Synthesis of Steroidal Thiadiazoles from Steroidal Ketones
Abstract
:Introduction
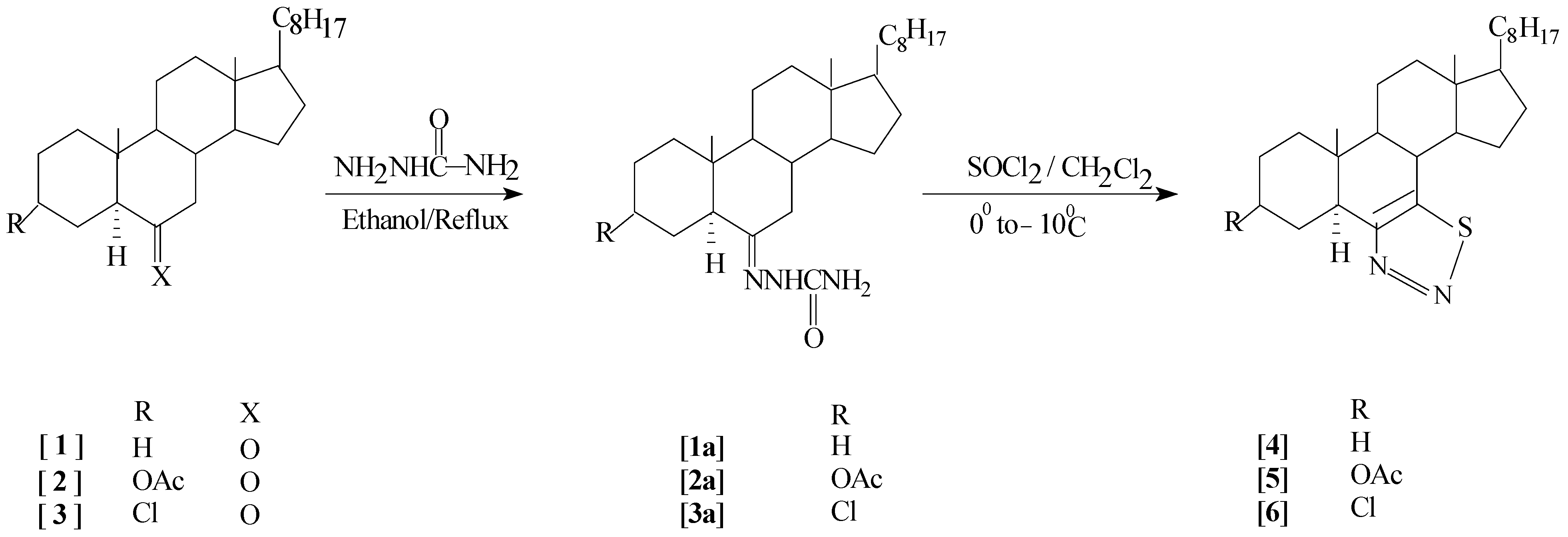
Results and Discussion

Acknowledgements
Experimental
General
General procedure for preparation of semicarbazones: [26]
General procedure for preparation of steroidal thiadiazoles
| Starting Ketone | Products | Yield (%) | M.p. (°C) | Rf | TLCa (mL) |
|---|---|---|---|---|---|
| α-cholestan-6-one (1) | α-cholestan-6-one-semicarbazone (1a) | 80 | 190 | 0.206 | 2: 1.00 :0.10 |
| 3β-acetoxy-α-cholestan-6-one (2) | 3β-acetoxy-α-cholestan-6-one-semicarbazone (2a) | 83 | 242 | 0.428 | 2 : 0.6: 0.10 |
| 3β-chloro-α-cholestan-6-one (3) | 3β-chloro-α-cholestan-6-one-semicarbazone (3a) | 75 | 152 | 0.603 | 2 : 0.6: 0.10 |
| Compound | Physical state | Molecularformula (Mol. Wt.) | Yield (%) | % found (calcd.) | Rf value | TLCa (mL) | |||
|---|---|---|---|---|---|---|---|---|---|
| C | H | N | S | ||||||
| 5α-cholest-6-eno[6,7- d] thiadiazole (4) | Glossy semi-solid | C27H44N2S (428) | 58 | 75.66 (75.70) | 10.24 (10.28) | 6.54 (6.50) | 7.42 (7.48) | 0.155 | 2:1.00:0.10 |
| 3β-acetoxy-5α-cholest-6-eno [6,7-d] thiadiazole (5) | Glossy semi-solid | C29H46N2OS (486) | 62 | 71.56 (71.60) | 9.41 (9.46) | 5.72 (5.76) | 6.51 (6.58) | 0.301 | 2 : 0.6: 0.10 |
| 3β-chloro-5α-cholest-6-eno [6,7-d] thiadiazole (6) | Glossy semi-solid | C27H43N2ClS (462/464) | 58 | 69.78 (70.08) | 9.48 (9.50) | 6.02 (6.05) | 6.78 (6.91) | 0.492 | |
| Compound | IR (KBr, cm-1) | 1H-NMR(δ, ppm) | MS (EI):mz (%) |
|---|---|---|---|
| 4 | 1615 (C=C) | 2.9 (t, C5-αH) | 428 (M+.), |
| 1380 (C-N) | 3.5 (dd, C8-βH) | 400 (100) (base peak), | |
| 1565 (N=N) | 1.12 (C10-CH3) | 368 (30) (M+-N2 and S), | |
| 715 (C-S) | 0.70 (C13-CH3) | 386 (10) (M+-N2 and CH3), | |
| 0.90 (C20-CH3) and | 287 (7) (M+-N2 and C8H17), | ||
| 0.83 (C25-CH3). | |||
| 5 | 1615 (C=C) | 4.9 (m, W1/2 =17 Hz, C3-αH A/B | 486 (M+.), |
| 1374 (C-N) | ring junction trans) [26] | 365 (100) (base peak), | |
| 1570 (N=N) | 2.75 (t, C5-αH), 2.95 (dd, C8-βH), | 458 (20) (M+-N2), | |
| 711 (C-S) | 2.1 (s, 3H, CH3COO), 1.13 (C10- | 442 (5) (M+-N2 and CH3), | |
| CH3), 0.67 (C13-CH3), 0.91 (C20- | 426 (8) (M+-AcOH), | ||
| CH3) and 0.87 (C25-CH3). | 398 (30) (M+-N2 and AcOH), | ||
| 345 (7) (M+-N2 and C8H17) | |||
| 6 | 1623 (C=C) | 2.7 (t, C5-αH), 3.0 (dd, C8-βH) | 462/464[35/37Cl] (M+.)/(M++2) |
| 1370 (C-N) | 4.2 (br, m, W1/2 =16Hz C3-αH | (3/0.96) (0.25/0.08 peak’s value), | |
| 1550 (N=N) | A/B ring junction trans) [ 26] | 394 (100) (base peak), | |
| 705 (C-S) | 1.15 (C10-CH3), 0.68 (C13-CH3), | 434/436 (12/3.8) (M+-N2), | |
| 0.93 (C20-CH3) and 0.83 (C25- | 418/420 (10/3.5) (M+-N2 and CH3), | ||
| CH3) | 400/402 (30/(9.1) (M+-N2 and S), | ||
| 321/323 (8/2.3) (M+-N2 and C8H17) |
References
- Lalezari, I.; Shafiee, A.; Yazdani, S. J. Pharm. Sci. 1974, 63, 628–631, and references cited therein. [CrossRef]
- Reddy, D.B.; Padmaja, A.; Reddy, M.M. J. Ecotox. Environ. Monit. 1993, 3, 87–90.
- Shafiee, A.; Lalezari, I.; Yazdani, S.; Pournorouz, A. J. Pharm. Sci. 1973, 62, 839–842. [CrossRef]
- Chopleo, C.V.; Mayers, P.L.; Smith, A.C.B.; Stilling, M.R.; Tulloch, I.F.; Walter, D.S. J. Med. Chem. 1988, 31, 7–13.
- Clemence, F.; Joliveau-Maushart, C.; Meirer, J.; Cered, J.; Cered, F.; Benzoni, J.; Deraedt, R. Eur. J. Med. Chem. Chim. Ther. 1985, 20, 257–260.
- Gadad, A.K.; Mahajanshetti, C.S.; Nimbalkar, S.; Raichurkar, A. Eur. J. Med. Chem. 2000, 35, 853–857. [CrossRef]
- Miyahara, M.; Nakadata, M.; Sueyoshi, S.; Tanno, M.; Kamio, S. Chem. Pharm. Bull. 1982, 30, 4402–4408. [CrossRef]
- Thomas, E.W.; Nishizawa, E.E.; Zimmermann, D.D.; Williams, C.J. J. Med. Chem. 1985, 28, 442–447. [CrossRef]
- Gaikwad, M.S.; Mane, A.S.; Hangarge, R.V.; Chavan, V.P.; Shingare, M.S. Ind. J. Chem. 2003, 42B, 189–191.
- Reddy, D.B.; Reddy, M.V.R.; Padmavathi, V. Synth. Commun. 1999, 29, 667–670.
- Reddy, D.B.; Balaiah, A.; Padmavathi, V.; Padmaja, A. Heterocycl. Commun. 1999, 5, 285–290.
- Hu, Y.; Baudort, S.; Porco, J.A. J. Org. Chem. 1999, 64, 1049–1054. [CrossRef]
- Dehaen, W.; Voets, M.; Bakulev, V.A. Adv. Nitrogen Heterocycl. 2000, 4, 37.
- Shafiee, A. J. Heterocycl. Chem. 1976, 13, 301–306. [CrossRef]
- Britton, T.C.; Iobal, T.J.; Chidester, G.C. J. Org. Chem. 1984, 49, 4773–4780. [CrossRef]
- Vasiliy, V.; Bakulev, A.; Dehaen, W. The Chemistry of 1,2,3-Thiadiazoles; John Wiley & Sons. Inc.: New York, 2004; pp. 1–92. [Google Scholar]
- Reddy, D.B.; Somasekhar, A.; Padmavathi, V. J. Chem. Res. (S). 1998, 784–785. [CrossRef]
- Oka, K.; Hara, S. J. Org. Chem. 1978, 43, 4533–4535. [CrossRef]
- Jones, D.N.; Lewis, J.R.; Shopee, C.W.; Summers, G.H.R. J. Chem. Soc. 1955, 2876–2881. [CrossRef]
- Dodson, R.M.; Riegel, B. J. Org. Chem. 1948, 13, 424–437. [CrossRef]
- Windaus, A.; Dalmer, O. Chem. Ber. 1919, 52, 162–167.
- Shafiullah; Ansari, S.A. J. Indian Chem. Soc. 1990, 62, 431–432.
- Hurd, C.D.; Mori, R.I. J. Am. Chem. Soc. 1955, 77, 5359–5364. [CrossRef]
- Butler, R.N.; O’ Donoghue, D.A. J. Chem. Soc., Perkin Trans. I 1995, 2079–2081.
- Stanetty, P.; Kremslehne, M. Heterocycles 1998, 48, 259–266.
- Vogel, A.I. A Text Book of Practical Organic Chemistry Including Organic Analysis, 3rd ed.; ELBS and Longman Group Ltd.: London, 1971; p. 344. [Google Scholar]
- Bhacca, N.S.; Williams, D.N. Application of NMR Spectroscopy in Organic Chemistry; Holden- Day: San Francisco, 1964; vol. IIC; p. 51. [Google Scholar]
- Sample availability:
© 2005 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Mushfiq, M.; Alam, M.; Akhtar, M. Synthesis of Steroidal Thiadiazoles from Steroidal Ketones. Molecules 2005, 10, 803-808. https://doi.org/10.3390/10070803
Mushfiq M, Alam M, Akhtar M. Synthesis of Steroidal Thiadiazoles from Steroidal Ketones. Molecules. 2005; 10(7):803-808. https://doi.org/10.3390/10070803
Chicago/Turabian StyleMushfiq, M., M. Alam, and M. Akhtar. 2005. "Synthesis of Steroidal Thiadiazoles from Steroidal Ketones" Molecules 10, no. 7: 803-808. https://doi.org/10.3390/10070803




