Role of Palladium in the Redox Electrochemistry of Ferrocene Monocarboxylic Acid Encapsulated Within ORMOSIL Networks
Abstract
:Introduction
Results and Discussion
Chemistry of the palladium chloride and trimethoxysilane interaction


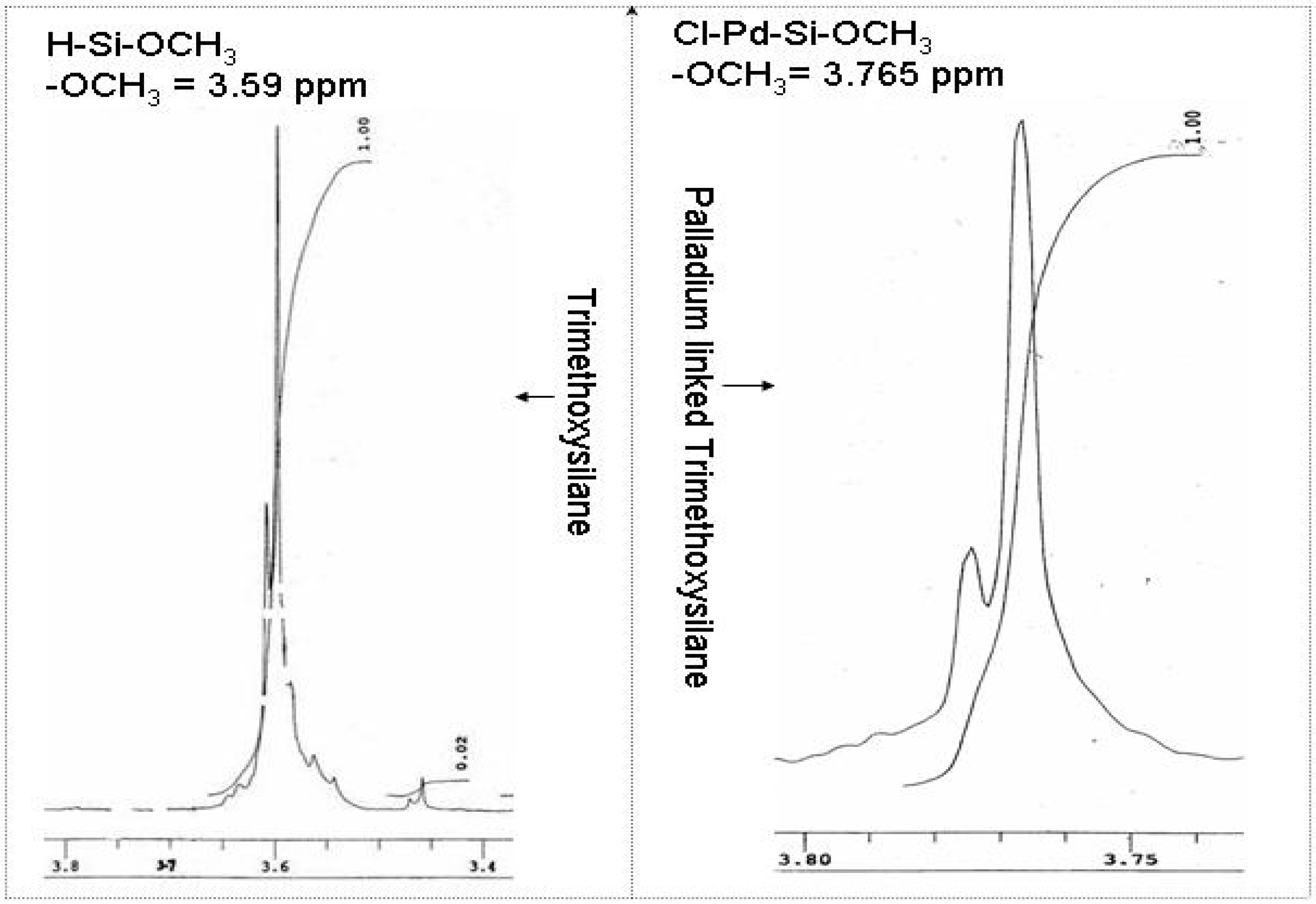
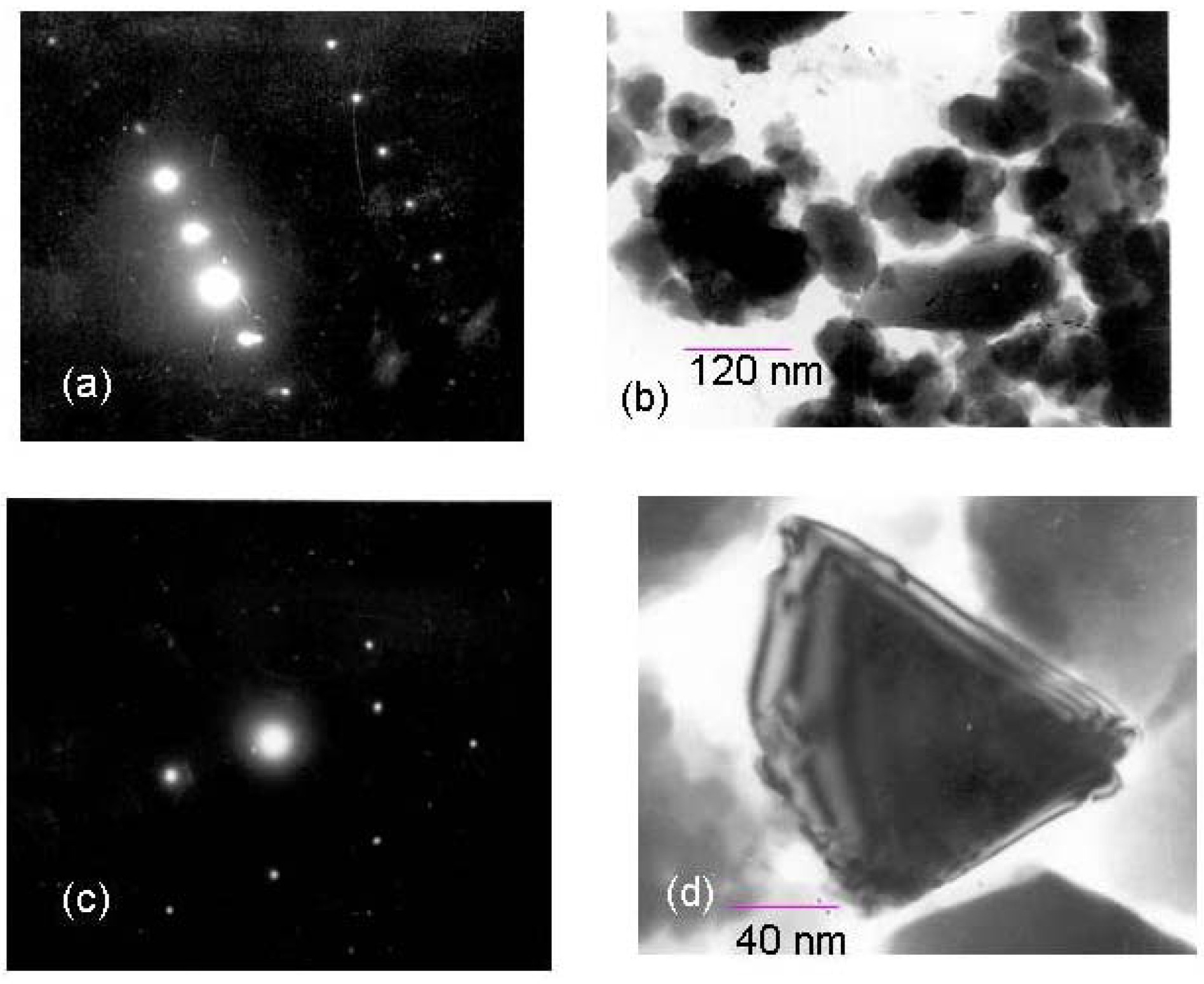
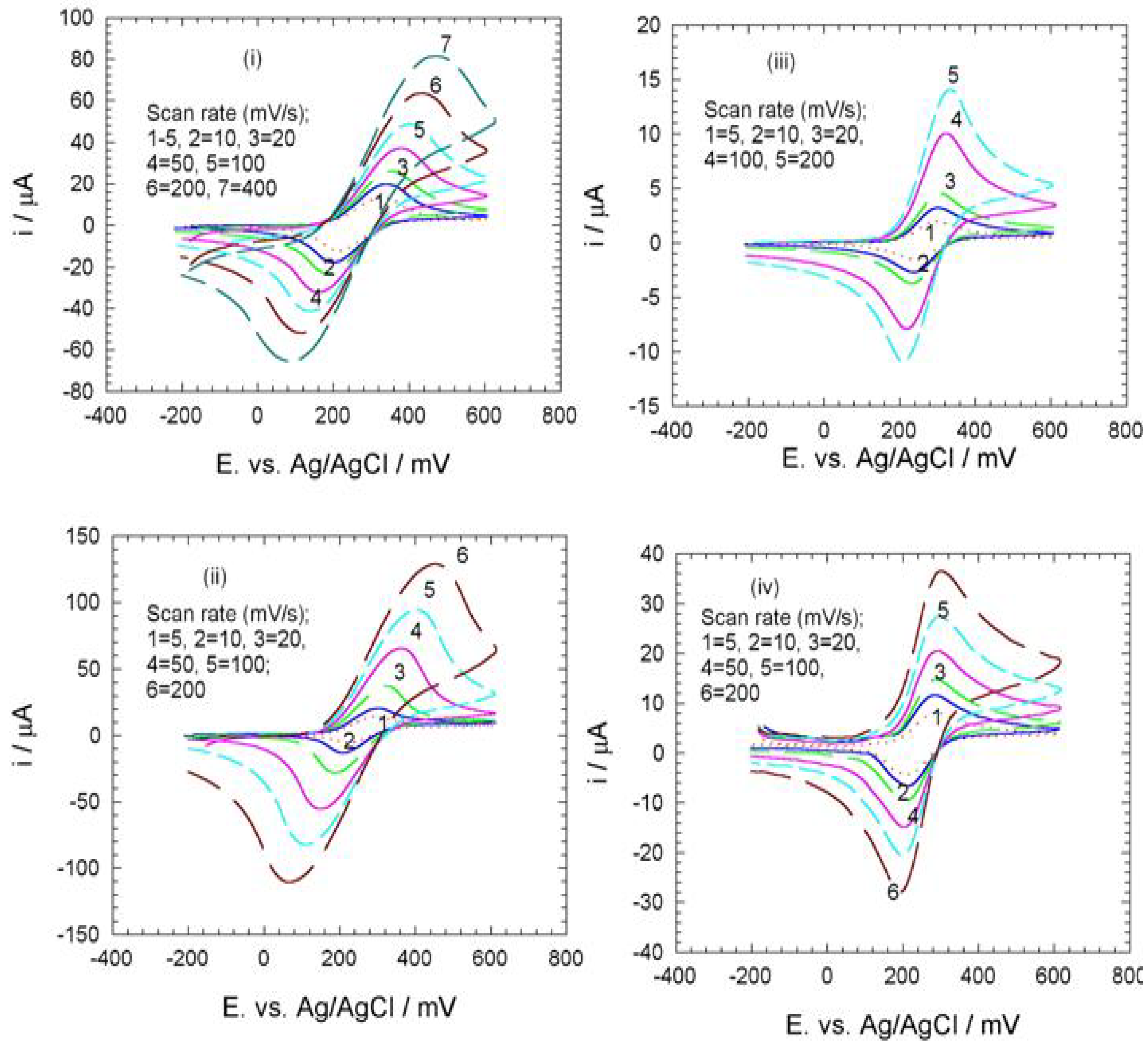
Electrochemistry of ferrocene monocarboxylic acid encapsulated ormosil-modified electrode based on palladium-silicon linkage
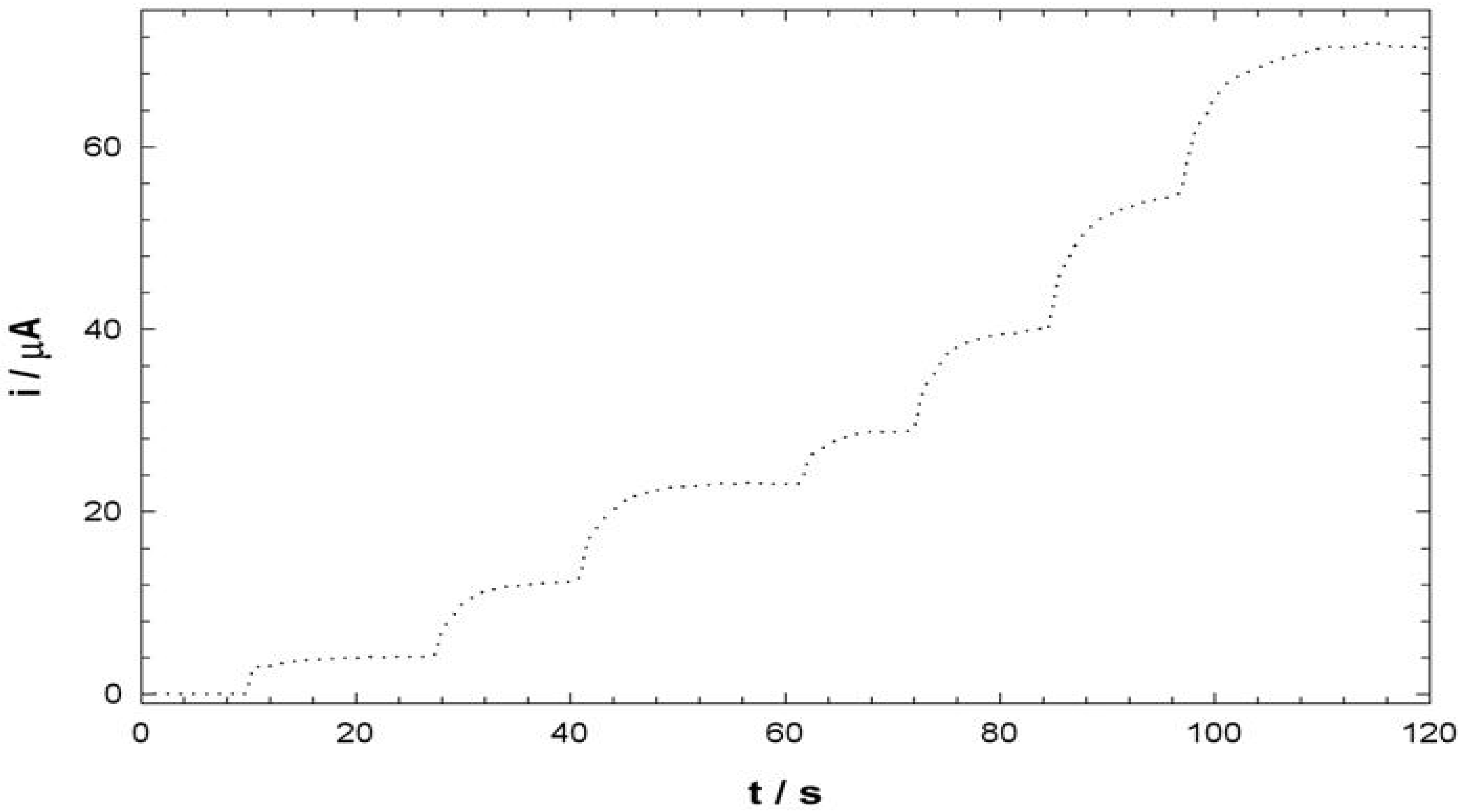
Electroanalysis of NADH
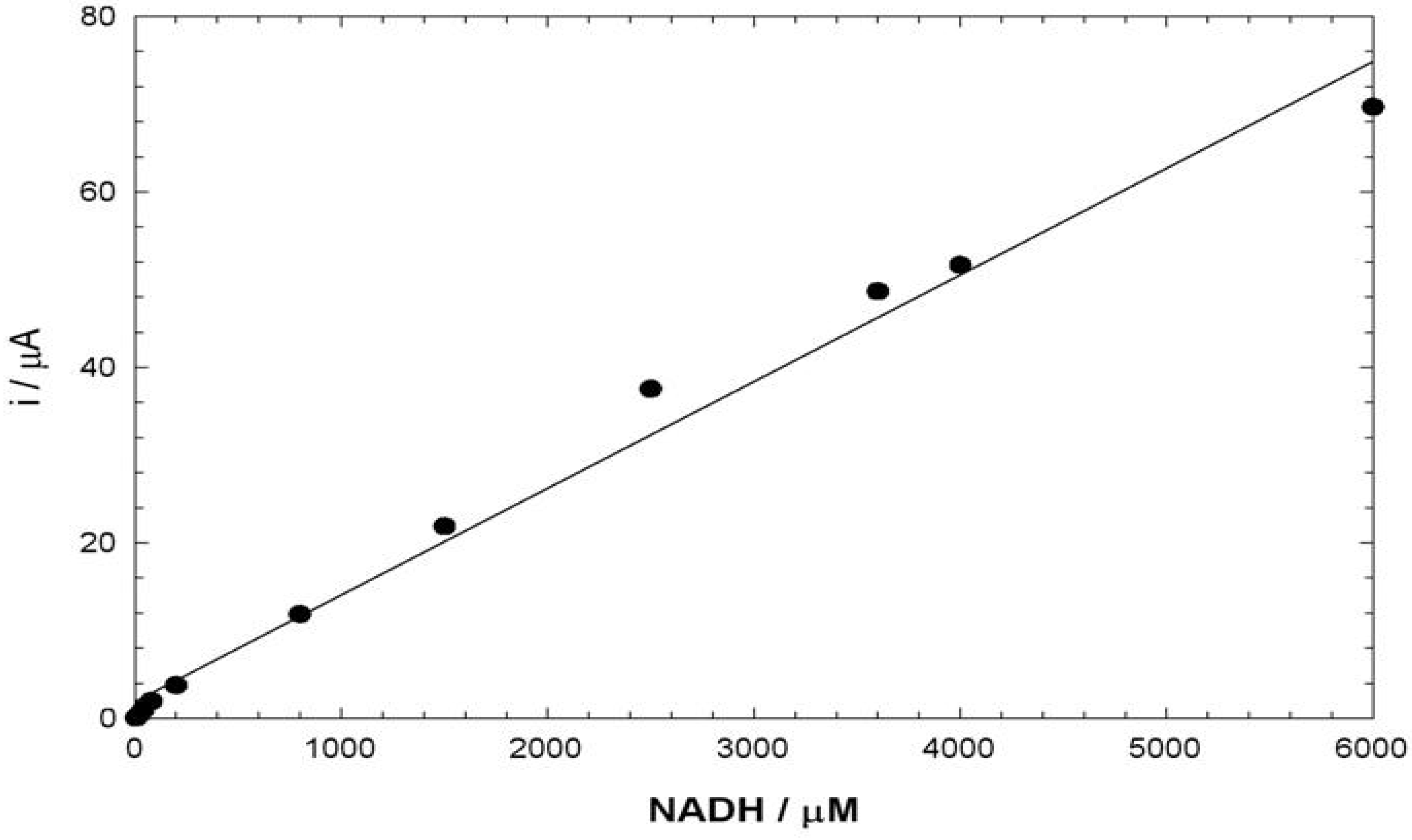
Conclusions
Experimental
General
Preparation of ORMOSIL having palladium-silicon linkages
Electrochemical measurements.
Acknowledgements
References and Notes
- Ghosh, P. K.; Bard, A. J. Clay-modified electrodes. J. Am. Chem. Soc. 1983, 105, 5691. [Google Scholar]
- Ege, D.; Ghosh, P. K.; White, J. R.; Equey, J. F.; Bard, A. J. Clay modified electrodes. 3. Electrochemical and electron spin resonance study of montmorillonite layers. J. Am. Chem. Soc. 1985, 107, 5644. [Google Scholar]
- Itaya, K.; Bard, A. J. Clay-modified electrodes. 5. Preparation and electrochemical characterization of pillared clay-modified electrodes and membranes. J. Phys. Chem. 1985, 89, 5565. [Google Scholar]
- White, J. R.; Bard, A. J. Clay modified electrodes: Part IV. The electrochemistry and electron spin resonance of methyl viologen incorporated into montmorillonite films. J. Electroanal. Chem. Interf. Electrochem. 1986, 197, 233. [Google Scholar]
- Itaya, K.; Chang, H.; Uchida, I. Anion-exchanged clay (hydrotalcite-like compounds) modified electrodes. Inorg. Chem. 1987, 26, 624. [Google Scholar] [CrossRef]
- Scavetta, E.; Berrettoni, M.; Giorgetti, M.; Tonelli, D. Electrochemical characterisation of Ni/Al---X hydrotalcites and their electrocatalytic behaviour. Electrochim. Acta 2002, 47, 2451. [Google Scholar]
- Scavetta, E.; Tonelli, D.; Giorgetti, M.; Nobili, F.; Marassi, R.; Berrettoni, M. AC impedance study of a synthetic hydrotalcite-like compound modified electrode in aqueous solution. Electrochim. Acta 2003, 48, 1347. [Google Scholar]
- Song, C. H.; Villemure, G. Preparation of clay-modified electrodes by electrophoretic deposition of clay films. J. Electroanal. Chem. 1999, 462, 143. [Google Scholar]
- Qiu, J.; Villemure, G. Anionic clay modified electrodes: electron transfer mediated by electroactive nickel, cobalt or manganese sites in layered double hydroxide films. J. Electroanal. Chem. 1997, 428, 165. [Google Scholar]
- Gaillon, L.; Bedioui, F.; Battioni, J. D-P. Electrochemical characterization of manganese porphyrins fixed onto silica and layered dihydroxide matrices. J. Electroanal.Chem. 1993, 347, 435. [Google Scholar]
- Salas, M.; Gordillo, B.; González, F. J. Current measurements as a tool to characterise the H-bonding between 1-ferrocenylmethylthymine and 9-octyladenine: a voltammetric and chronoamperometric analysis. J. Electroanal. Chem. 2004, 574, 33–39. [Google Scholar] [CrossRef]
- Camm, K. D.; Furtado, S. J.; Gott, A. L.; McGowan, P. C. Synthesis and structural studies of bis-amino-functionalised ferrocene salts and ferrocenium salts. Polyhedron 2004, 23, 2929–2936. [Google Scholar] [CrossRef]
- Padeste, C.; Steiger, B.; Grubelnik, A.; Tiefenauer, L. Molecular assembly of redox-conductive ferrocene–streptavidin conjugates — towards bio-electrochemical devices. Biosens. Bioelectron. 2004, 20, 545–552. [Google Scholar]
- Zhang, F-F.; Wan, Q.; Wang, X.-L.; Sun, Z.-D.; Zhu, Z.-Q.; Xian, Y.-Z.; Jin, L.-T.; Yamamoto, K. Amperometric sensor based on ferrocene-doped silica nanoparticles as an electron transfer mediator for the determination of glucose in rat brain coupled to in vivo microdialysis. J. Electroanal. Chem. 004, 571, 133–138. [Google Scholar]
- Asaftei, S.; Walder, L. Covalent layer-by-layer type modification of electrodes using ferrocene derivatives and crosslinkers. Electrochim. Acta 2004, 49, 4679–4685. [Google Scholar]
- Pandey, P. C.; Upadhyay, S.; Upadhyay, A. K. Electrochemical sensors based on functionalized ormosil-modified electrodes—role of ruthenium and palladium on the electrocatalysis of nadh and ascorbic acid. Sensor. Actuat. B: Chem. 2004, 102, 126–131. [Google Scholar]
- Pandey, P. C.; Upadhyay, S.; Sharma, S. Studies on the electrochemical performance of glucose biosensor based on ferrocene encapsulated ormosil and glucose oxidase modified graphite paste electrode. Biosens.Bioelectron. 2003, 18, 1257–1268. [Google Scholar]
- Pandey, P. C.; Upadhyay, S.; Tiwari, I. A novel ferrocene-encapsulated palladium-linked ormosil-based electrocatalytic biosensor. The role of the reactive functional group. Electroanal 2001, 13(18), 1519–1527. [Google Scholar]
- Pandey, P. C.; Upadhyay, S.; Pathak, H. C. Studies on ferrocene immobilized sol-gel glasses and its application in the construction of a novel solid-state ion sensor. Electroanal. 1999, 11, 950–956. [Google Scholar] [CrossRef]
- Mino, T.; Segawa, H.; Yamashita, M. Palladium-catalyzed asymmetric allylic alkylation using chiral hydrazone ligands with ferrocene skeleton. J. Organomet. Chem. 2004, 689, 2833–2836. [Google Scholar] [CrossRef]
- Bianchini, C.; Meli, A.; Oberhauser, W.; Parisel, S.; Gusev, O. V.; Kal'sin, A. M.; Nikolai, V. V.; Fedor, M. D. Methoxycarbonylation of styrene to methyl arylpropanoates catalyzed by palladium(II) precursors with 1,1′-bis(diphenylphosphino)metallocenes. J. Mol. Catal. A: Chem. 2004, 224, 35. [Google Scholar]
- i>Pessˆoa, C. H. A.; Gushikem, Y.; Kubota, L. T. Ferrocenecarboxylic acid adsorbed on Nb2O5 film grafted on a SiO2 surface: NADH oxidation study. Electrochim. Acta 2004, 46, 2499. [Google Scholar]
- Pfisterer, S. Z. Metallkunde 1950, 41, 358.
© 2005 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Pandey, P.; Upadhyay, B. Role of Palladium in the Redox Electrochemistry of Ferrocene Monocarboxylic Acid Encapsulated Within ORMOSIL Networks. Molecules 2005, 10, 728-739. https://doi.org/10.3390/10060728
Pandey P, Upadhyay B. Role of Palladium in the Redox Electrochemistry of Ferrocene Monocarboxylic Acid Encapsulated Within ORMOSIL Networks. Molecules. 2005; 10(6):728-739. https://doi.org/10.3390/10060728
Chicago/Turabian StylePandey, P., and B. Upadhyay. 2005. "Role of Palladium in the Redox Electrochemistry of Ferrocene Monocarboxylic Acid Encapsulated Within ORMOSIL Networks" Molecules 10, no. 6: 728-739. https://doi.org/10.3390/10060728



