (Ph), 137.2, 137.3 (i-Ph), 158.0 (C-4, C-6), 162.8 (C=O); MS (EI): m/z (%): 366 (62) [M]+, 215 (85) ([M]–HOCH2CHOBn) +, 91 (89); Anal. Calcd. for C21H22N2O4: C, 68.84; H, 6.05; N, 7.65. Found: C, 68.58; H, 6.59; N, 7.69. 6-[1R,2S-1,2-Bis(benzyloxy)-3-hydroxy-propyl][1,2,4]triazolo[1,5-a]pyrimidine (5)
 : – 30.1 (c = 1.0, CHCl3); IR (capillary, cm–1): 3373 (OH); 1H-NMR (250.13 MHz, CDCl3): δ = 3.21 (br, 1H, OH), 3.60 (dt, 1H, J1´,2´ = 8.0 Hz, J2´,3´ = 4.0 Hz, H-2´), 3.88 (m, center of AB part of ABX, J3´a,3´b = 11.8 Hz, 2H, H-3´a, H-3´b), 4.28 (d, 1H, J = 11.9 Hz, CHHPh), 4.43 (d, 1H, J = 11.3 Hz, CHHPh), 4.53 (d, 1H, J = 11.9 Hz, CHHPh), 4.53 (d, 1H, J = 11.3 Hz, CHHPh), 4.63 (d, 1H, H-1´), 6.90–6.93 (m, 2H, Ph), 7.00–7.09 (m, 3H, Ph), 7.22–7.33 (m, 5H, Ph), 8.49 (s, 1H, H-2), 8.65 (d, 1H, H-7), 8.74 (d, 1H, J5,7 = 2.2 Hz, H-5); 13C-NMR (62.9 MHz, CDCl3): δ = 60.6 (C-3´), 72.1, 72.5 (CH2Ph), 75.9 (C-1´), 80.5 (C-2´), 123.3 (C-6), 127.9, 128.0, 128.2, 128.2, 128.3, 128.7 (Ph), 134.5 (C-7), 1.36.4, 136.5 (i-Ph), 154.8 (C-3a), 155.5 (C-5), 156.1 (C-2); MS (CI): m/z (%): 391 (90) [M+H]+, 91 (100); Anal. Calcd. for C22H22N4O3: C, 67.69; H, 5.64; N, 14.35. Found: C, 67.35; H, 5.71; N, 14.45.
: – 30.1 (c = 1.0, CHCl3); IR (capillary, cm–1): 3373 (OH); 1H-NMR (250.13 MHz, CDCl3): δ = 3.21 (br, 1H, OH), 3.60 (dt, 1H, J1´,2´ = 8.0 Hz, J2´,3´ = 4.0 Hz, H-2´), 3.88 (m, center of AB part of ABX, J3´a,3´b = 11.8 Hz, 2H, H-3´a, H-3´b), 4.28 (d, 1H, J = 11.9 Hz, CHHPh), 4.43 (d, 1H, J = 11.3 Hz, CHHPh), 4.53 (d, 1H, J = 11.9 Hz, CHHPh), 4.53 (d, 1H, J = 11.3 Hz, CHHPh), 4.63 (d, 1H, H-1´), 6.90–6.93 (m, 2H, Ph), 7.00–7.09 (m, 3H, Ph), 7.22–7.33 (m, 5H, Ph), 8.49 (s, 1H, H-2), 8.65 (d, 1H, H-7), 8.74 (d, 1H, J5,7 = 2.2 Hz, H-5); 13C-NMR (62.9 MHz, CDCl3): δ = 60.6 (C-3´), 72.1, 72.5 (CH2Ph), 75.9 (C-1´), 80.5 (C-2´), 123.3 (C-6), 127.9, 128.0, 128.2, 128.2, 128.3, 128.7 (Ph), 134.5 (C-7), 1.36.4, 136.5 (i-Ph), 154.8 (C-3a), 155.5 (C-5), 156.1 (C-2); MS (CI): m/z (%): 391 (90) [M+H]+, 91 (100); Anal. Calcd. for C22H22N4O3: C, 67.69; H, 5.64; N, 14.35. Found: C, 67.35; H, 5.71; N, 14.45. Acknowledgments
References
- Borrmann, D. Houben-Weyl, Methoden der Organischen Chemie; Müller, E., Ed.; Thieme: Stuttgart, 1969; Vol. VII/4. [Google Scholar]
- Schaumann, E. Houben-Weyl, Methoden der Organischen Chemie; Klamann, D., Ed.; Thieme: Stuttgart, New York, 1985; Vol. E 11. [Google Scholar]
- Tominaga, Y. J. Heterocycl. Chem. 1989, 26, 1167–1204.
- Peseke, K.; Feist, H.; Quincoces, J. Targ. Heterocycl. Syst. 2001, 5, 299–325.
- Ramesh, N. G.; Balasubramanian, K. K. Tetrahedron Lett. 1991, 32, 3875–3878.
- Rudloff, I.; Peseke, K.; Reinke, H. J. Prakt. Chem. 1998, 340, 334–340.
- Ramesh, N. G.; Balasubramanian, K. K. Eur. J. Org. Chem. 2003, 4477–4487.
- Montero, A.; Feist, H.; Michalik, M.; Quincoces, J.; Peseke, K. Synthesis 2002, 664–669.
- Montero, A.; Feist, H.; Michalik, M.; Quincoces, J.; Peseke, K. J. Carbohydr. Chem. 2002, 21, 305–312. [CrossRef]
- Rudloff, I.; Bari, A.; Feist, H.; Michalik, M.; Reinke, H.; Peseke, K. Z. Naturforsch. 2004, 59b, 398–405.
- Montero, A.; Michalik, M.; Feist, H.; Reinke, H.; Rudloff, I.; Peseke, K. J. Carbohydr. Chem. 2004, 23, 317–327. [CrossRef]
- Bari, A.; Feist, H.; Michalik, D.; Michalik, M.; Peseke, K. Synthesis 2004, 2863–2868.
- Sample Availability: Not available.
© 2005 by MDPI (http://www.mdpi.org). Reproduction is permitted for non commercial purposes.
Share and Cite
Bari, A.; Feist, H.; Michalik, M.; Peseke, K. Pyrimidine Acyclo-C-Nucleosides by Ring Transformations of 2-Formyl-L-arabinal. Molecules 2005, 10, 837-842. https://doi.org/10.3390/10080837
Bari A, Feist H, Michalik M, Peseke K. Pyrimidine Acyclo-C-Nucleosides by Ring Transformations of 2-Formyl-L-arabinal. Molecules. 2005; 10(8):837-842. https://doi.org/10.3390/10080837
Chicago/Turabian StyleBari, Ahmed, Holger Feist, Manfred Michalik, and Klaus Peseke. 2005. "Pyrimidine Acyclo-C-Nucleosides by Ring Transformations of 2-Formyl-L-arabinal" Molecules 10, no. 8: 837-842. https://doi.org/10.3390/10080837
APA StyleBari, A., Feist, H., Michalik, M., & Peseke, K. (2005). Pyrimidine Acyclo-C-Nucleosides by Ring Transformations of 2-Formyl-L-arabinal. Molecules, 10(8), 837-842. https://doi.org/10.3390/10080837



