A Survey on the Reactivity of Phenyliodonium Ylide of 2-Hydroxy-1,4-Naphthoquinone with Amino Compounds
Abstract
:Introduction
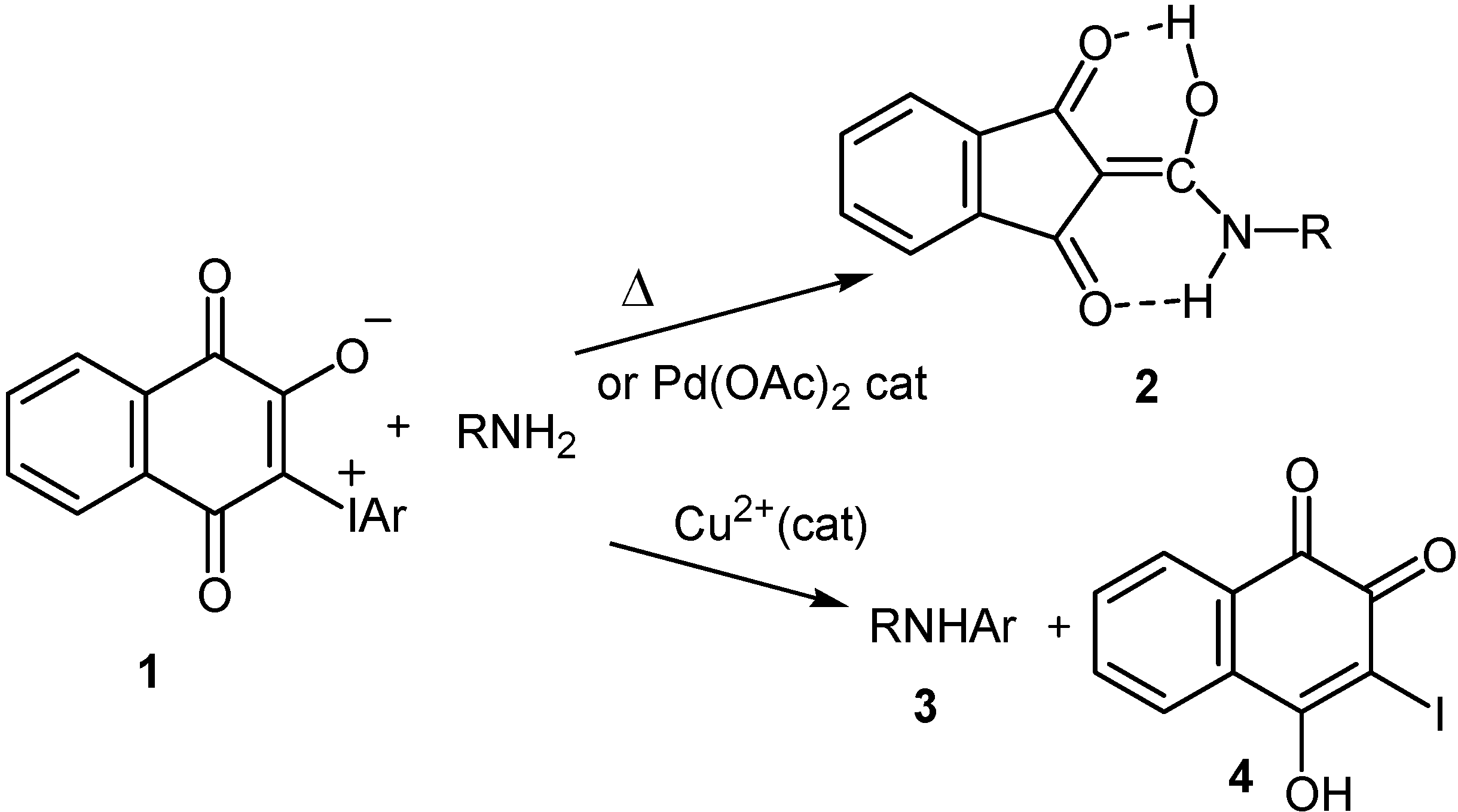
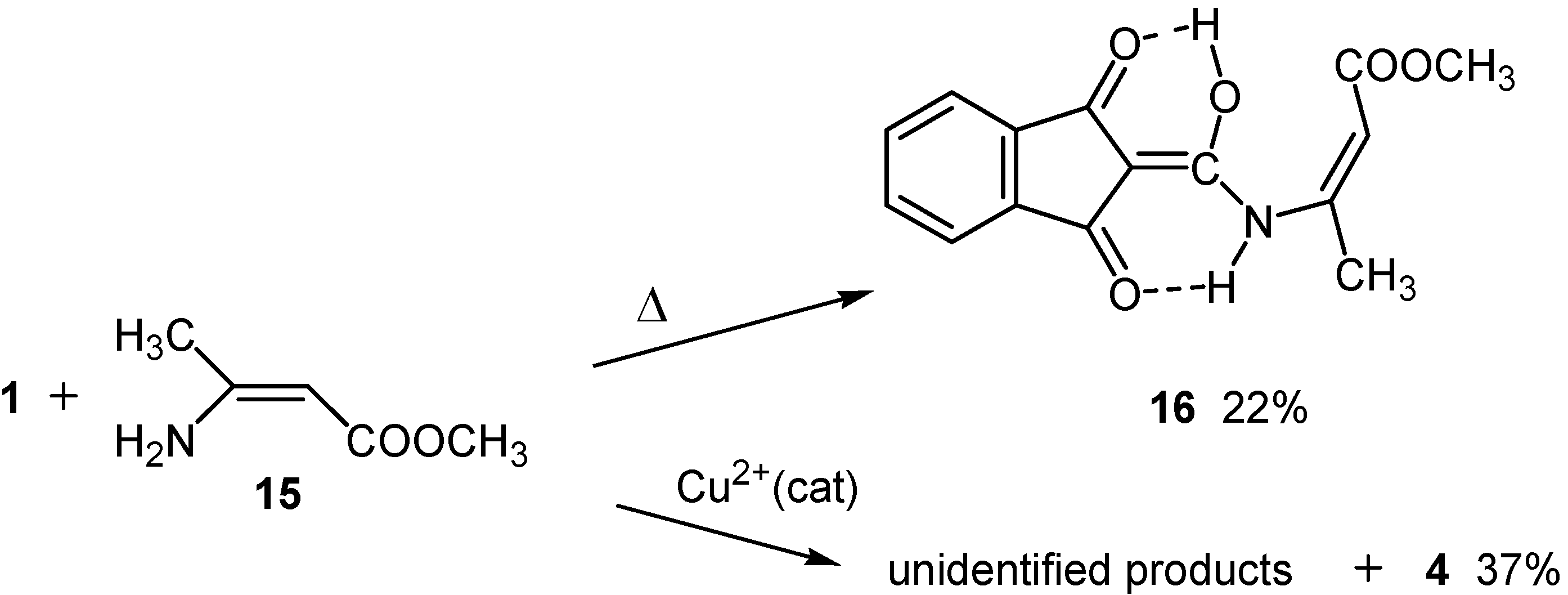
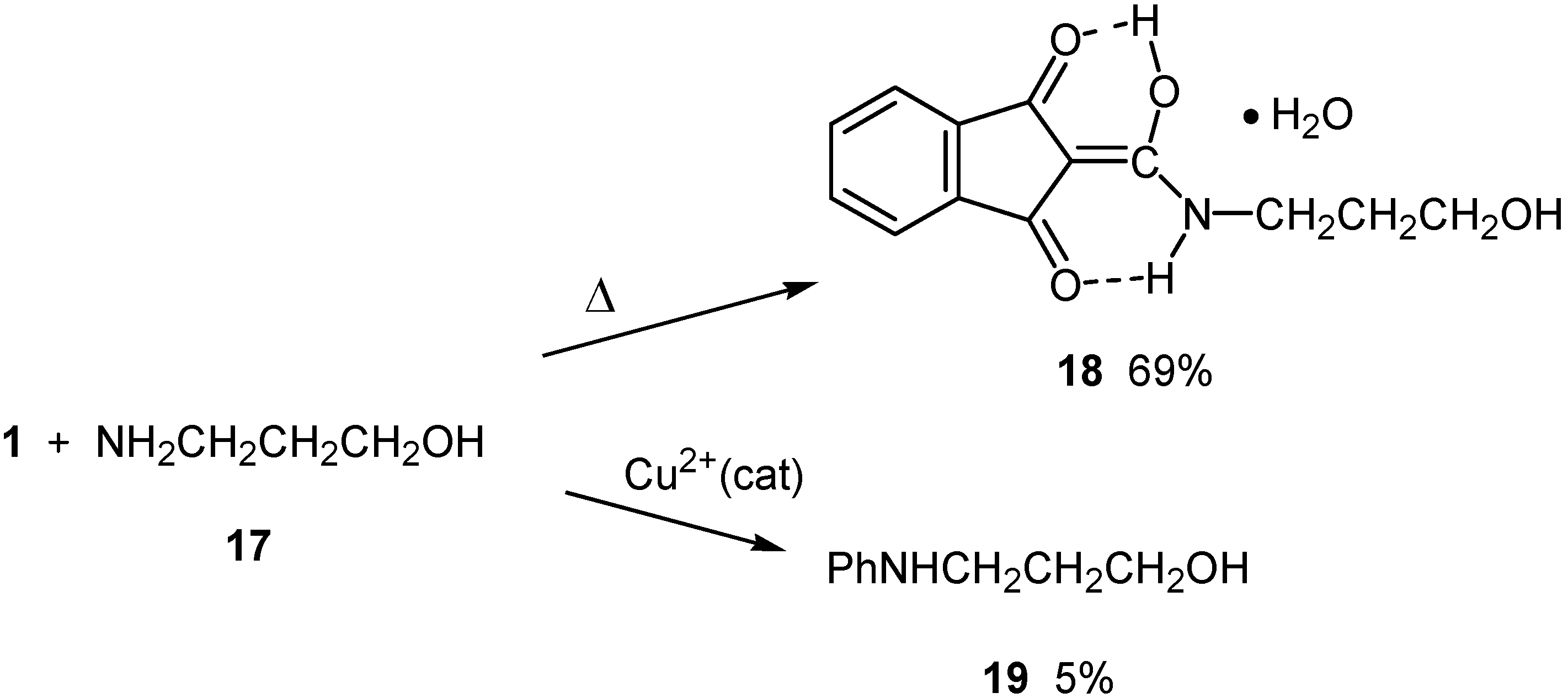
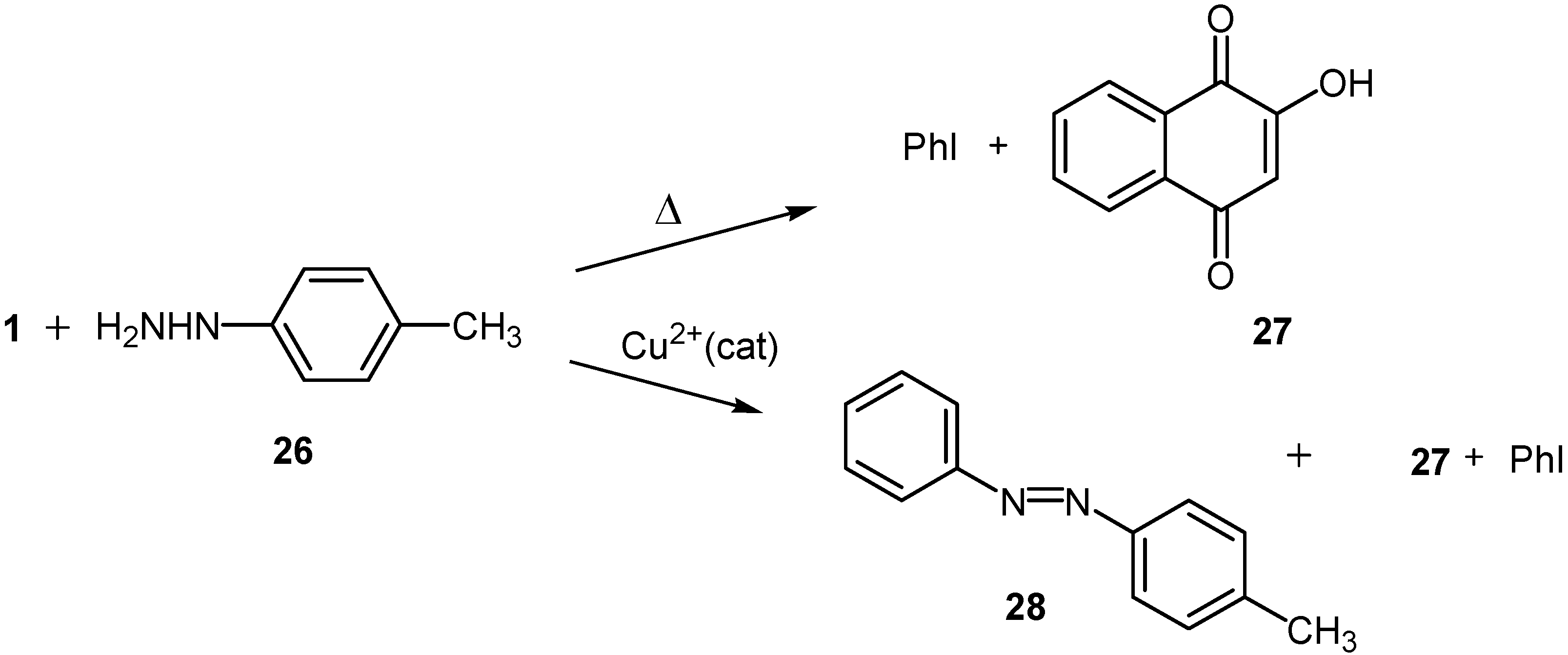
Results and Discussion
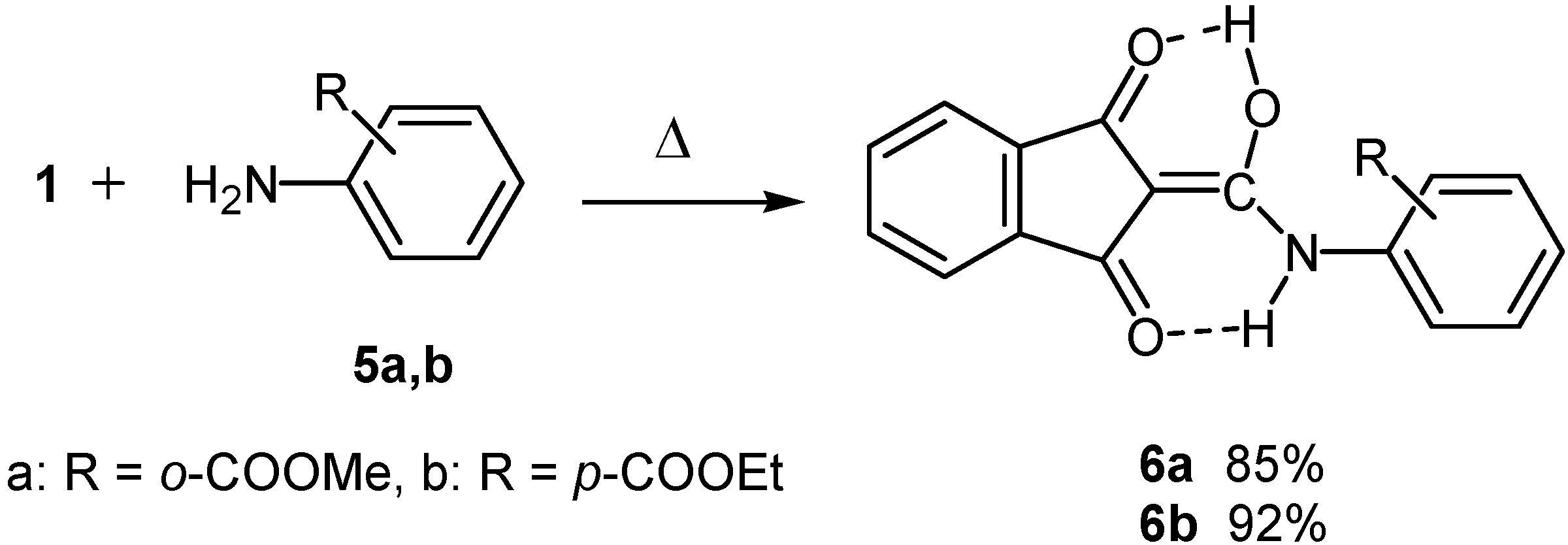
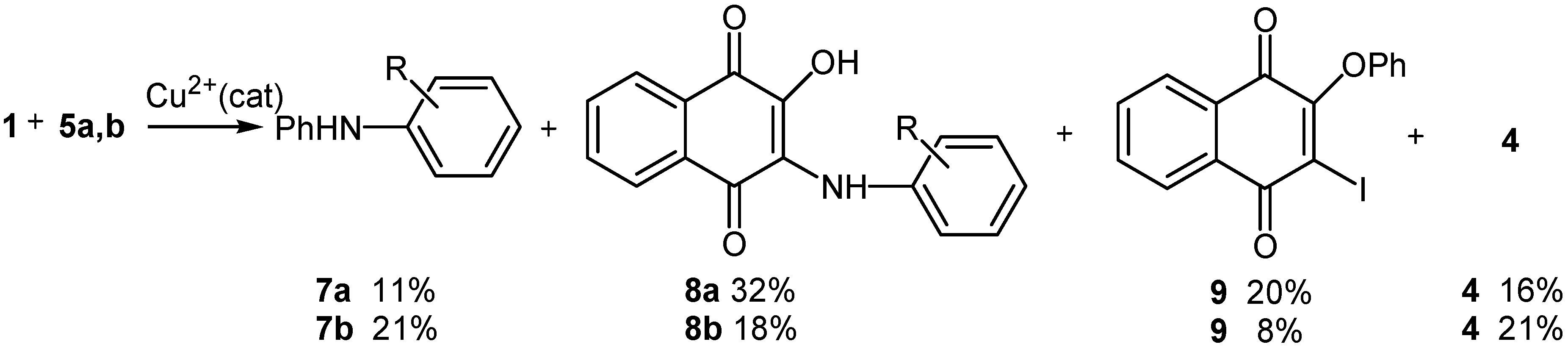
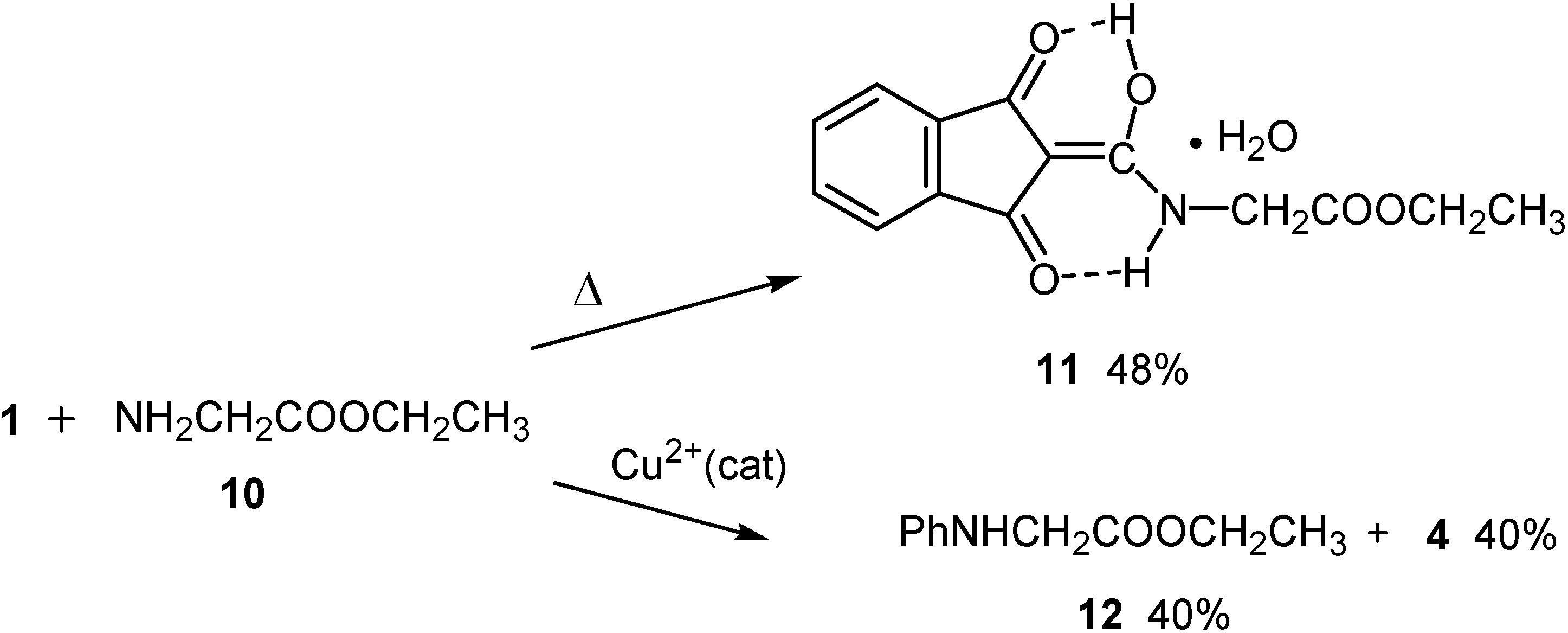



Conclusions
Experimental
General
General procedure for the thermal reaction of 1 with amino compounds.
General procedure for the copper-catalyzed reaction of 1 with amino compounds.
Reaction with methyl 2-aminobenzoate (5a) and ethyl 4-aminobenzoate (5b)
Reaction with ethyl glycinate (10)
Reaction with glycine (13)
Reaction with methyl 3-aminocrotonate (15)
Reaction with 3-aminopropanol (17)
Reaction with 3-aminophenol (20)
Reaction with urea (24)
Reaction with p-tolylhydrazine (26)
References
- Thomson, R.H. Naturally Occurring Quinones IV; Blackie Academic & Professional: London, 1997. [Google Scholar]
- Spyroudis, S. Hydroxyquinones: Synthesis and Reactivity. Molecules 2000, 5, 1291–1330. [Google Scholar]
- Hatzigrigoriou, E.; Spyroudis, S.; Varvoglis, A. Derivatives of 1,4-Naphthoquinone via 3- (phenyliodonio)-1,2,4-trioxo-1,2,3,4-tetrahydronaphthalenide. Liebigs Ann. Chem. 1988, 167–170. [Google Scholar] Papoutsis, I.; Spyroudis, S.; Varvoglis, A. The Chemistry of 2-Oxido-3-phenyliodonio-1,4-benzoquinones: Transformation to 2-Cyclopentene-1,4-diones and Cycloadditions. Tetrahedron Lett. 1994, 35, 8449–8452. [Google Scholar] Kobayashi, K.; Uneda, T.; Kawakita, M.; Morikawa, O.; Konishi, H. One-Pot Synthesis of Naphtho[2,3-b]furan-4,9-diones by Sequential Coupling/Ring Closure Reactions. Tetrahedron Lett. 1997, 38, 837–840. [Google Scholar] Stagliano, K.W.; Malinakova, H.C. Regiospecific Synthesis of Unsymmettrical 2,3-Diarylquinones via Stepwise Pd(0)-Catalyzed Couplings of Arylstananes to Doubly Activated Quinone Equivalents. Tetrahedron Lett. 1997, 35, 6617–6620. [Google Scholar] Stagliano, K.W.; Malinakova, H.C. Regiospesific Synthesis of 2,3-Bisnaphtho-pyranyl Quinones related to Conocurvone. Effect of Substituents on Palladium-Catalyzed Cross-Coupling of Organostannanes to Naphthopyranyl Hydroxyquinone Triflates. J. Org. Chem. 1999, 64, 8034–8040. [Google Scholar] Emadi, A.; Harwod, J.S.; Kohanim, S.; Stagliano, K.W. Regiocontroled Synthesis of the Trimeric Quinone Framework of Conocurvone. Org. Lett. 2002, 4, 521–524. [Google Scholar] Spyroudis, S.; Xanthopoulou, N. Triptycene Quinones in Synthesis: Preparation of Triptycene Cyclopentanedione and Its Reactivity as a Dienophile. J. Org. Chem. 2002, 67, 4612–4614. [Google Scholar] Spyroudis, S.; Xanthopoulou, N. Triptycene Quinones in Synthesis: Preparation of Triptycene Bis- cyclopentanedione. ARKIVOC. 2003, (vi), 95–105. [Google Scholar]
- Malamidou-Xenikaki, E.; Spyroudis, S.; Tsanakopoulou, M. Studies on the Reactivity of 2-Hydroxy-1,4-naphthoquinone: Reaction with Amines. J. Org. Chem. 2003, 68, 5627–5631. [Google Scholar]
- Ma, D.; Zhang, Y.; Yao, J.; Wu, S.; Tao, F. Accelerating Effect Induced by the Structure of α-Amino Acid in the Copper-Catalyzed Coupling Reaction of Aryl Halides with α-Amino Acids. Synthesis of Benzolactam-V8. J. Am. Chem. Soc. 1998, 120, 12459–12467. [Google Scholar]
- Andrews, L.J. Aromatic Molecular Complexes of the Electron Donor-Aceptor Type. Chem. Rev. 1954, 54, 713–776. [Google Scholar]
- Ley, S.V.; Thomas, A.W. Modern Synthetic Methods for Copper-Mediated C(aryl) -O,C(aryl)-N, and C(aryl)-S Bond Formation. Angew. Chem. 2003, 42, 5400–5449. [Google Scholar] Job, G. E.; Buchwald, S.L. Copper-catalyzed Arylation of β-Amino Alcohols. Org. Lett. 2002, 4, 3703–370. [Google Scholar] Lam, P.Y.S.; Bone, D.; Vincent, G.; Clark, C.G.; Combs, A.P. N-Arylation of α- aminoesters with p-tolylboronic acid promoted by copper (II) acetate. Tetrahedron Lett. 2003, 44, 1691–1694. [Google Scholar] Nandakumar, M.V. Copper catalyzed arylation of urea. Tetrahedron Lett. 2004, 45, 1989–1990. [Google Scholar]
- Craig, G. The Nitro and Amino Derivatives of t-Butylbenzene. J. Am. Chem. Soc. 1935, 57, 195–198. [Google Scholar]
- Tien, J.M.; Hunsberger, I.M. The Preparation of Substituted Hydrazines. III. A General Method for Preparing N-Substituted Glycines. J. Am. Chem. Soc. 1955, 77, 6696–6998. [Google Scholar]
- Searles, S.; Gregory, V.P. The Reaction of Trimethyl Oxide with Amines. J. Am. Chem. Soc. 1954, 76, 2789–2890. [Google Scholar]
- Burns, J.; McCombie, H.; Scarborough, H.B. Some Substitution Products of Azobenzene. J. Chem. Soc. 1928, 2929–2933. [Google Scholar]
- Sample availability: Not available.
© 2005 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Spagou, K.; Malamidou-Xenikaki, E.; Spyroudis, S. A Survey on the Reactivity of Phenyliodonium Ylide of 2-Hydroxy-1,4-Naphthoquinone with Amino Compounds. Molecules 2005, 10, 226-237. https://doi.org/10.3390/10010226
Spagou K, Malamidou-Xenikaki E, Spyroudis S. A Survey on the Reactivity of Phenyliodonium Ylide of 2-Hydroxy-1,4-Naphthoquinone with Amino Compounds. Molecules. 2005; 10(1):226-237. https://doi.org/10.3390/10010226
Chicago/Turabian StyleSpagou, Konstantina, Elizabeth Malamidou-Xenikaki, and Spyros Spyroudis. 2005. "A Survey on the Reactivity of Phenyliodonium Ylide of 2-Hydroxy-1,4-Naphthoquinone with Amino Compounds" Molecules 10, no. 1: 226-237. https://doi.org/10.3390/10010226



