The Preparation and Characterization of 5-Substituted-4-chloro-1,2,3-dithiazolium Salts and their Conversion into 4-Substituted-3-chloro-1,2,5-thiadiazoles
Abstract
:Introduction
Results and Discussion

1,2,3-Dithiazolium perchlorates 2

Electrochemical Study of the 1,2,3-dithiazolium perchlorates 2c-f


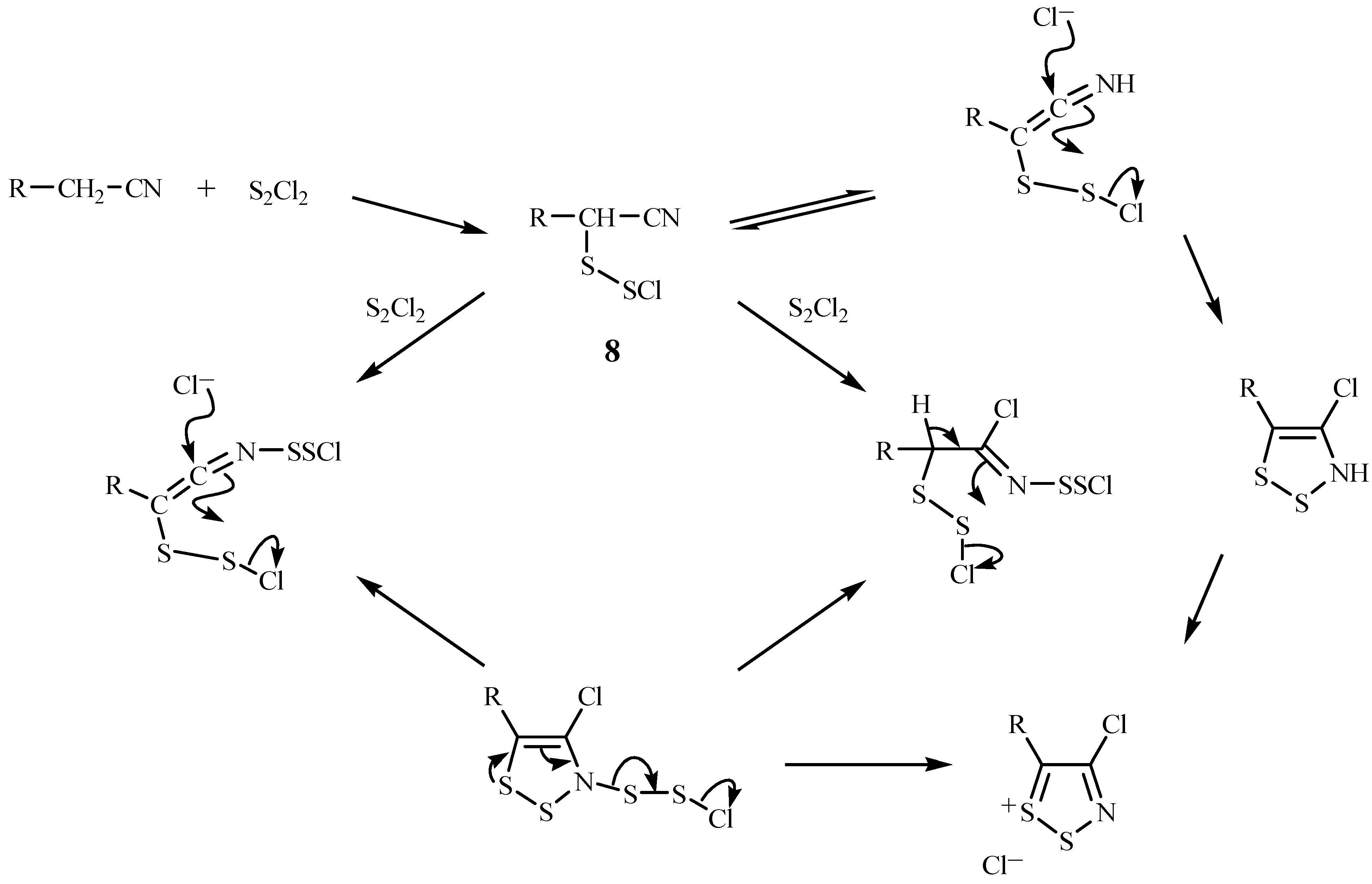
Mechanism of Dithiazole Ring Formation.
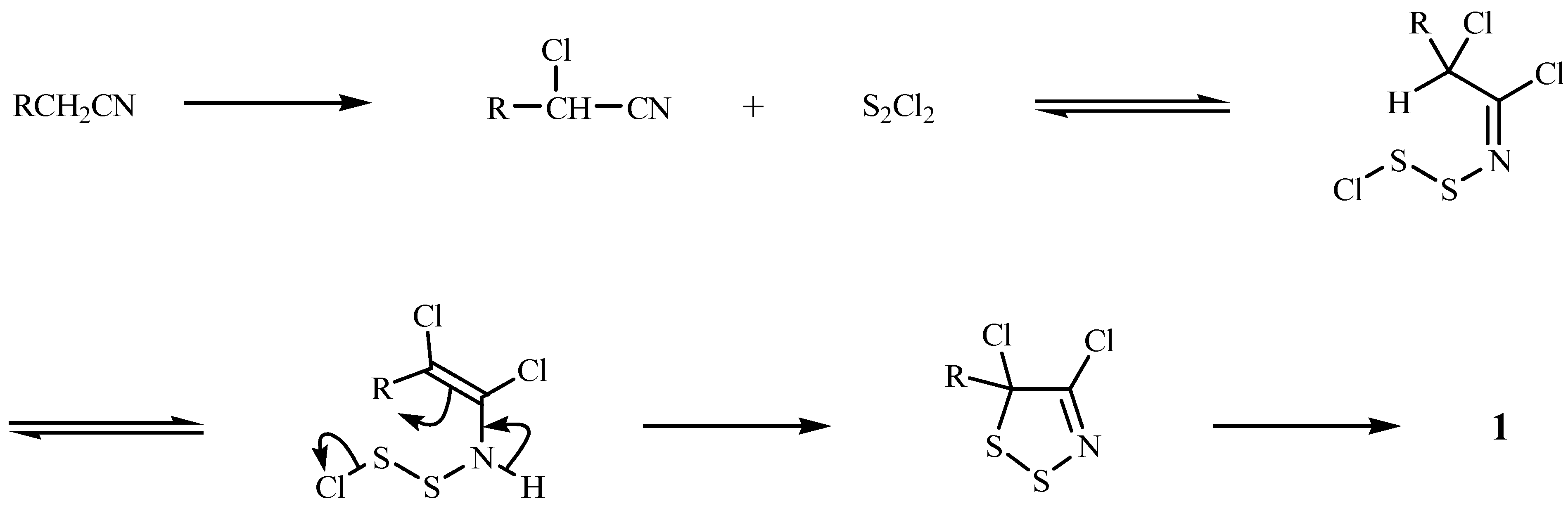
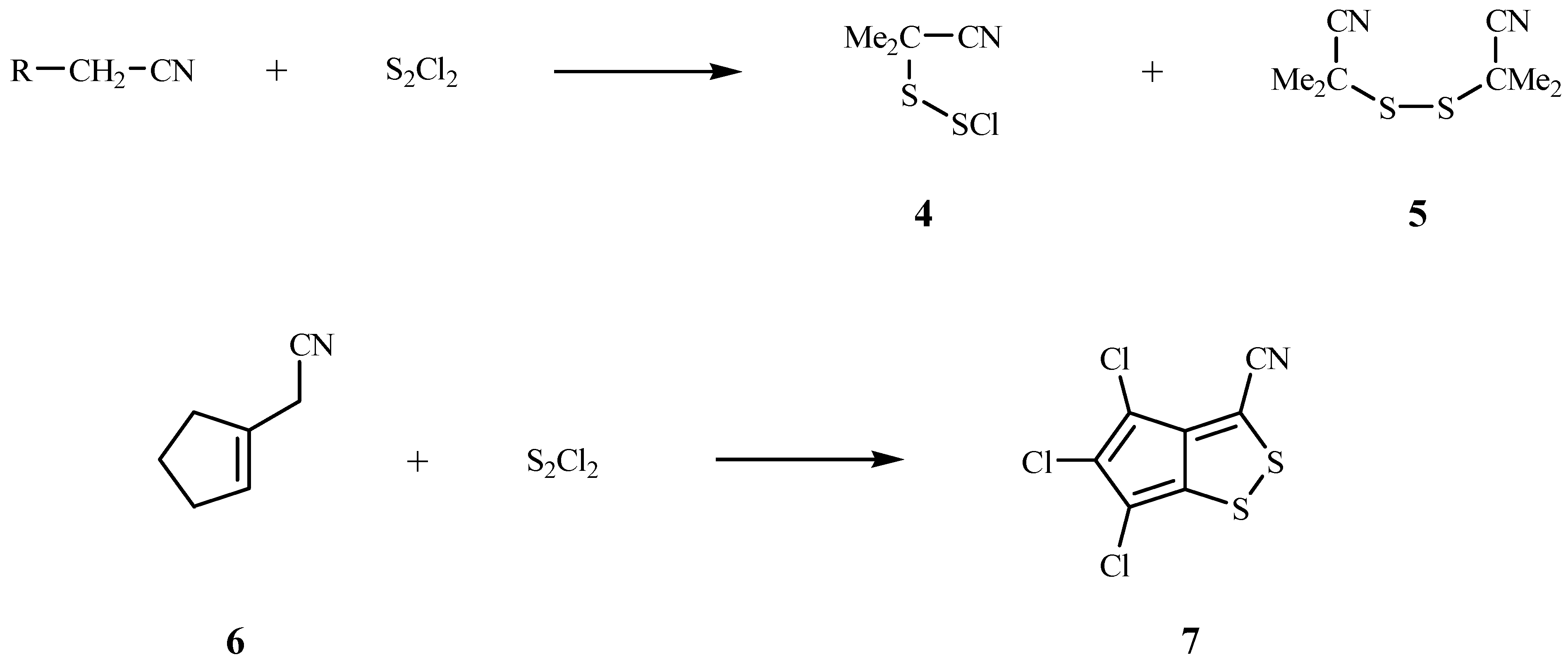
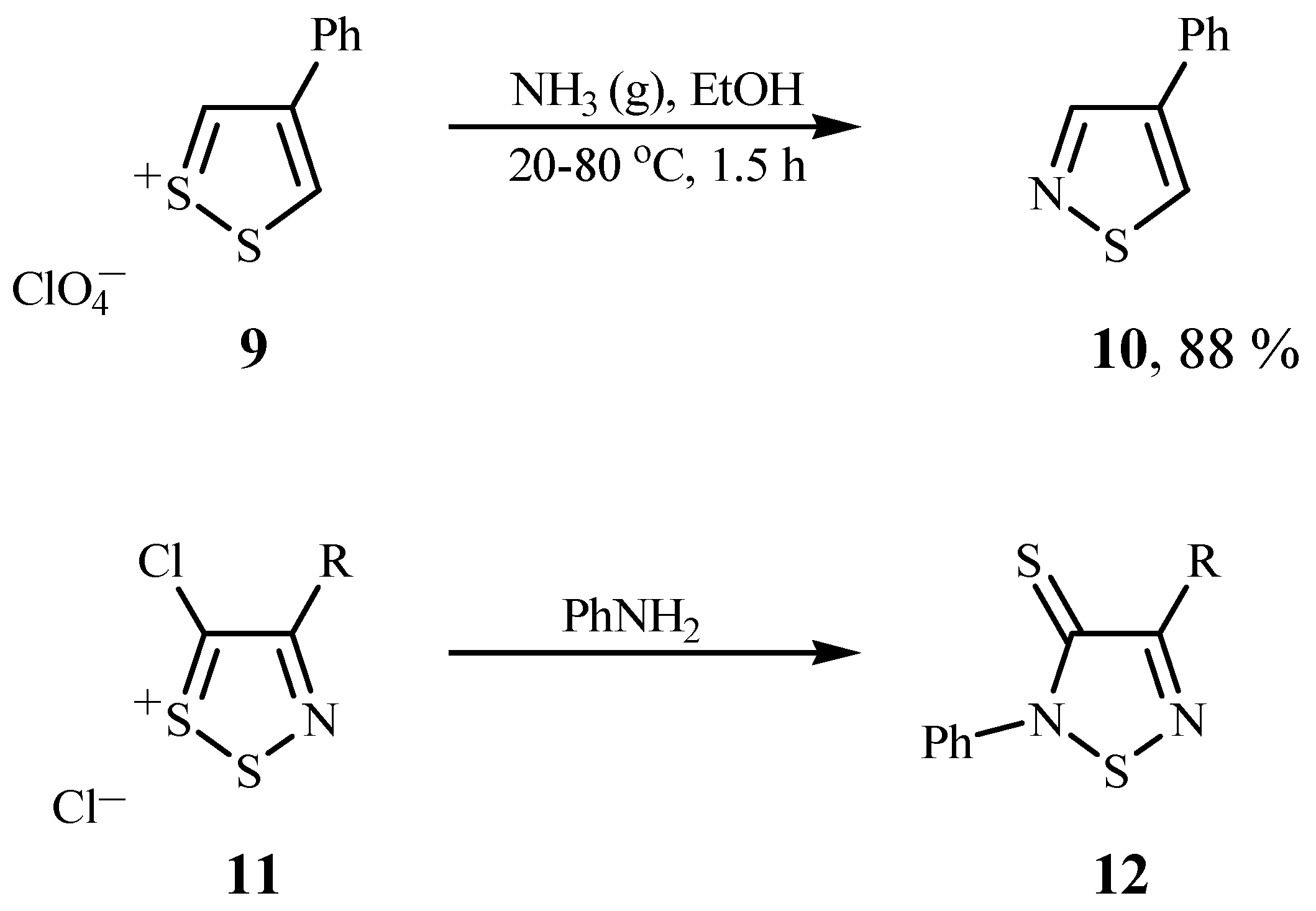
A Route to 1,2,5-Thiadiazoles 13


Conclusions
Experimental
General
Cyclic voltammetry of the 1,2,3-dithiazolium perchlorates 2c-f
1,2,3-Dithiazolium chlorides (1) (see Table 1)
1,2,3-Dithiazolium perchlorates (2): Typical procedure
Reaction of 4-chloro-5-cyano-1,2,3-dithiazolium chloride (1b) with aqueous perchloric acid.
Reaction of 1,2,3-dithiazolium chlorides (1) with aqueous ammonia: Typical procedure
Acknowledgements
References and Notes
- Appel, R.; Janssen, H.; Siray, M.; Knoch, F. Chem. Ber. 1985, 118, 1632–1643. [CrossRef]
- Lee, H.; Kim, K.; Whang, D.; Kim, K. J. Org. Chem. 1994, 59, 6179–6183.Jeon, M-K.; Kim, K.; Kim, S. H. Tetrahedron 1998, 54, 2459–2476, and references therein.
- Besson, T.; Rees, C. W. J. Chem. Soc., Perkin Trans. 1 1996, 2857–2860.Besson, T.; Guillard, J.; Rees, C. W.; Thiéry, V. J. Chem. Soc., Perkin Trans. 1 1998, 889–892, and references therein.
- Warburton, W. K. Chem. Rev. 1957, 57, 1011–1020.Schneller, S.W. Int. J. Sulfur Chem. 1976, 8, 579–597.
- Gray, M. A.; Rees, C. W.; Williams, D. J. Heterocycles 1994, 37, 1827–1851.Emayan, K.; Rees, C. W. Bull. Soc. Chim. Belg. 1997, 106, 605–611.
- Barclay, T. M.; Beer, L.; Cordes, A. W.; Oakley, R. T.; Preuss, K. E.; Taylor, N. J.; Reed, R. W. Chem. Commun 1999, 531–532.Beer, L.; Cordes, A. W.; Haddon, R. C.; Itkis, M. E.; Oakley, R. T.; Reed, R. W.; Robertson, C. M. Chem. Commun 2002, 1872–1873.
- Cairns, T. L.; Carboni, R. A.; Coffman, D. D.; Engelhardt, V. A.; Heckert, R. E.; Little, E. L.; McGeer, E. G.; McKusick, B. C.; Middleton, W. J.; Scribner, R. M.; Theobald, C. W.; Winberg, H. E. J. Am. Chem. Soc. 1958, 80, 2775–2778. [CrossRef]
- Ireland, C. J.; Pizey, J. S. J. Chem. Soc., Chem. Commun. 1972, 4.
- White, A. J. P.; Williams, D. J. unpublished results.
- English, R. F.; Rakitin, O. A.; Rees, C. W.; Vlasova, O. G. J. Chem. Soc., Perkin Trans. 1 1997, 201–205.
- Roe, D.G. Ph.D. Thesis, University of London, 1994.
- Rieger, P. H. Electrochemistry, 2nd Edition ed; Chapman & Hall: New York, 1994; p. 184. [Google Scholar]
- Aherne, C. M.; Banister, A. J.; Gorrell, I. B.; Hansford, M. I.; Hauptman, Z. V.; Luke, A. W.; Rawson, J. M. J. Chem. Soc., Dalton Trans. 1993, 967–972.
- Korshak, V. V.; Liseenko, A. F. J. Gen. Chem. USSR (Engl. Transl.) 1939, 9, 1329, [Chem. Abstr., 1940, 34, 741]..
- Hageman, H. A. US Patent 3,832,378, 1974. [Chem. Abstr., 1974, 81, 135485]..
- Rakitin, O. A.; Rees, C. W.; Williams, D. J.; Torroba, T. J. Org. Chem. 1996, 61, 9178–9185.
- Olofson, R. A.; Landesberg, J. M.; Berry, R. O.; Leaver, D.; Robertson, W. A. H.; McKinnon, D. M. Tetrahedron 1966, 22, 2119–2134.
- Markovskii, L. N.; Dubinina, T. N.; Barashenkov, G. C.; Romanenko, E. A.; Shermolovich, Yu. G. Zh. Org. Khim. 1993, 29, 491.
- Koutentis, P. A. 1,2,5-Thiadiazoles and Related Compounds. In Science of Synthesis; Volume 13, Product Class 11; Editors Storr, R. C., Gilchrist, T. L., Eds.; 2003; pp. 297–348. [Google Scholar]
- Weinstock, L. M.; Davis, P.; Handelsman, B.; Tull, R. J. Org. Chem. 1967, 32, 2823–2829.
- Sample Availability: Available from the author.
© 2005 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Koutentis, P. The Preparation and Characterization of 5-Substituted-4-chloro-1,2,3-dithiazolium Salts and their Conversion into 4-Substituted-3-chloro-1,2,5-thiadiazoles. Molecules 2005, 10, 346-359. https://doi.org/10.3390/10020346
Koutentis P. The Preparation and Characterization of 5-Substituted-4-chloro-1,2,3-dithiazolium Salts and their Conversion into 4-Substituted-3-chloro-1,2,5-thiadiazoles. Molecules. 2005; 10(2):346-359. https://doi.org/10.3390/10020346
Chicago/Turabian StyleKoutentis, P. 2005. "The Preparation and Characterization of 5-Substituted-4-chloro-1,2,3-dithiazolium Salts and their Conversion into 4-Substituted-3-chloro-1,2,5-thiadiazoles" Molecules 10, no. 2: 346-359. https://doi.org/10.3390/10020346




