Facile Preparation of Peracetates and Per-3-bromobenzoates of α-Mono- and Disaccharides
Abstract
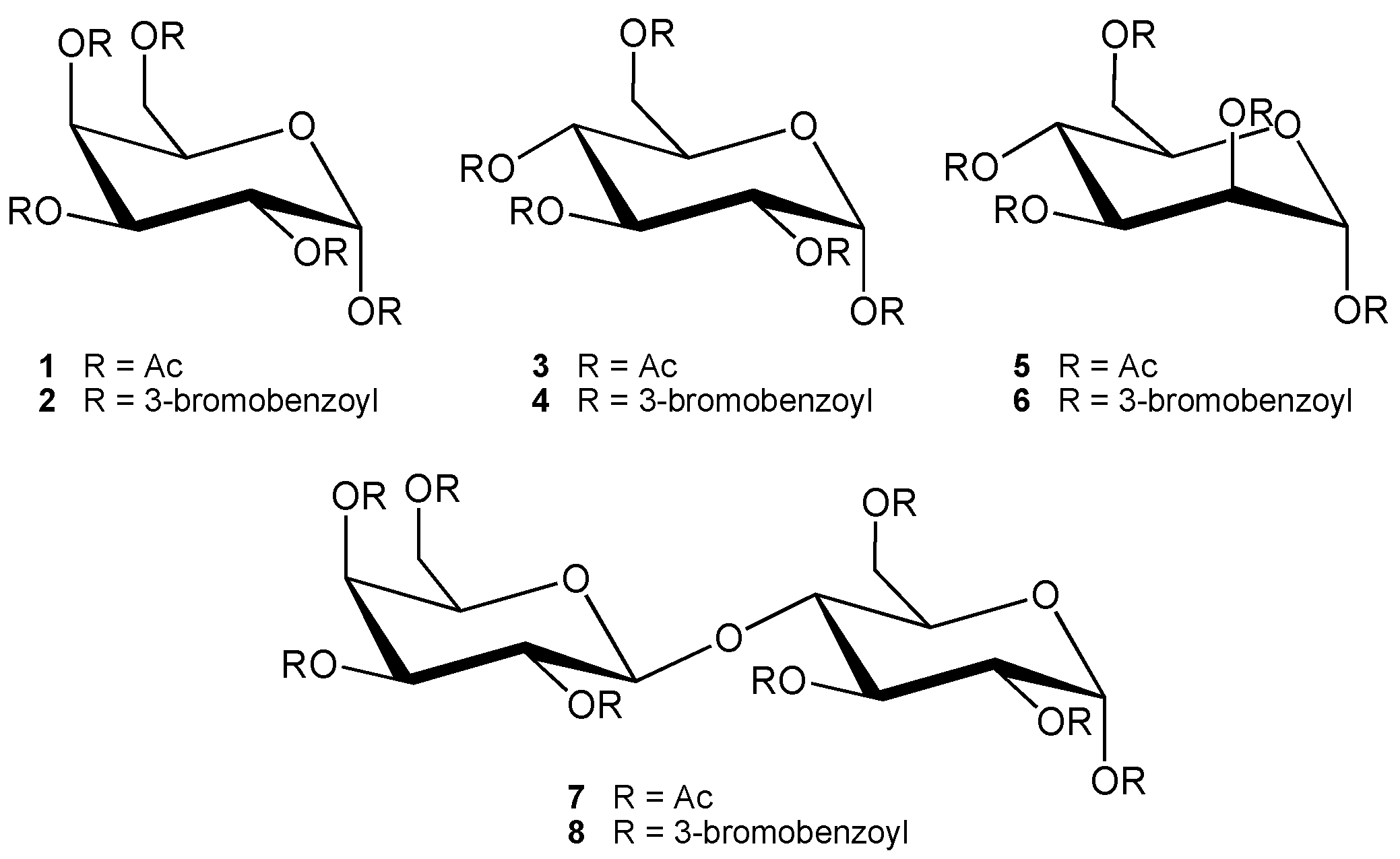
:Introduction
Results and Discussion
Conclusions
| Entry | Sugar | Amount (g) | Acylation Reagent | Amount (mL) | Product a | Rf | Yield(%)b |
|---|---|---|---|---|---|---|---|
| 1 | Galactose | 0.313 (1.74 mmol) | AcCl | 0.68 (5.5 eq) | 0.468 | 0.64 c | 69.0 |
| 2 | Glucose | 0.237 (1.32 mmol) | AcCl | 0.51 (5.4 eq) | 0.391 | 0.61 c | 76.3 |
| 3 | Mannose | 0.158 (0.88 mmol) | AcCl | 0.34 (5.4 eq) | 0.249 | 0.63 c | 72.9 |
| 4 | Lactose | 0.257 (0.75 mmol) | AcCl | 0.54 (10.1 eq) | 0.367 | 0.43 c | 71.9 |
| 5 | Galactose | 0.328 (1.82 mmol) | Ac2O | 0.94 (5.5 eq) | 0.518 | 72.7 | |
| 6 | Glucose | 0.164 (0.91 mmol) | Ac2O | 0.47 (5.5 eq) | 0.257 | 72.3 | |
| 7 | Mannose | 0.159 (0.88 mmol) | Ac2O | 0.46 (5.5 eq) | 0.296 | 85.8 | |
| 8 | Galactose | 0.505 (2.81 mmol) | 3-bromo-BzCl | 2.10 (6.1 eq) | 2.463 | 0.37 d | 80.2 |
| 9 | Glucose | 0.509 (2.83 mmol) | 3-bromo-BzCl | 2.10 (6.1 eq) | 2.310 | 0.45 d | 74.7 |
| 10 | Mannose | 0.504 (2.80 mmol) | 3-bromo-BzCl | 2.10 (6.1 eq) | 2.173 | 0.26 d | 70.9 |
| 11 | Lactose | 0.503 (1.47 mmol) | 3-bromo-BzCl | 1.80 (10.0 eq) | 1.815 | 0.25 d | 68.4 |
Experimental
General
General Experimental Procedure
Spectral Data
Acknowledgements
References and Notes
- Peachey, N. M.; Eckhardt, C. J. Energetics of Organic Solid-State Reactions: The Topochemical Principle and the Mechanism of the Oligomerization of the 2,5-Distyrylpyrazine Molecular Crystal. J. Am. Chem. Soc. 1993, 115, 3519–3526. [Google Scholar] [CrossRef]
- Rothenberg, G.; Downie, A. P.; Raston, C. L.; Scott, J. L. Understanding Solid/Solid Organic Reactions. J. Am. Chem. Soc. 2001, 123, 8701–8708. [Google Scholar]
- Tanaka, K.; Toda, F. Solvent-Free Organic Synthesis. Chem. Rev. 2000, 100, 1025–1074. [Google Scholar]
- Tanaka, Y.; Zhang, Q. W.; Saito, F. Mechanochemical Decomposition of an Aromatic Polyamide Film. Ind. Eng. Chem. Res. 2003, 42, 5018–5023. [Google Scholar] Zhang, Q.; Matsumoto, H.; Saito, F.; Baron, M. Debromination of Hexabromobenzene by Its Co-grinding with CaO. Chemosphere 2002, 48, 787–793. [Google Scholar] Zhang, Q.; Matsumoto, H.; Saito, F. Decomposition of Polytetrafluoroethylene by Grinding with Strontium Oxide. Chem. Lett. 2001, 148–149. [Google Scholar] Zhang, Q. W.; Lu, J. F.; Saito, F.; Baron, M. Mechanochemical Solid-Phase Reaction between Polyvinylidene Fluoride and Sodium Hydroxide. J. Appl. Polym. Sci. 2001, 81, 2249. [Google Scholar]
- Monagheddu, M.; Mulas, G.; Doppiu, S.; Cocco, G.; Raccanelli, S. Reduction of Polychlorinated Dibenzodioxins and Dibenzofurans in Contaminated Muds by Mechanically Induced Combustion Reactions. Environ. Sci. Technol. 1999, 33, 2485–2488. [Google Scholar] Cao, G.; Doppiu, S.; Monagheddu, M.; Orru, R.; Sannia, M.; Cocco, G. Thermal and Mechanochemical Self-Propagating Degradation of Chloro-organic Compounds: The Case of Hexachlorobenzene over Calcium Hydride. Ind. Eng. Chem. Res. 1999, 38, 3218–3224. [Google Scholar] Loiselle, S.; Branca, M.; Mulas, G.; Cocco, G. Selective Mechanochemical Dehalogenation of Chlorobenzenes over Calcium Hydride. Environ. Sci. Technol. 1997, 31, 261–265. [Google Scholar] Hall, A. K.; Harrowfield, J. M.; Hart, R. J.; McCormick, P. G. Mechanochemical Reaction of DDT with Calcium Oxide. Environ. Sci. Technol. 1996, 30, 3401–3407. [Google Scholar] Rowlands, S. A.; Hall, A. K.; McCormick, P. G.; Street, R.; Hart, R. J.; Ebell, G. F.; Donecker, P. Destruction of Toxic Materials. Nature 1994, 367, 223–223. [Google Scholar]
- Schaffer, G. B.; McCormick, P. G. Reduction of Metal Oxides by Mechanical Alloying. Appl. Phys. Lett. 1989, 55, 45. [Google Scholar] Schaffer, G. B.; McCormick, P. G. The Direct Synthesis of Metals and Alloys by Mechanical Alloying. Mater. Sci. Forum 1992, 88-90, 779. [Google Scholar]
- Toda, F. Solid State Organic Chemistry: Efficient Reactions, Remarkable Yields, and Stereoselectivity. Acc. Chem. Res. 1995, 28, 480–486. [Google Scholar] Paul, C.; Curtin, D. Y. Thermally Induced Organic Reactions in the Solid State. Acc. Chem. Res. 1973, 6, 217–225. [Google Scholar] Komatsu, K.; Wang, G. W.; Murata, Y.; Tanaka, T.; Fujiwara, K.; Yamamoto, K.; Saunders, M. Mechanochemical Synthesis and Characterization of the Fullerene Dimer C120. J. Org. Chem. 1998, 63, 9358–9366. [Google Scholar] Schmeyers, J.; Toda, F.; Boy, J.; Kaupp, G. Quantitative Solid–Solid Synthesis of Azomethines. J. Org. Chem. 1998, 63, 9358–9366. [Google Scholar] Singh, N. B.; Singh, N. P.; Kumar, V. A.; Nethaji, M. Study of the Reaction between 1,2,3-Trihydroxybenzene and 8-Hydroxyquinoline in the Solid State. J. Chem. Soc. Perkin Trans. 2 1994, 361–366. [Google Scholar]
- Castricum, H. L.; Bakker, H.; van der Linden, B.; Poels, E. K. Mechanochemical Reactions in Cu/ZnO Catalysts Induced by Mechanical Milling. J. Phys. Chem. B, 2001; 105, 7928–7937. [Google Scholar]
- Collins, P. M.; Ferrier, R. J. Monosaccharides, Their Chemistry and Their Roles in Natural Products; John Wiley & Sons: New York, 1995; pp. 360–369. [Google Scholar]
- Toshima, K.; Tatsuta, K. Recent Progress in O-Glycosylation Methods and Its Application to Natural Products Synthesis. Chem. Rev. 1993, 93, 1503–1531. [Google Scholar] Kováč, P. Efficient Chemical Synthesis of Methyl β-Glycosides of β-(1−6)-Linked D-galacto-oligosaccharides by a Stepwise and a Blockwise Approach. Carbohydr. Res. 1986, 153, 237–251. [Google Scholar]
- Ellervik, U.; Magnusson, G. Guanidine/Guanidinium Nitrate; a Mild and Selective O-Deacetylation Reagent that Leaves the N-Troc Group Intact. Tetrahedron Lett. 1997, 38, 1627–1628. [Google Scholar] Herzig, J.; Nudelman, A.; Gottlieb, H. E.; Fischer, B. Studies in Sugar Chemistry. 2. A Simple Method for O-Deacylation of Polyacylated Sugars. J. Org. Chem. 1986, 51, 727–730. [Google Scholar] Lemieux, R. U.; Stick, R. V. 1,2:5,6-Di-O-isopropylidene-α-D-galactofuranose. Aust. J. Chem. 1975, 28, 1799–1801. [Google Scholar] Lemieux, R. U.; Hendriks, K. B.; Stick, R. V.; James, K. Halide Ion Catalyzed Glycosidation Reactions. Syntheses of α-Linked Disaccharides. J. Am. Chem. Soc. 1975, 97, 4056–4062. [Google Scholar]
- Zemplén, G.; Gerecs, A.; Hadácsy, I. Über die Verseifung Acetylierter Kohlenhydrate. Ber. Dtsch. Chem. Ges. 1936, 69, 1827–1830. [Google Scholar] Zemplén, G.; Pacsu, E. Uber die Verseifung acetylierter Zucker und Verwandter Substanzen. Ber. Dtsch. Chem. Ges. 1929, 62b, 1613–1617. [Google Scholar] Zemplén, G. Abbau der Reduzlerenden Blosen I.: Direk Kohstitutions-Ermittlung der Cellobiose. Ber. Dtsch. Chem. Ges. 1926, 59, 1254–1260. [Google Scholar] Zemplén, G.; Kunz, A. Über die Natriumverbindungen der Glucose und die Verseifung der Aoylierten Zucker. Ber. Dtsch. Chem. Ges. 1923, 56, 1705–1710. [Google Scholar]
- Byramova, N. E.; Ovchinnikov, M. V.; Backinowsky, L. V.; Kochetkov, N. K. Selective Removal of O-Acetyl Groups in the Presence of O-Benzoyl Groups by Acid-Catalysed Methanolysis. Carbohydr. Res. 1983, 124, C8–C11. [Google Scholar]
- Brauns, D. H. Optical Rotation and Atomic Dimension VI. J. Am. Chem. Soc. 1926, 48, 2776–2788. [Google Scholar] Hudson, C. S.; Dale, J. K. The Isomeric Pentacetates of Mannose. J. Am. Chem. Soc. 1915, 37, 1280–1282. [Google Scholar] Erwig, E.; Koenigs, W. Notiz über Pentacetyldextrose. Ber. Dtsch. Chem. Ges. 1989, 24, 1464–1467. [Google Scholar]
- Wolfrom, M. L.; Thompson, A. Methods in Carbohydrate Chemistry; John Wiley & Sons: New York, 1963; Volume 2, p. 211. [Google Scholar] Erwig, E.; Koenigs, W. Ueber fünffach Acetylirte Galactose und Dextrose. Ber. Dtsch. Chem. Ges. 1889, 22, 2207–2214. [Google Scholar] Franchimont, A. P. N. Uber Kohlehydrate. Ber. Dtsch. Chem. Ges. 1979, 12, 1938–1942. [Google Scholar] Liebermann, C.; Hörmann, O. Ueber die Formeln des Rhamnetins und Xanthorhamnins. Ber. Dtsch. Chem. Ges. 1978, 11, 1618–1622. [Google Scholar]
- Christensen, B. E.; Clarke, R. A. Microdtermination of Hydroxyl Content of Sugars and Glycosides. Ind. Eng. Chem. Anal. Ed. 1945, 17, 265–265. [Google Scholar] Freed, M.; Wynne, A. M. Determination of Hydroxyl Groups in Organic Compounds. Ind. Eng. Chem. Anal. Ed. 1936, 8, 278–279. [Google Scholar]
- Fischer, E.; Noth, H. Teilweise Aoylierung der Mehrwertigen Alcohols und Zucker. IV: Derivate der d-Glucose und d-Fructose. Ber. Dtsch. Chem. Ges. 1918, 51, 321–332. [Google Scholar]
- Sen, A. K; Banerji, N. Synthesis of 4-O-beta-D-Xylopyranosyl-D-Glucopyranoses and 6-O-beta-D-Xylopyranosyl-D-Glucopyranoses and Their Protein Conjugates. Indian J. Chem. 1989, 28B, 818–823. [Google Scholar]
- Kartha, K. P. R.; Field, R. A. Iodine: A Versatile Reagent in Carbohydrate Chemistry IV. Per-O-acetylation, Regioselective Acylation and Acetolysis. Tetrahedron 1997, 53, 11753–11766. [Google Scholar] Dauben, W. G.; Vaughan, C. W. A Study of the Reaction of β-D-Glucose Pentaacetate with Phosphorus Pentachloride. J. Am. Chem. Soc. 1955, 77, 1674–1675. [Google Scholar]
- Hudson, C. S.; Johnson, J. M. A Fourth Crystalline Pentaacetate of Galactose and Some Related Compounds. J. Am. Chem. Soc 1916, 38, 1223–1228. [Google Scholar] Hudson, C. S. The Existence of a Third Crystalline Pentacetate of Galactose. J. Am. Chem. Soc. 1915, 37, 1591–1593. [Google Scholar] Hudson, C. S.; Parker, H. O. Conversion of Galactose Pentacetate to an Isomeric Form. J. Am. Chem. Soc. 1915, 37, 1589–1591. [Google Scholar]
- Haines, A. H. Relative Reactivities of Hydroxyl Groups in Carbohydrates. Adv. Carbohydr. Chem. Biochem. 1976, 33, 11–109. [Google Scholar] Carey, F. A.; Hodgson, K. O. Efficient Syntheses of Methyl 2-O-benzoyl-4,6-O-benzylidene-α-D-glucopyranoside and Methyl 2-O-benzoyl-4,6-O-benzyl- idene-α-D-ribo-hexopyranosid-3-ulose. Carbohydr. Res. 1970, 12, 463–465. [Google Scholar] Williams, J. M.; Richardson, A. C. Selective Acylation of Pyranoside—I.: Benzoylation of Methyl α-D-Glycopyranosides of Mannose, Glucose and Galactose. Tetrahedron 1967, 23, 1369–1378. [Google Scholar]
- Eagle, A. J.; Herrington, T. M.; Isaacs, N. S. The Effectiveness of High-pressure in the Syntheses of Pure Hexose Penta-carbamates and Penta-carboxylates. J. Chem. Research (S) 1993, 390, Chem. Research (M) 1993, 2663-2679. [Google Scholar]
- Conchie, J.; Levvy, G. A.; Marsh, C. A. Methyl and Phenyl Glycosides of the Common Sugars. Adv. Carbohydr. Chem. 1957, 12, 157–187. [Google Scholar]
- Fernandez-Lorente, G.; Palomo, J. M.; Cocca, J.; Mateo, C.; Moro, P.; Terreni, M.; Fernandez-Lafuente, R.; Guisan, J. M. Regio-selective Deprotection of Peracetylated Sugars via Lipase Hydrolysis. Tetrahedron 2003, 59, 5705–5711. [Google Scholar] Nóbrega, C.; Vázquez, J. T. Conformational Study of the Hydroxymethyl Group in α-D-mannose Derivatives. Tetrahedron: Assymetr. 2003, 14, 2793–2801. [Google Scholar]
- Sample Availability: Available from the authors.
© 2005 by MDPI (http:www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Wang, Z.; Matin, M.; Sheikh, S. Facile Preparation of Peracetates and Per-3-bromobenzoates of α-Mono- and Disaccharides. Molecules 2005, 10, 1325-1334. https://doi.org/10.3390/10101325
Wang Z, Matin M, Sheikh S. Facile Preparation of Peracetates and Per-3-bromobenzoates of α-Mono- and Disaccharides. Molecules. 2005; 10(10):1325-1334. https://doi.org/10.3390/10101325
Chicago/Turabian StyleWang, Zerong, Mahfuza Matin, and Samia Sheikh. 2005. "Facile Preparation of Peracetates and Per-3-bromobenzoates of α-Mono- and Disaccharides" Molecules 10, no. 10: 1325-1334. https://doi.org/10.3390/10101325




