3-Formylchromones IV. The Rearrangement of 3-Formylchromone Enamines as a Simple, Facile Route to Novel Pyrazolo[3,4-b]pyridines and the Synthetic Utility of the Latter
Abstract
:Introduction
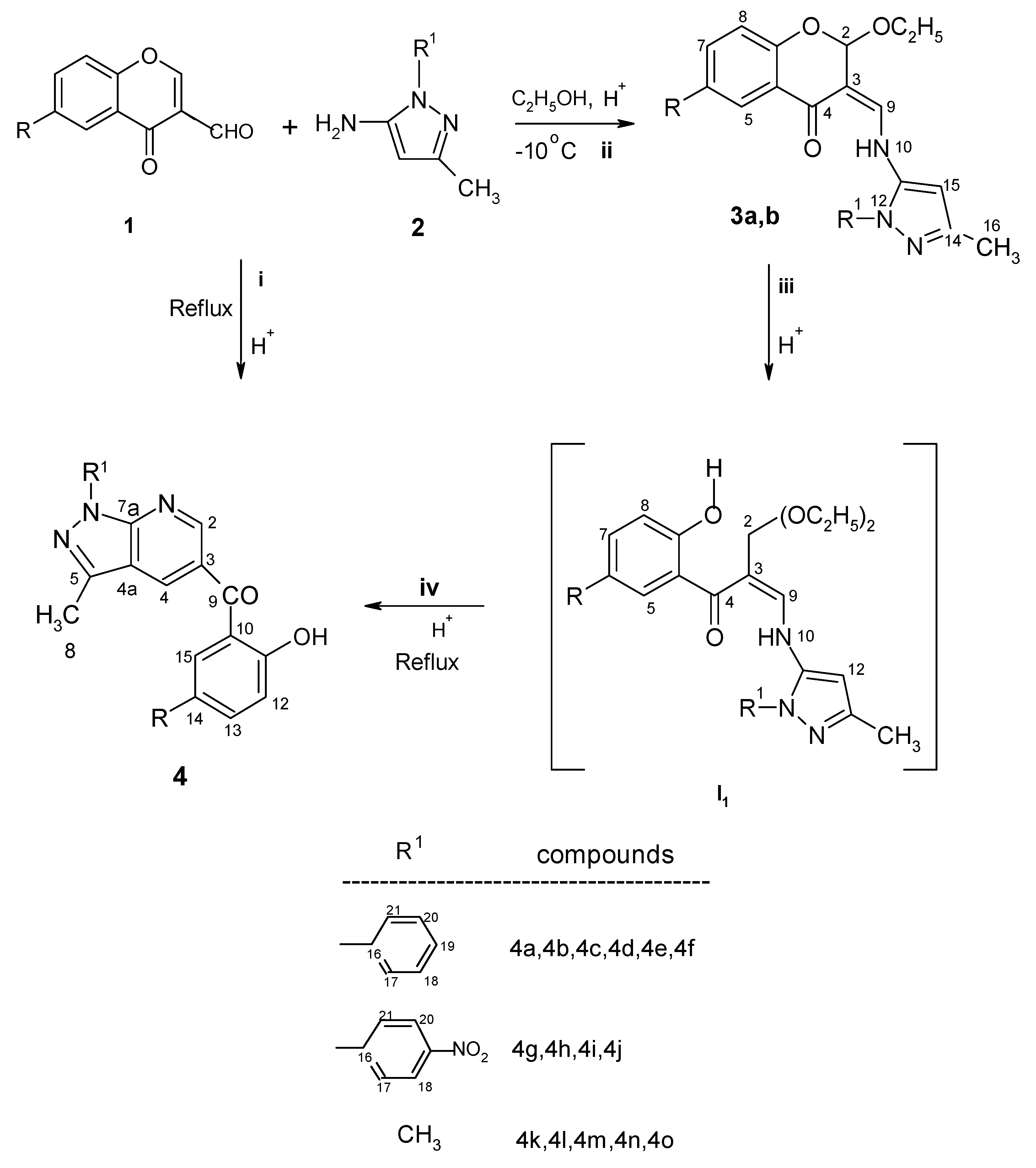
Results and Discussion
Conclusions
Acknowledgements
Experimental
General
Synthesis of enamine adduct 3a
Synthesis of enamine adduct 3b
Preparation of pyrazolo[3,4-b]pyridine derivatives 4a-4e,4g-4k (Classical conditions)
Preparation of pyrazolo[3,4-b]pyridine derivatives 4f, 4l,4m,4n and 4o (Classical conditions)
Microwave procedure for preparation of 4a-4o
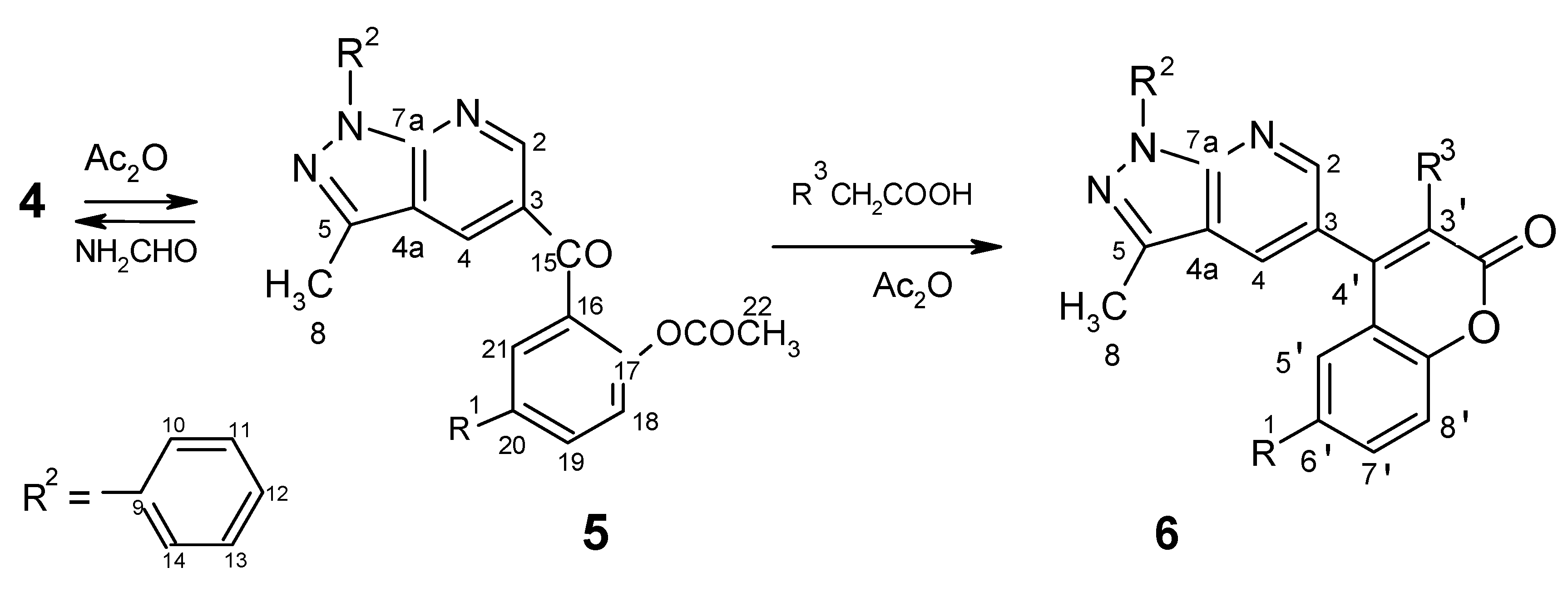
Preparation of compounds 5 by acylation: procedure for 5a,5b,5c (with acetic anhydride)
Procedure for 5d,5e,5f (with acid chlorides)
Preparation of coumarin derivatives 6a, 6b
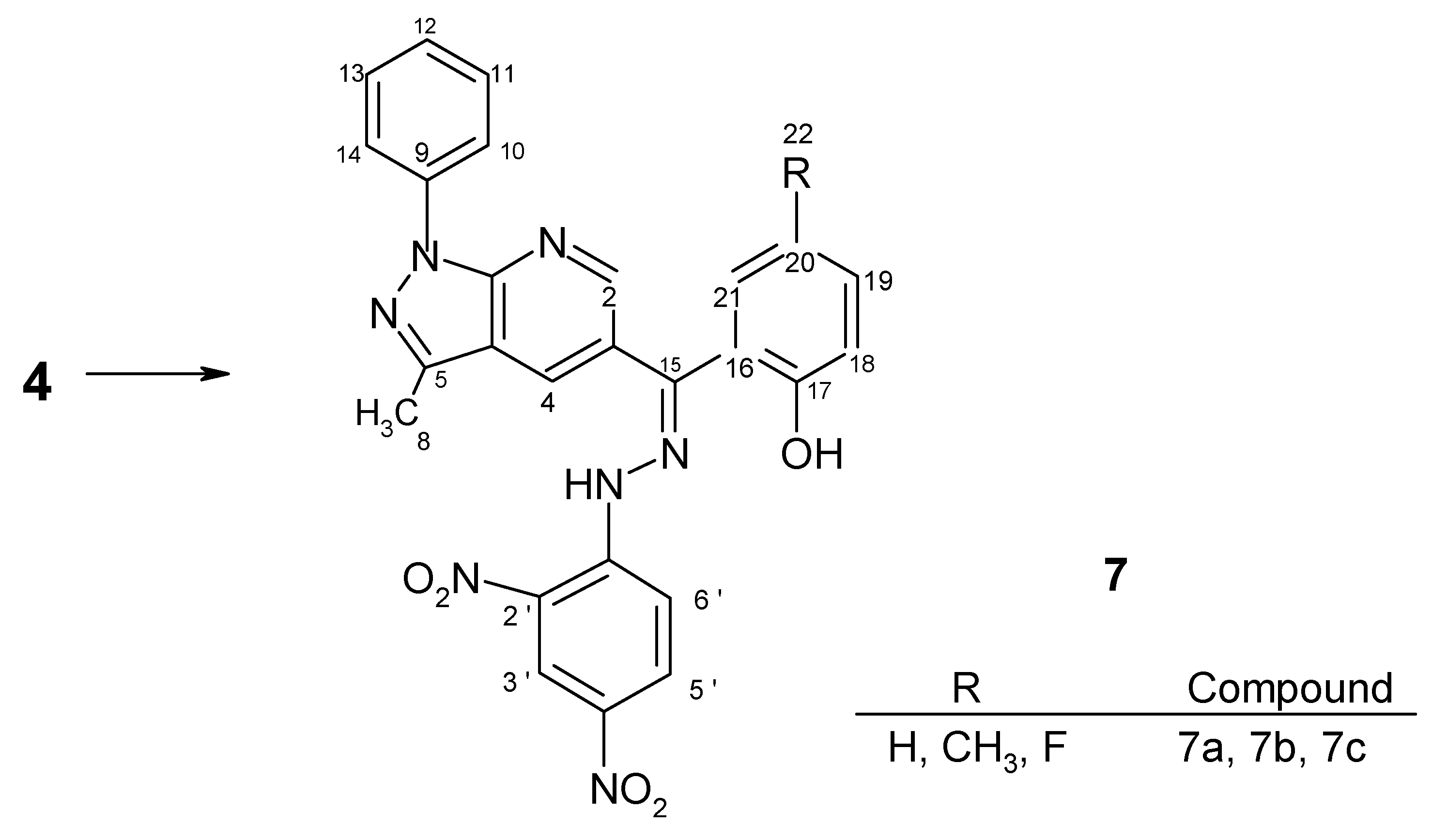
Preparation of hydrazone derivatives 7
| No
------- Yield | Compound name | |||||||
|---|---|---|---|---|---|---|---|---|
| Formula / MW | Melting point | Elemental analysis | ||||||
| 4a ------- 89% | 3-(2-hydroxybenzoyl)-5-methyl-7-phenylpyrazolo[3,4-b]pyridine | |||||||
| C20H15N3O2 329.4 | 120 –121 °C | Calc.: 72.94 %C; 4.59 %H; 12.76 %N
Found: 73.12 %C; 4.54 %H; 12.87 %N | ||||||
| 13C-NMR (CDCl3) δ(ppm), J(Hz): 12.73 q, J(C,H)=127.9(C-8);
116.57q, 3J(C,H)=2.9(C-4a); 118.98dt, J(C,H)=162.3, 3J(C,H)=7.3 (C-12); 119.23dd, J(C,H)=163.6, 3J(C,H)=9.2(C-14); 119.59dt, 3 J(C,H)=4.7, 3J(C,H)=7.6(C-10); 121.45dt, J(C,H)=161.6, 3J(C,H)=7.5(C-17,21); 126.55dt, J(C,H)=162.9, 3J(C,H)=7.6(C-19); 127.54d, 3J(C,H-4)=8.0(C-5); 129.41dd, J(C,H)=161.5, 3J(C,H) = 8.3(C-18,20); 131.87dd, J(C,H)=166.1, 3J(C,H)=6.0(C-4); 133.27dd, J(C,H)=160.2, 3J(C,H)=9.0(C-15); 136.85dd, J(C,H)=159.8, 3J(C,H)=9.2(C-13); 139.24t, 3 J(C,H)=8.0(C16);144.50qd, 2J(C,H)=7.1, 3J(C,H)=2.5(C-16); 150.30dd, J(C,H)=183.3, 3J(C,H)=5.5(C-2); 151.54dd, 3J(C,H-2)=13.4, 3J(C,H-4)=6.8(C-7a); 163.43dd, 3J(C,H) 7.5,6.8 (C-11), 199.11t, 3J(C,H) = 4.4(C-9). | ||||||||
| 4b | 3-(2-hydroxy-5-methylbenzoyl)-5-methyl-7-phenylpyrazolo[3,4-b]pyridine | |||||||
| C21H17N3O2 343.4 | 142-144 °C | Calc.: 73.38 %C; 4.99 %H; 12.23%N
Found: 73.22 %C; 4.84 %H; 12.27 %N | ||||||
| ------- | ||||||||
| 92% | ||||||||
| 4c | 3-(2-hydroxy-5-fluorobenzoyl)-5-methyl-7-phenylpyrazolo[3,4-b]pyridine | |||||||
| C20H14FN3O2 339.4 | 155-156 °C | ----- | ||||||
| ------- | ||||||||
| 87% | ||||||||
| 4d | 3-(2-hydroxy-5-bromobenzoyl)-5-methyl-7-phenylpyrazolo[3,4-b]pyridine | |||||||
| C20H14 BrN3O2 408.4 | 162-163° C | Calc.: 58.82 %C; 3.43 %H; 10.21%N
Found: 58.62 %C; 3.21 %H; 10.01 %N | ||||||
| ------- | ||||||||
| 87% | ||||||||
| 4e | 3-(2-hydroxy-5-chlorobenzoyl)-5-methyl-7-phenylpyrazolo[3,4-b]pyridine | |||||||
| C20H14 ClN3O2 363.2 | 160-162 °C | Calc.: 65.97 %C; 3.84 %H; 11.54%N; 9.74 %Cl
Found: 66.22 %C; 3.88 %H;11.34%N; 9.70%Cl | ||||||
| ------- | ||||||||
| 89% | ||||||||
| 4f | 3-(2-hydroxy-5-nitrobenzoyl)-5-methyl-7-phenylpyrazolo[3,4-b]pyridine | |||||||
| C20H14N4O4 374.4 | 207-208 °C | Calc.: 77.36 %C; 4.52 %H; 18.07%N
Found: 77.48 %C; 4.58 %H; 18.14%N | ||||||
| ------- | ||||||||
| 70% | ||||||||
| 4g | 3-(2-hydroxy-5-methylbenzoyl)-5-methyl-7-(4-nitrophenyl)pyrazolo[3,4-b] pyridine | |||||||
| C21H16N4O4 388.4 | 224-226 °C | Calc.: 64.94 % C; 4.15 % H; 14.43 % N
Found: 64.78 % C; 4.03 % H; 14.37 % N | ||||||
| ------- | ||||||||
| 73% | ||||||||
| 4h | 3-(2-hydroxy-5-fluorobenzoyl)-5-methyl-7-4-(nitrophenyl)pyrazolo[3,4-b] pyridine | |||||||
| C20H13 FN4O4 392.4 | 222 -224 °C | ---------- | ||||||
| ------- | ||||||||
| 87% | ||||||||
| 4i | 3-(2-hydroxy-5-bromobenzoyl)-5-methyl-7-(4-nitrophenyl)pyrazolo[3,4-b] pyridine | |||||||
| C20H13 BrN4O4 329.4 | 260-261 °C | Calc.: 53.00 % C; 2.89 % H; 12.36 % N; 17.63 %Br
Found: 53.19% C; 2.68 % H; 12.11% N;17.42 %Br | ||||||
| ------- | ||||||||
| 82% | ||||||||
| 4j | 3-(2-hydroxy-5-chlorobenzoyl)-5-methyl-7-4-(nitrophenyl)pyrazolo[3,4-b] pyridine | |||||||
| C20H13 ClN4O4 408.8 | 239-241 °C | Calc.: 58.76% C; 3.21% H; 13.71% N; 8.67%Cl
Found: 58.67 %C; 3.11% H; 13.68% N; 8.58%Cl | ||||||
| ------- | ||||||||
| 88% | ||||||||
| 4k | 3-(2-hydroxy-5-methylbenzoyl)-5,7-dimethylpyrazolo[3,4-b]pyridine | |||||||
| C16H15 N3O2 281.3 | 176-177 °C | Calc.: 68.31 %C; 5.37 % H; 14.94 % N
Found: 68.22 %C; 5.21 % H; 14.67 % N | ||||||
| ------- | ||||||||
| 87% | ||||||||
| 4l | 3-(2-hydroxy-5-fluorobenzoyl)-5,7-dimethylpyrazolo[3,4-b]pyridine | |||||||
| ------- | C15H12 FN3O2 285.3 | 160-161 °C | ------------- | |||||
| 87% | ||||||||
| 4m | 3-(2-hydroxy-5-bromobenzoyl)-5,7-dimethylpyrazolo[3,4-b]pyridine | |||||||
| ------- | C15H12BrN3O2 346.2 | 150-151 °C | Calc.: 52.04 %C; 3.49 %H; 12.14 % N; 23.08 % Br
Found : 52.19 %C; 3.32 %H; 12.11 % N; 23.17 % Br | |||||
| 89% | ||||||||
| 4n | 3-(2-hydroxy-5-chlorobenzoyl)-5,7-dimethylpyrazolo[3,4-b]pyridine | |||||||
| ------- | C15H12ClN3O2 301.7 | 170-171 °C | Calc.: 59.71 % C; 4.01% H; 13.93 % N; 11.75 %Cl
Found: 59.58 % C; 4.16% H; 14.11 % N; 11.42 %Cl | |||||
| 87% | ||||||||
| 4o | 3-(2-hydroxy-5-nitrobenzoyl)-5,7-dimethylpyrazolo[3,4-b] pyridine | |||||||
| ------- | C15H12 N4O4 312.3 | 213-215 °C | Calc.: 57.69 % C; 3.87 %H; 17.94 % N
Found : 57.53 % C; 3.68 %H; 18.11 % N | |||||
| 86% | ||||||||
| No | R | R1/8 | OH | 2 | 4 | 12 | 13 | 15 | 17(21)(2H) | 18(20)(2H) | 19 |
| 4a | H-14 7.63dd, J= 8.0 J= 1.4 | 2.71s | 11.86s | 8.96d, J=2.1 | 8.46d, J = 2.1 | 7.13dd, J = 8.2, J = 1.1 | 7.57td J= 8.2, J = 1.4 | 7.63dd, J = 8.0 J= 1.4 | 8.26dd J= 7.5 J=1.1 | 7.55t J= 7.5 | 7.34t J= 7.5 J= 1.1 |
| 4b | CH3 | 2.28s 2.64s | 11.06s | 8.94d J=1.9 | 8.47d J=1.9 | 7.04d J=7.7 | 7.38dd J=7.8 J=0.8 | 7.39t J=3.1 J=2.6 | 8.26dd,2H J=7.6 J=1.1 | 7.55t,2H J=7.6 J=1.1 | 7.34tJ=7.6 J=1.1 |
| 4c | F | 2.72s | 11.57s | 8.95d J=1.9 | 8.45d J=1.9 | 7.10dd J11,12=10.2 J11,F = 4.4 | 7.2-7.4 m 3H* 13,15,19 | * | 8.25dd,2H J=8.0 J=1.1 | 7.55t,2H J=8.0 J=1.1 | * |
| 4d | Br | 2.73s | 11.75s | 8.94d J=1.9 | 8.46d J=1.9 | 7.05d J=8.8 | 7.64dd J=8.8 J=2.5 | 7.38t J=7.7 | 8.25dd,2H J=7.7 J=1.1 | 7.56t,2H J=7.7 J=1.1 | 7.38t J=7.7 J=1.1 |
| 4e | Cl | 2.73s | 11.74s | 8.95d J=2.1 | 8.47d J=2.1 | 7.10d J=9.0 | 7.51dd J=9.0 J=2.5 | 7.60d J=2.5 | 8.25dd,2H J=7.7 J=1.1 | 7.56t,2H J=7.7 J=1.1 | 7.35t J=8.0 J=1.1 |
| 4f | NO2 | 2.73s | 12.60s | 8.99d J=2.0 | 8.49d J=2.0 | 7.23d J=9.0 | 8.46dd J=9.0 J=2.6 | 8.65d J=2.6 | 8.25dd,2H J=7.6 J=1.1 | 7.56t,2H J=7.5 J=1.1 | 7.55t J=7.5 J=1.1 |
| 4g | CH3 | 2.28s | 10.21s | 8.95d | 8.71d | 6.92d | 7.29dd | 7.33d | 8.48dd,2H | 8.67dd,2H | --- |
| 2.68s | J=1.9 | J=1.9 | J=8.2 | J=8.2 | J=2.2 | J=9.4 | J=9.4 | ||||
| J=2.2 | J=1.9 | J=1.9 | |||||||||
| 4h | F | 2.67s | 10.08s | 8.97d | 8.69d | 7.05dd | 7.2-7.3m | H-13,15 | 8.41dd,2H | 8.62dd,2H | |
| J=2.2 | J=2.2 | J=8.8 | 2H | J=9.3 | J=9.3 | --- | |||||
| J=4.4 | J=1.1 | J=1.9 | |||||||||
| 4i | Br | 2.67s | 10.37s | 8.95d | 8.68d | 7.00d | 7.53dd | 7.33d | 8.41dd,2H | 8.60dd,2H | |
| J=1.9 | J=1.9 | J=8.6 | J=8.6 | J=2.2 | J=9.2 | J=9.2 | --- | ||||
| J=2.6 | J=2.0 | J=2.0 | |||||||||
| 4j | Cl | 2.68s | 10.37s | 8.96d J=1.9 | 8.69d J=1.9 | 7.05d J=8.5 | 7.47dd J=8.5 J=2.7 | 7.45d J=2.7 | 8.43dd,2H J=9.3 J=2.2 | 8.62dd,2H J=9.3 J=2.2 | --- |
| No | R | R1 | 8 | OH | 2 | 4 | 12 | 13 | 15 | ||
| 4k | CH3 | 2.28s 2.64s | 4.15s | 11.67 s | 8.87d J=1.9 | 8.40d J=1.9 | 7.04t J=9.1 J=4.4 | 7.34-7.39m, (2H) 13, 15 | * | ||
| 4l | F | 2.63s | 4.15s | 11.56s | 8.87d J=2.0 | 8.39d J=2.0 | 7.11dd J=8.9 J=4.6 | 7.31td J=8.9 J=3.1 J=2.6 | 7.29t J=3.1 J=2.6 | ||
| 4m | Br | 2.63s | 4.15s | 11.74s | 8.86d J=1.9 | 8.38d J=1.9 | 7.04d J=8.8 | 7.63dd J=8.8 J=2.4 | 7.69d J=2.4 | ||
| 4n | Cl | 2.65s | 4.16s | 11.74s | 8.87d J=2.0 | 8.39d J=2.0 | 7.09d J=8.9 | 7.51dd J=8.9 J=2.5 | 7.56d J=2.4 | ||
| 4o | NO2 | 2.62s | 4.17s | 12.50s | 8.91d J=2.0 | 8.42d J=2.0 | 7.22d J=8.9 | 7.44dd J=8.9 J=2.7 | 8.61d J=2.7 |
| No | R | N-CH3 | C 14(CH3) | 8(CH3) | 2 | 3 | 4 | 4a |
| 4b | CH3 | 20.73 | 12.72 | 150.23 | 127.76 | 130.92 | 116.72 | |
| 4c | F | 12.76 | 150.14 | 127.02 | 131.86 | 116.63 | ||
| 4d | Br | 12.76 | 150.11 | 126.92 | 132.01 | 116.73 | ||
| 4e | Cl | 12.76 | 150.11 | 126.91 | 131.96 | 116.57 | ||
| 4m | Br | 33.73 | 12.52 | 149.75 | 125.86 | 132.21 | 114.41 | |
| No | R | 5 | 7a | 9 | 10 | 11 | 12 | 13 |
| 4b | CH3 | 144.48 | 151.5 | 199.04 | 119.31 | 161.37 | 118.74 | 137.93 |
| 4c | F | 144.61 | 151.6 | 198.19d
4JC,F=2,5 | 119.11d
JC,F=6.3 | 159.59 | 120.38d
3JC,F =7,4 | 124.45d
2JC,F=23.8 |
| 4d | Br | 144.61 | 151.6 | 198.08 | 120.87 | 161.30 | 121.01 | 139.52 |
| 4e | Cl | 144.61 | 151.6 | 198.14 | 120.2 | 161.84 | 120.62 | 136.72 |
| 4m | Br | 142.93 | 151.9 | 198.28 | 120.89 | 162.18 | 120.56 | 139.02 |
| No | R | 14 | 15 | 16 | 17,21 | 18,20 | 19 | |
| 4b | CH3 | 128.45 | 132.88 | 139.25 | 121.48 | 129.41 | 126.54 | |
| 4c | F | 155.02d
1JC,F=246.5 | 117.98d
2JC,F=23.8 | 139.11 | 121.54 | 129.45 | 126.68 | |
| 4d | Br | 110.89 | 135.07 | 139.21 | 121.58 | 129.46 | 126.72 | |
| 4e | Cl | 124.07 | 132.07 | 139.24 | 121.54 | 129.44 | 126.69 | |
| 4m | Br | 110.51 | 135.13 | ------- | -------- | -------- | ------- |
| No ------- Yield | Compound name | |||
|---|---|---|---|---|
| Formula / MW | Melting point | Elemental analysis | ||
| NMR data (CDCl3 unless otherwise indicated) [δ(ppm) , J (Hz )] | ||||
| 3a ------- 72% | 2-Ethyloxy-6-methyl-3-(3-methyl-1-phenylpyrazol-5-ylaminomethylene)chroman-4-one | |||
| C23H23N3O3 383.5 | 164 – 166 °C | Calc.: 70.93%C; 5.95%H; 10.79%N
Found: 70.90%C; 6.01%H; 10.59%N | ||
| 1H-NMR: 1.24 (t, 3H, CH3); 2.17 (s, 3H, CH3 on C-14), 2.49 (s, 3H, CH3 -6); 3.71 (q, 2H, CH2); 6.25 (s, 1H, H-2); 7.37- 7.42 (m, 3H, H-18,19,20); 7.52 (dd, 2H, 3J =8.4, 4J =2, H-17,21); 7.56 (d, 1H, 3J =8.4, H-8); 7.65 (dd, 1H, 3J =8.4, 4J =2, H-7); 7.67 (d, 1H, 4J =2, H-5); 8.08 (s, 1H, H-9); 8.67 (s, 1H, H-10); 8.99 (d, 1H, 4J =2, H-15); 9.02 (d, 1H, 4J =2.4, NH). | ||||
| 3b ------- 61% | 2-Ethyloxy-6-nitro-3-(3-methyl-1-phenylpyrazol-5-ylaminomethylene)chroman-4-one | |||
| C22H20N4O5 420.4 | 187 – 189 °C | Calc.: 62.85%C; 4.75%H; 13.33%N
Found: 61.40%C; 4.63%H; 13.19%N | ||
| 1H-NMR: 1.25 (t, 3H, CH3); 2.70 (s, 3H, CH3 on C-14); 3.91 (q, 2H, CH2); 6.37 (s, 1H, H-2); 7.35 (t, 1H, 3J =7.4, H-19); 7.52-758 (m, 2H, H-18,20); 8.24 (m, 2H, H-17,21); 8.35 (d, 1H , 3J =7.7, H-8); 8.42 (dd, 1H, 3J =7.7, 4J =2.7, H-7); 8.48 (d, 1H, 3J = 2.4, H-9); 8.64 (d,1H, 4J =2.7, H-5); 8.98 (d,1H,4J =2.0, H-15); 9.02 (d, 1H, 4J =2.4, NH) | ||||
| 5a ------- 68% | 3-(2-acetyloxybenzoyl)-5-methyl-7-phenylpyrazolo[3,4-b]pyridine | |||
| C22H17N3O3 371.2 | 109-111 °C | Calc.: 71.12 %C; 4.58 %H, 11.31%N
Found: 71.26 %C; 4.50 %H, 11.28%N | ||
| 1H-NMR: 2.03 (s, 3H, CH3CO); 2.68 (s, 3H, CH3-8); 7.30 (dd, 1H, 3J=7.3, 4J=2.1, H-13); 7.32-7.39 (m, 3H, H-11,18,20); 7.49-7.56 (m, 2H,H-10,12), 7.55 (t,1H, 3J=7.6, H-14); 8.26 (dd, 2H, 3J=7.7, 4J= 2.1, H-19,21); 8.48 (d, 1H, 4J=1.2,H-4); 9.05 (d, 1H, 4J=1.2, H-2) | ||||
| 5b ------- 61% | 3-(2-acetyloxy-5-methylbenzoyl)-5-methyl-7-phenylpyrazolo[3,4-b]pyridine | |||
| C23H19N3O3 385.2 | 125-127 ° C | Calc.: 71.65 %C; 4.93 %H, 10.90%N
Found: 71.44 %C; 5.09 %H, 10.81%N | ||
| 1H-NMR: 1.99 (s, 3H, CH3CO); 2.40 (s, 3H, CH3 on C-20); 2.67 (s, 3H, CH3-8); 7.11 (d, 1H, 3J=8.3, H-18); 7.30 (dd, 1H, 3J=7.3, 4J=2.1, H-19); 7.35 (d, 1H, 4J=2.2, H-21); 7.39 (m, 1H, H-14); 7.55 (t, 2H, 3J=7.6, H-10,12); 8.23 (dd, 2H, 3J=7.6, 4J=1.1, H-11,13); 8.49 (d, 1H, 4J(2,4)=1.2, H-4); 9.04 (d, 1H, 4J=1.2, H-2). | ||||
| 5c ------ 58% | 3-(2-ethyloxycarbonyloxy-5-methylbenzoyl)-5-methyl-7-phenylpyrazolo[3,4-b]-pyridine | |||
| C24H21N3O4 415.4 | 154-156 ° C | Calc.: 69.39 %C; 5.09 %H; 10.11%N
Found: 69.48 %C; 5.00 %H; 10.02%N | ||
| 1H-NMR:1.07 (t,3H, 3J=7,3,CH3); 2.55 (s,3H,CH3); 2.66 (s, 3H, CH3-8); 4.09 (q,2H, 3J=7,3, CH2 ); 7.23 (t, 1H, 3J=8.3, H-18); 7.37 (d, 1H, 3J=8.3, H-12); 8.09 (tt, 2H, 3J=7.6, H-11,13); 8.24 (dd, 2H, 3J=8.8, 4J=1.1, H-17,21); 8.49 (d, 1H, 4J=1.3, H-4); 9.04 (d, 1H, 4J=1.3Hz, H-2). | ||||
| 5d ------- 63% | 3-[2-(3-methylphenyloxyacetyloxy-5-methyl)benzoyl]-5-methyl-7-phenylpyrazolo[3,4-b]-pyridine | |||
| C30H25N3O4 491.3 | 118-120 ° C | Calc.: 73.27%C; 5.09 %H, 8.55%N
Found: 73.24%C; 5.19 %H, 8.41%N | ||
| 1H-NMR: 2.41 (s, 3H, CH3); 2.25 (s, 3H, CH3); 2.64 (s, 3H, CH3-8); 4.62 (s, 2H, CH2); 6.73 (d, 1H, 4J=2.1, HPhO); 7.06-7.13 (m, 3H, HPhO); 7.3-7.4 (m, 3H, H-12,18,19); 7.45 (dd, 2H, 3J=7.3, 4J=2.1, H-11,13); 8.23 (dd, 2H, 3J=7.3, 4J=2.1, H-10,14); 8.47 (d, 1H, 4J=1.1, H-4); 9.04 (d, 1H, 4J=1.1, H-2). | ||||
6a ------- 68% | 3-(6'methyl-3'-phenylcoumarin-4'-yl)-5-methyl-7-phenylpyrazolo[3,4-b]pyridine | |||
| C29H21N3O2 443.3 | 222-224 °C | Calc.: 78.50 %C; 4.73 %H, 9.47%N
Found: 78.26 %C; 4.69 %H, 9.28%N | ||
| 1H-NMR: 2.29 (s, 3H, CH3); 2.59 (s, 3H, CH3-8); 6.95 (s, 1H, H-5'); 7.1-7.2 (m, 5H, Ph on 3'); 7.29 (t, 1H, 3J=7.3, 4J=1.9, H-12); 7.38 (dd, 2H, 3J=7.3, J=1.9, H-11,13); 7.51 (d, 1H, 3J=7.4, H-8'); 7.54 (dd, 3J=7.4, 4J=1.9, H-7'); 7.78 (d, 1H, 4J=1.2, H-4); 8.23 (dd, 2H, 3J=7.6, 4J=2.0, H-10,14); 8.39 (d, 1H, 4J=1.2, H-2). | ||||
| 6b ------- 61% | 3-[(6'methyl-3'-phenylthio)coumarin-4'-yl]-5-methyl-7-phenylpyrazolo[3,4-b]pyridine | |||
| C29H21N3O2S 475.2 | 319-323 ° C | Calc.: 67.12 %C; 4.52 %H, 11.18%N
Found: 66.98 %C; 4.35 %H, 11.28%N | ||
| 1H-NMR (DMSO-d6): 2.25 (s, 3H, CH3 ); 2.62 (s, 3H, CH3-8 ); 6.97 (s, 1H, H-5'); 7.1-7.2 (s, 5H, Ph on 3'); 7.33 (t, 1H, 3J=7.5, 4J=1.9, H-12); 7.48 (d,1H, 3J=8.1, H-8'); 7.52-7.55 (m, 2H, H-11,13 ); 7.56 (dd, 1H, 3J=8.3, 4J=1.9, H-7'); 8.26 (dd, 2H, 3J=8.6, 4J=2.0, H-10,14); 8.39 (d, 1H, 4J=1.2, H-4); 8.64 (d, 1H, 4J=1.2, H-2). | ||||
7a ------- 63% | 3-(2-hydroxybenzoyl)-5-methyl-7-phenylpyrazolo[3,4-b]pyridine-15-(2',4'-dinitrophenyl)hydrazone | |||
| C26H19N7O5 509.5 | 280-281 °C | Calc.: 61.23 %C; 3.73 %H, 19.24%N
Found: 61.45 %C; 3.40 %H, 19.02%N | ||
| 1H-NMR: 2.72 (s, 3H, CH3); 6.82-6.86 (m, 2H, H-19,21); 7.13 (dd, 1H, 3J=8.3Hz, 4J=2 Hz, H-18); 7.32-7.43 (m, 2H, H-12,20); 7.56 (t, 2H, 3J=7.9Hz, 4J=2Hz, H-11,13); 8.17 (d, 1H, 4J=1.6Hz, H-4); 8.27 (d, 1H, 3J=8.9Hz, H-6'); 8.28 (dd, 2H, 3J=7.9Hz, 4J=1.9Hz, H-10, 14); 8.45 (dd, 1H, 3J=9 Hz, 4J=2Hz, H-5'); 8.59 (d, 1H, 4J=1.6Hz, H-2); 9.09 (d, 1H, 4J=2Hz, H-3'); 11.26 (s, NH); 11.37 (s, OH). | ||||
| 7b ------- 68% | 3-(2-hydroxy-5-methylbenzoyl)-5-methyl-7-phenylpyrazolo[3,4-b]pyridine-15-(2', 4'-dinitrophenyl)hydrazone | |||
| C27H21N7O5 523.5 | 290-292 °C | Calc.: 61.89 %C; 4.01 %H, 18.72%N
Found: 61.78 %C; 4.11 %H, 18.53%N | ||
| 1H-NMR: 2.26 (s, 3H, CH3); 2.75 (s, 3H, CH3-8); 6.58 (s, 1 H, H-21); 7.05 (d, 1H, 3J=8.5Hz, H-18); 7.17 (dd, 1H, 3J=8.6Hz, 4J=2.4Hz, H-19); 7.34 (t, 1H, 3J=7.6Hz, 4J=1.1Hz, H-12); 7.55 (dd, 2H, 3J=8.5Hz, 4J=2Hz, H-11,13); 7.75 (dd, 1H,3J=8.4Hz, 4J=2.5Hz, H-6'); 8.32 (dd, 2H, 3J=8.1Hz, 4J=2Hz, H-10,14); 8.44 (dd, 1H, 3J=9.1Hz, 4J=2.6Hz, H-5'); 8.15 (d, 1H, 4J=2.8Hz, H-4); 8.57 (d, 1H, 4J=2.0, H-2); 9.07 (d, 1H, 4J=2.6 H-3'); 11.13 (s, NH); 11.20 (s, OH). | ||||
| 7c ------- 70% | 3-(2-hydroxy-5-fluorobenzoyl)-5-methyl-7-phenylpyrazolo[3,4-b]pyridine-15-(2', 4'-dinitrophenyl)hydrazone | |||
| C26H18FN7O5 528.4 | 291-292 °C | ----------------- | ||
| 1H-NMR: 2.66 (s, 3H, CH3); 6.94 (dd, 1H, 3J=8.8Hz, 4J=2.4Hz, H-19); 7.12 (d, 1H, 3J=8.8Hz, H-18); 7.51 (t, 1H, 3J=8.8Hz, 4J=2Hz, H-12); 7.56 (d, 1 H, 4J=2.4Hz, H-21); 8.14 (d, 1H, 4J=1.9Hz, H-4); 8.16 (dd, 2H, 3J=8.8Hz, 4J=2 Hz, H-11,13); 8.39 (dd, 1H, 3J=9.1Hz, 4J=2.6 Hz, H-5'); 8.43 (dd, 2H, 3J=8.1Hz, 4J=2Hz, H-10,14); 9.07 (d, 1H, 4J=1.9Hz, H-2); 9.09 (d, 1H, 4J=2.6 Hz, H-3'); 11.06 (s, NH); 11.27 (s, OH). | ||||
References
- Stankovičová, H.; Lácová, M.; Gáplovský, A.; Chovancová, J.; Pronayová, N. Tetrahedron 2001, 57, 3455.
- Stankovičová, H.; Fabian, W.M.F.; Lácová, M. Molecules 1996, 1, 223.
- Stankovičová, H.; Gašparová, R; Lácová, M.; Chovancová, J. Collect. Czech. Chem. Commun. 1997, 62, 781.
- El-Shaaer, H.M.; Perjessy, A.; Záhradník, P.; Lácová, M. Collect. Czech. Chem. Commun. 1994, 59, 1673.
- El-Shaaer, H.M.; Perjessy, A.; Záhradník, P.; Lácová, M.; Šusteková, Z. Monatsh. Chem. 1993, 124, 539.
- Stankovičová, H.; Gáplovský, A.; Lácová, M.; Puchala, A.; Chovancová, J. J. Am. Chem. Soc. (in press).
- Gáplovský, A.; Donovalová, J.; Lácová, M.; Mračnová, R.; El-Shaaer, H.M. J. Photochem. Photobio.- A: Chem. 2000, 136, 61.
- El-Shaaer, H.M.; Foltínová, P.; Lácová, M.; Chovancová, J.; Stankovičová, H. Il Farmaco 1998, 53, 224. [PubMed]
- Gašparová, R.; Lácová, M.; EL-Shaaer, H.M. Il Farmaco 1997, 52, 251. [PubMed]
- Gosh, Ch.K. J. Hetrocycl. Chem. 1983, 20, 1437. [CrossRef]
- Sabitha, G. Aldrichim. Acta 1996, 29, 15.
- Gašparová, R.; Lácová, M. Collect. Czech. Chem. Commun 1995, 60, 1178. [PubMed]
- Lácová, M.; Gašparová, R.; Loos, D.; Liptay, T.; Pronayová, N. Molecules 2000, 5, 167.
- Nohara, A.; Umetani, T.; Sanno, Y. Tetrahedron Lett. 1973, 14, 1995.
- Nohara, A.; Umetani, T.; Sanno, Y. Tetrahedron 1974, 30, 3553. [CrossRef]
- Sample Availability: Contact the authors
© 2005 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Lacova, M.; Puchala, A.; Solcanyova, E.; Lac, J.; Kois, P.; Chovancova, J.; Rasala, D. 3-Formylchromones IV. The Rearrangement of 3-Formylchromone Enamines as a Simple, Facile Route to Novel Pyrazolo[3,4-b]pyridines and the Synthetic Utility of the Latter. Molecules 2005, 10, 809-821. https://doi.org/10.3390/10070809
Lacova M, Puchala A, Solcanyova E, Lac J, Kois P, Chovancova J, Rasala D. 3-Formylchromones IV. The Rearrangement of 3-Formylchromone Enamines as a Simple, Facile Route to Novel Pyrazolo[3,4-b]pyridines and the Synthetic Utility of the Latter. Molecules. 2005; 10(7):809-821. https://doi.org/10.3390/10070809
Chicago/Turabian StyleLacova, M., A. Puchala, E. Solcanyova, J. Lac, P. Kois, J. Chovancova, and D. Rasala. 2005. "3-Formylchromones IV. The Rearrangement of 3-Formylchromone Enamines as a Simple, Facile Route to Novel Pyrazolo[3,4-b]pyridines and the Synthetic Utility of the Latter" Molecules 10, no. 7: 809-821. https://doi.org/10.3390/10070809
APA StyleLacova, M., Puchala, A., Solcanyova, E., Lac, J., Kois, P., Chovancova, J., & Rasala, D. (2005). 3-Formylchromones IV. The Rearrangement of 3-Formylchromone Enamines as a Simple, Facile Route to Novel Pyrazolo[3,4-b]pyridines and the Synthetic Utility of the Latter. Molecules, 10(7), 809-821. https://doi.org/10.3390/10070809




