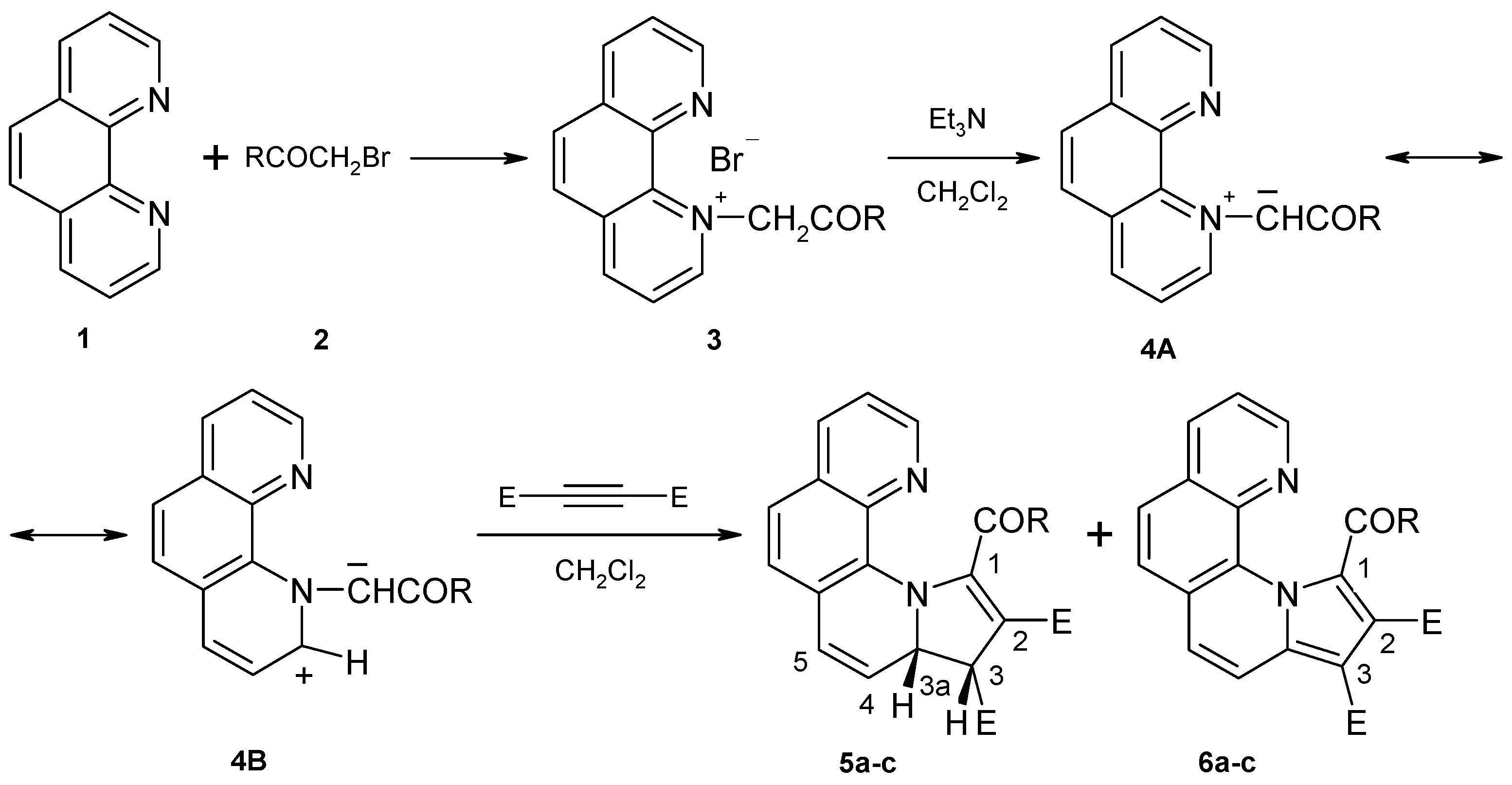
1-(4-Phenylphenacyl)-1,10-phenanthrolinium bromide (3)
1,10-Phenanthroline hydrate (4 g, 20 mmol) and 2’-bromo-4-phenylacetophenone (5.5 g, 20 mmol) were refluxed in acetone (80 mL) for 24 hrs. The precipitate formed was filtered by suction and washed with acetone (50 mL). Yield 75%, m.p. 227-230 ºC (from ethanol); Anal. Calcd. For C26H19BrN2O: C 68.58; H 4.21; Br 17.55; N 6.15. Found C 68.91, H 4.53, Br 17.93; N 6.42; 1H-NMR (DMSO-d6, δ, ppm, J, Hz): 7.36 (bs, 2H, CH2); 7.48-7.51 (m, 1H, H-4”); 7.54-7.59 (m, 2H, H-3”, H-5”); 7.85-7.88 (m, 2H, H-2”, H-6”); 7.91 (dd, 1H, 8.2, 4.3, H-8); 8.04 (d, 2H, 8.5, H-3’, H-5’); 8.28 (d, 2H, 8.5, H-2’, H-6’); 8.48 and 8.51 (2d, 2H, 8.9, H-5, H-6); 8.53 (dd, 1H, 4.3, 1.8, H-9); 8.64 (dd, 1H, 8.2, 5.9, H-3); 8.78 (dd, 1H, 8.2, 1.8, H-7); 9.62 (dd, 1H, 8.2, 1.4, H-4); 9.71 (dd, 1H, 5.9, 1.4, H-2); 13C-NMR (DMSO-d6, δ, ppm): 69.6 (CH2); 124.8 (C-3); 125.5 (C-8); 127.0 (C-6); 127.1 (C-2”, C-6”); 127.4 (C-3’, C-5’); 128.7 (C-4”); 128.9 (C-2’, C-6’); 129.2 (C-3”, C-5”); 130.7 (C-5); 131.5 (C-4a); 132.0 (C-6a); 133.1 (C-1’); 136.3 (C-10b); 138.0 (C-7); 138.4 (C-10a); 138.6 (C-1”); 145.3 (C-4’); 148.1 (C-4); 148.9 (C-9); 152.1 (C-2); 190.2 (COAr).
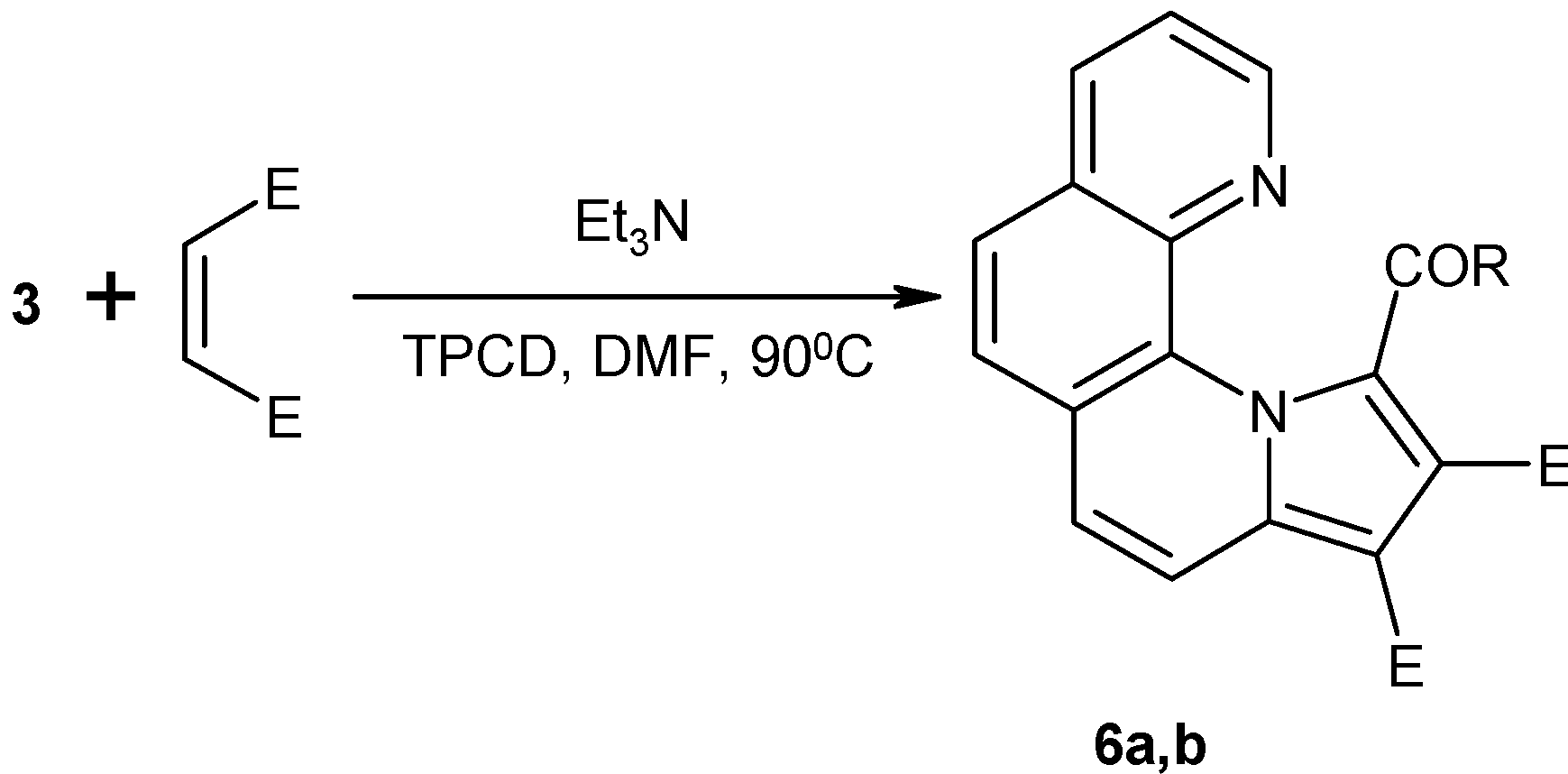
Diesters of 1-(4-phenylbenzoyl)-pyrrolo[1,2-a][1,10]phenanthroline-2,3-dicarboxylate (6a-c): General procedure:
Phenanthrolinium salt 3 (2.3 g, 5 mmol) was suspended in dichloromethane (25 mL) and then dimethyl (diethyl or diisopropyl) acetylenedicarboxylate (5.5 mmol) was added. Under vigorous stirring, triethylamine (0.7 mL, 5 mmol, dissolved in 5 mL of methylene chloride) was added dropwise. After 20 min the reaction mixture was washed twice with water (50 mL) and the solvent evaporated. The residue was refluxed in ethanol for an hour and the precipitate was isolated by filtration.
Dimethyl 1-(4-phenylbenzoyl)-pyrrolo[1,2-a][1,10]phenanthroline-2,3-dicarboxylate (6a). Yellow crystals (from DMF). Yield 60%, m.p. 286-7°C; Anal. Calcd. for C32H22N2O5: C 74.70, H 4.31, N 5.44. Found C 75.02, H 4.62, N 5.71; 1H-NMR (CDCl3+TFA, δ, ppm, J, Hz): 3.68, 3.96 (2s, 6H, CH3); 7.38-7.42 (m, 1H, H-4”); 7.43-7.47 (m, 2H, H-3”, H-5”); 7.50-7.55 (m, 2H, H-2”, H-6”); 7.51 (d, 2H, 8.3, H-3’, H-5’); 7.62 (d, 2H, 8.3, H-2’, H-6’); 7.96 (d, 1H, 9.5, H-5); 8.22 (dd, 1H, 8.2, 6.3, H-9); 8.29, 8.38 (2d, 2H, 8.9, H-6, H-7); 8.58 (d, 1H, 9.5, H-4); 9.15 (dd, 1H, 8.1, 1, H-8); 9.42 (dd, 1H, 6.3, 1, H-10); 13C-NMR (CDCl3+TFA, δ, ppm): 52.7, 53.6 (2 CH3); 96.5 (C-3); 117.7, 119.2, 122.5, 126.4, 126.9, 128.5, 130.5 (C-1, C-2, C-3a, C-5a, C-7a, C-11a, C-11b); 124.3 (C-5); 124.6 (C-9); 124.7 (C-4); 125.9 (C-7); 126.1 (C-3', C-5'); 127.0 (C-2", C-6"); 127.8 (C-2', C-6'); 128.4 (C-4"); 129.0 (C-3", C-5"); 130.2 (C-6); 139.0, 139.4 (C-1', C-1"); 144.4 (C-4'); 144.5 (C-10); 147.1 (C-8); 164.0 (2-CO2Me); 166.3 (3-CO2Me); 184.2 (COAr).
Diethyl 1-(4-phenylbenzoyl)-pyrrolo[1,2-a][1,10]phenanthroline-2,3-dicarboxylate (6b). Yellow crystals (from nitromethane). Yield 84%, m.p. 228-31ºC. Anal. Calcd. for C34H26N2O5: C 75.26, H 4.83, N 5.16. Found C 75.55, H 5.09, N 5.38; 1H-NMR (CDCl3, δ, ppm, J, Hz): 1.07 (t, 3H, 7.1, 2-CH2CH3); 1.38 (t, 3H, 7.1, 3-CH2CH3); 3.77, 3.80 (2q, 1H, 10.2, 7.1, 2-CHAHBCH3); 3.92, 3.95 (2q, 1H, 10.4, 7.1, 2-CHAHBCH3); 4.13-4.47 (m, 2H, 3-CH2CH3); 7.32 (dd, 1H, 8.1, 4.3, H-9); 7.41-7.44 (m, 1H, 7.4, H-4”); 7.47-7.52 (m, 2H, 7.4, H-3”, H-5”); 7.68 (d, 1H, 9.2, H-5); 7.69-7.71 (m, 1H, 7.4, H-2”, H-6”); 7.75 (d, 2H, 8.4, H-3’, H-5’); 7.78 and 7.84 (2d, 2H, 8.6, H-6, H -7); 8.08 (dd, 1H, 4.3, 1.7, H-10); 8.15 (dd, 1H, 8.1, 1.7, H-8); 8.23 (d, 2H, 8.4, H-2’, H-6’); 8.56 (d, 1H, 9.2, H-4); 13C-NMR (CDCl3, δ, ppm): 13.7, 14.3 (2 CH3); 60.2, 61.6 (2 CH2); 104.1 (C-3); 120.3 (C-4); 122.5 (C-9); 125.3 (C-7); 125.7, 125.9, 127.7, 129.0, 130.8, 137.3, 137.4 (C-1, C-2, C-3a, C-5a, C-7a, C-11a, C-11b); 126.0 (C-5); 126.6 (C-3’, C-5’); 126.7 (C-6); 127.3 (C-2”, C-6”); 128.2 (C-4”); 129.0 (C-3”, C-5”); 130.6 (C-2’, C-6’); 136.0 (C-8); 136.8 (C-1’); 140.1 (C-1”); 144.8 (C-4’); 145.8 (C-10); 163.6 (2-CO2Et); 165.6 (3-CO2Et); 184.1 (COAr).
Diisopropyl 1-(4-phenylbenzoyl)-pyrrolo[1,2-a][1,10]phenanthroline-2,3-dicarboxylate (6c). Yellow crystals (from acetonitrile). Yield 80%, m.p. 193-5°C. Anal. Calcd. for C36H30N2O5: C 75.77, H 5.30, N 4.91. Found C 76.1, H 5.58, N 5.07; 1H-NMR (CDCl3, δ, ppm, J, Hz): 0.90, 1.12 (2d, 6H, 6.3, 2-CH(CH3)2); 1.37, 1.40 (2d, 6H, 6.3, 3-CH(CH3)2); 4.78 (sep, 1H, 6.3, 2-CH(CH3)2); 5.32 (sep, 1H, 6.3, 3-CH(CH3)2); 7.32 (dd, 1H, 8.2, 4.3, H-9); 7.41-7.43 (m, 1H, 7.3, H-4”); 7.47-7.52 (m, 2H, 7.3, H-3”, H-5”); 7.68 (d, 1H, 9.2, H-5); 7.69-7.72 (m, 2H, 7.3, H-2”, H-6”); 7.75 (d, 2H, 8.2, H-3’, H-5’); 7.79, 7.86 (2d, 2H, 8.5, H-6, H-7); 8.01 (dd, 1H, 4.3, 1.7, H-10); 8.15 (dd, 1H, 8.2, 1.7, H-8); 8.26 (d, 2H, 8.2, H-2’, H-6’); 8.59 (d, 1H, 9.2, H-4); 13C-NMR (CDCl3, δ, ppm): 20.9, 21.5, 22.0, 22.1 (4 CH3); 67.8, 69.6 (2CH); 104.1 (C-3); 120.4 (C-4); 122.4 (C-9); 125.1 (C-7); 125.6 (C-5); 125.6, 125.8, 127.6, 129.1, 130.8, 137.2, 137.4 (C-1, C-2, C-3a, C-5a, C-7a, C-11a, C-11b); 126.7 (C-6); 126.8 (C-3’, C-5’); 127.0 (C-2”, C-6”); 128.0 (C-4”); 128.9 (C-3”, C-5”); 130.8 (C-2’, C-6’); 135.9 (C-8); 136.7 (C-1’); 140.2 (C-1”); 144.9 (C-4’); 145.6 (C-10); 163.1 (2-CO2iPr); 165.2 (3-CO2iPr); 183.8 (COAr).
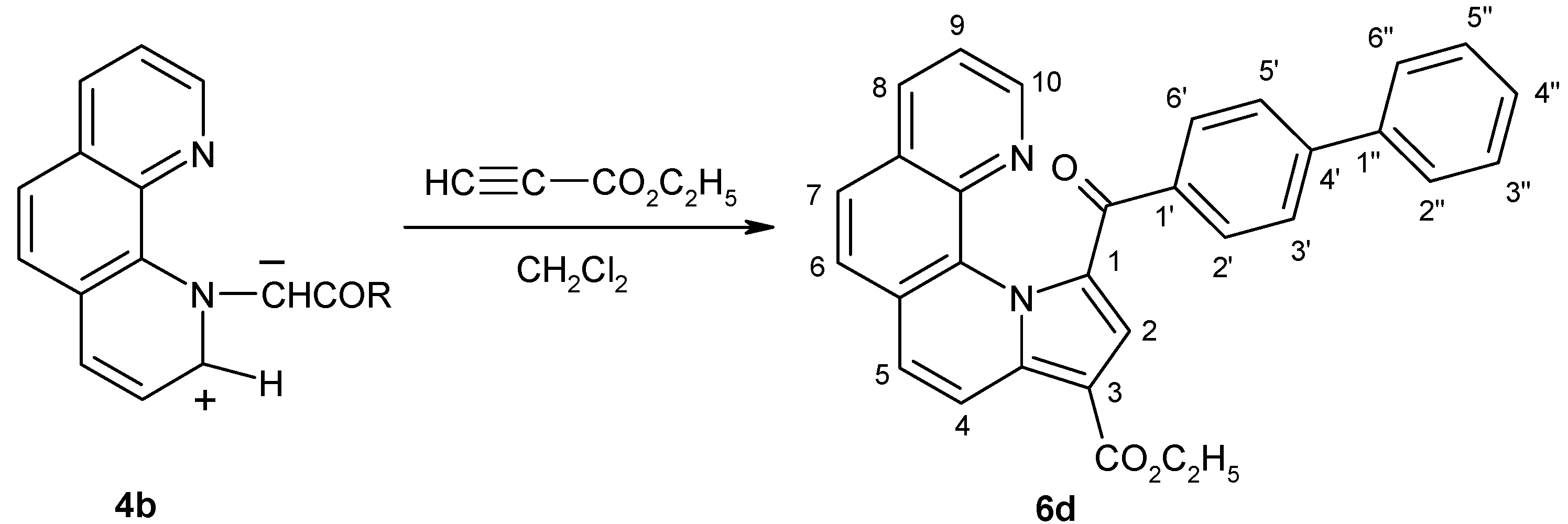
Ethyl 1-(4-phenylbenzoyl)-pyrrolo[1,2-a][1,10]phenanthroline-3-carboxylate (6d)
Phenanthrolinium salt 3 (2.3 g, 5 mmol) was suspended in dichloromethane (25 mL) and then of ethyl propiolate (0.6 mL, 6 mmol) were added. Under vigorous stirring triethylamine (0.75 mL, 5 mmol, dissolved in 5 mL of methylene chloride) were added dropwise. After 20 min the reaction mixture was washed with water (50 mL) and the solvent evaporated. The residue was purified by column chromatography on neutral Al2O3 using CH2Cl2 as eluent. The product was recrystallized from an acetonitrile and ethanol mixture (2:1) to give yellow crystals.Yield 37%, m.p. 234-6°C. Anal. Calcd. for C31H22N2O3: C 79.13, H 4.71, N 5.95. Found C 79.41, H 5.02, N 6.24; 1H-NMR (CDCl3, δ, ppm, J, Hz): 1.44 (t, 3H, 7.1, CH3); 4.38-4.50 (m, 2H, CH2); 7.36 (dd, 1H, 8.2, 4.2, H-9); 7.41-7.47 (m, 1H, H-4"); 7.49-7.56 (m, 2H, H-3", H-5"); 7.65 (s, 1H, H-2); 7.71 (d, 1H, 9.3, H-5); 7.73-7.78 (m, 3H, H-6/H-7, H-2", H-6"); 7.82 (d, 2H, 8.5, H-3', H-5'); 7.87 (d, 1H, 8.6, H-6/H-7); 8.14 (dd, 1H, 8.2, 1.8, H-8); 8.28 (dd, 1H, 4.2, 1.8, H-10); 8.34 (d, 2H, 8.5, H-2', H-6'); 8.60 (d, 1H, 9.3, H-4); 13C-NMR (CDCl3, δ, ppm): 14.5 (CH3); 60.0 (CH2); 106.2 (C-3); 120.0 (C-4); 121.6 (C-2); 122.3 (C-9); 124.9, 126.5 (C-6, C-7); 125.4, 127.7, 129.6, 132.7, 136.5, 138.1 (C-1, C-3a, C-5a, C-7a, C-11a, C-11b); 125.6 (C-5); 126.9 (C-3', C-5'); 127.3 (C-2", C-6"); 128.0 (C-4"); 128.9 (C-3", C-5"); 130.7 (C-2', C-6'); 135.7 (C-8); 138.7, 140.2, 145.0 (C-1', C-4', C-1"); 146.0 (C-10); 164.6 (CO2Et); 184.7 (COAr).