Introduction
Thermal efficiency is an important performance characteristic of Brayton cycles and, in practice, has a major impact on operating cost. It is well known that reheating in gas turbine engines limits the extent to which an isothermal heat addition is approached. With respect to simple heat addition, when a compressible gas with subsonic velocity flows through a frictionless constant area duct with heat addition, the temperature of the gas increases along the duct. Also with respect to simple area change, when a compressible fluid/gas with subsonic velocity flows through a frictionless adiabatic duct with decreasing area, the temperature of the gas decreases along the duct. Based on the nature of these two flows, simple heat addition (Rayleigh flow) and simple area change (Isentropic flow) may be combined in such a way so as to yield an isothermal heat addition process [
1]. The idealized isothermal process consists of a compressible gas with subsonic velocity flowing through a frictionless converging duct such that while heated all along the duct, any infinitesimal decrease in temperature due to simple area change is exactly compensated by the simple heat addition. It is noted that, since temperature of the gas is constant during the isothermal heat addition, the kinetic energy of the gas (and hence, the Mach number) must increase in order to satisfy the conservation of energy. The appropriate application of the idealized isothermal process is to gas turbine engines operating with air. It is equally desirable that the Brayton cycle be modified by the isothermal heat addition.
Vecchiarelli et al. [
1] indicated that the hypothetical modification of gas turbine engines including two heat additions (rather than one) may result some efficiency improvement as compared with conventional engines. In recent years, Göktun and Yavuz, [
2], Tyagi, [
3], Tyagi et al., [
4], Erbay et al., [
5] and Kaushik et al, [
6] have studied the effect of isothermal heat addition using open/closed cycle regenerative Brayton heat engines and showed that there is a significant improvement in the thermal efficiency (above 15%) of a Brayton cycle with isothermal heat addition over the conventional Brayton engine.
Angulo-Brown [
7] proposed an ecological criteria E = P - T
LS
gen for finite time Carnot heat engine where P is the power output, T
L is the sink temperature and Sgen is the entropy generation rate in the cycle and found that the thermal efficiency of Carnot heat engine at maximum power output is the average of the Carnot and Curzon-Ahlborn [
8,
9] efficiency. Yan [
10] proposed that it is more reasonable to use ambient temperature (T
0) in place of the sink temperature (T
L) when the sink temperature is not equal to the ambient temperature. Cheng and Chen [
11,
12,
13] and Tyagi et al. [
14] have studied the ecological optimization of endoreversible and irreversible heat engines and found some useful results for these cycles
In this paper we have presented an ecological optimization along with a detailed parametric study of an irreversible regenerative closed cycle Brayton heat engine with isothermal heat addition.
System Description
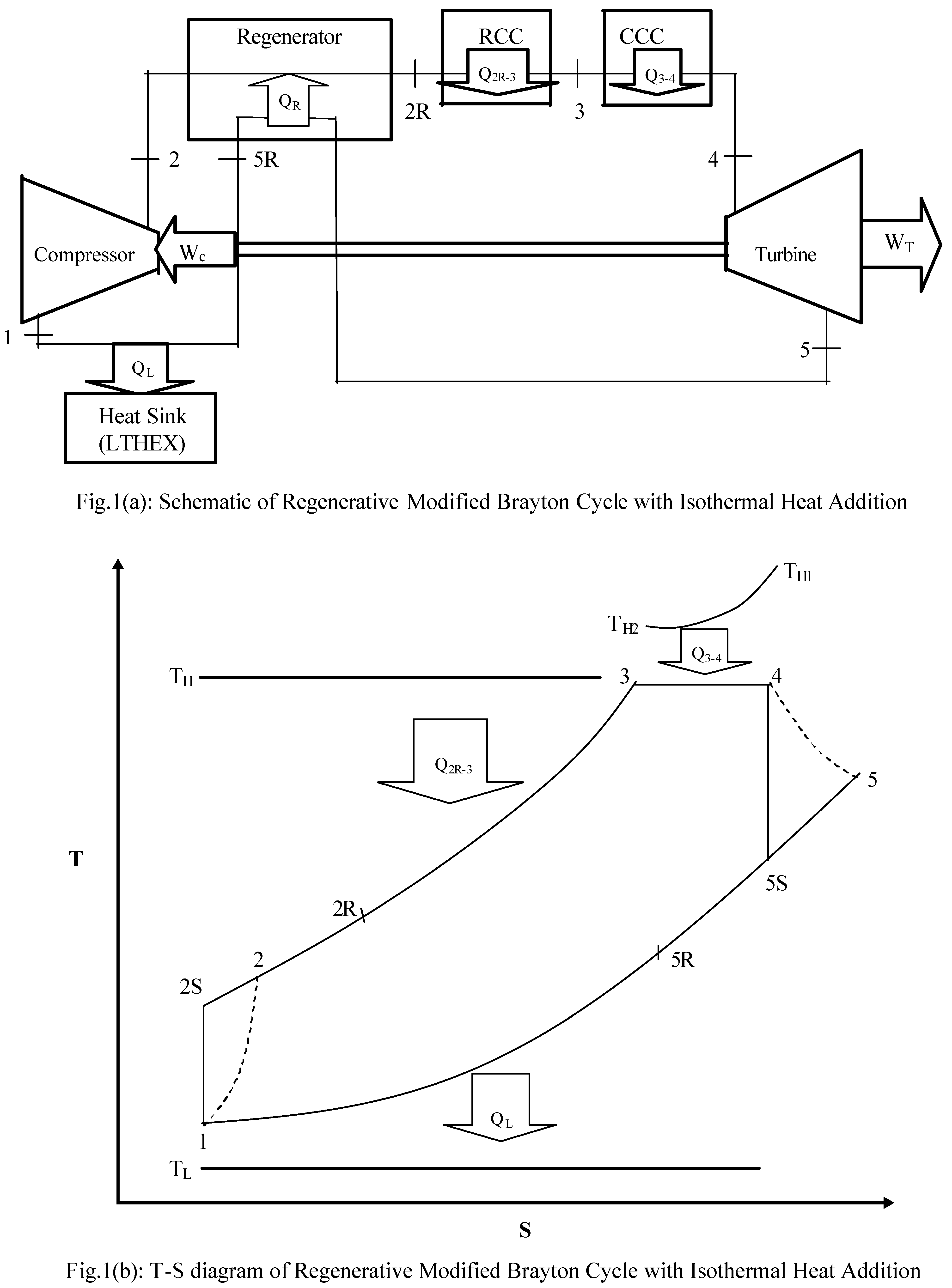
The Schematic and T-S diagrams of closed cycle regenerative Brayton heat engine with isothermal heat addition are shown in
Figs.1(a-b) respectively. The basic components of this cycle are the compressor, regenerator, regular combustion chamber (RCC), converging combustion chamber (CCC), turbine and the heat sink (LTHEX). The gas enters the compressor at state 1 and compressed to state 2 (really) and/or 2S (ideally). At state 2, the cold gas leaving the compressor enters the regenerator, where it is heated to 2R. In an ideal regenerator, the gas leaves the regenerator at the turbine exhaust (state 5) i. e. T
2R = T
5. The isobaric heat addition process takes place between 2R-3 in RCC from an infinite heat capacity source at temperature T
H. Further heat addition is accomplished in the CCC isothermally between state points 3-4 from a heat source of finite heat capacity whose temperature varies from T
H1 to T
H2. When the gas leaves the CCC at state 4 it has lower pressure than at state 3, but the velocity and hence, the kinetic energy of the gas has increased enormously due to the nature of CCC. The gas enters the turbine at state 4, expands nonisentropically to state 5 (ideally to state 5S). The hot gas leaves the turbine at state 5, enters the regenerator where it is cooled to state 5R at constant pressure, by supplying the heat to the regenerator. Finally the working fluid enters the LTHEX at state 5R and cooled to state 1 at constant pressure rejecting the heat to the heat sink at temperature T
L thereby, completing the cycle. Thus we have considered here the theoretical model of a modified regenerative Brayton cycle 1-2-2R-3- 4-5-5R-1 with real processes.
Thermodynamic Analysis
Let QH, QL and QR are the heat transfer rates to and from the heat engine and the regenerative heat transfer rate respectively, then:
where
where C
H is the heat capacitance rate in the external fluids on CCC, and C
W is the heat capacitance rate of the working fluid. U
HA
H, U
H1A
H1, U
LA
L and U
RA
R are the overall heat transfer rates and areas products between the external reservoirs and RCC, CCC, heat sink and in the regenerator respectively. V3 and V4 are the velocities of the working fluid at state points 3 and 4 respectively, m is the mass flow rate of the working fluid and (LMTD)’s are the Log Mean Temperature Difference between the cycle and external reservoirs and defined as:
The isothermal heat addition during process 3-4 can also be defined in terms of Mach number (M) as:
where Vs is the speed of sound and for a perfect gas:
where γ is the adiabatic exponent and R is the gas constant. Using Eqs.(5, 9) and (13) we have:
From Eqs.(4-11) we have:
where ε’s are the effectiveness of the various heat exchangers and defined as:
where N
H, N
H1, N
L and N
R are the number of heat transfer units viz. N
H = U
HA
H/C
W; N
H1 = U
H1A
H1/C
H; N
L = U
LA
L/C
W and N
R = U
RA
R/C
W. The compressor and turbine efficiencies are defined as:
Now from Eqs.(15-24) we get:
But in this case for a Brayton cycle, we have:
where R
T is the cycle temperature ratio, r
t (= p
4/p
3 <1) is the isothermal pressure drop ratio and r
p (= p
2/p
1 >1) is the cycle pressure ratio, p denotes the pressure. From Eq.( 31) we have:
where α = (r
t)
(γ-1)/γ. Substituting the values of T
1, T
2S, T
3 and T
5S from Eqs.(25-30) in Eq.(32) we get a quadratic equation in T
2 (treating T
5 as constant) as:
which on solving for T
2 gives:
where A, B, C are constants and given in the
Appendix. Now by the first law of thermodynamics, we have:
The objective function of ecological optimization proposed by Angulo-Brown [
7] and modified by Yan [
10] is defined as:
where T
0 is the ambient temperature and Sgen is the entropy generation rate. Considering the relationship between the heat transfer and entropy generation rate we have:
Substituting Eqs.(17) and (35) into Eq.(37) which on simplifying yields:
where different pa rameters are given in the
Appendix. Substituting Eq.(34) into Eq.( 38) we see that E is a function of a single variable T
5 (as T
2 is also function of T
5). Thus maximizing E with respect to T
5 i.e.
= 0 yields:
Solving Eq.( 39) for T
5, we get the optimum value of temperature T
5 as:
where the various parameters are given in the
Appendix. Substituting the value of T
5,opt into Eq.(34) the value of T
2,opt and other parameters (viz. E
max, P
m and η
m) can be calculated for a given set of operating parameters.
Discussion of Results
In order to have a numerical appreciation of the results for an irreversible regenerative Brayton heat engine with isothermal heat addition, we have investigated the effects of different cycle parameters (TH, TH1, TL, CH1, CW, εH, εH1, εR, εL,ηT and ηC) on the ecological function and the corresponding power output and thermal efficiency. The effects of each one of these parameters have been examined while the others are kept as constant (viz. TH = 900 K, TH1 = 1250 K, TL = 300 K, T0 = 295 K, εH = εH1 = εR = εL = ηT = ηC = 0.80, CW = 1.05 kW/K and CH1 = 1.0 kW/K) and obtained the following results:
Effects of the different effectiveness
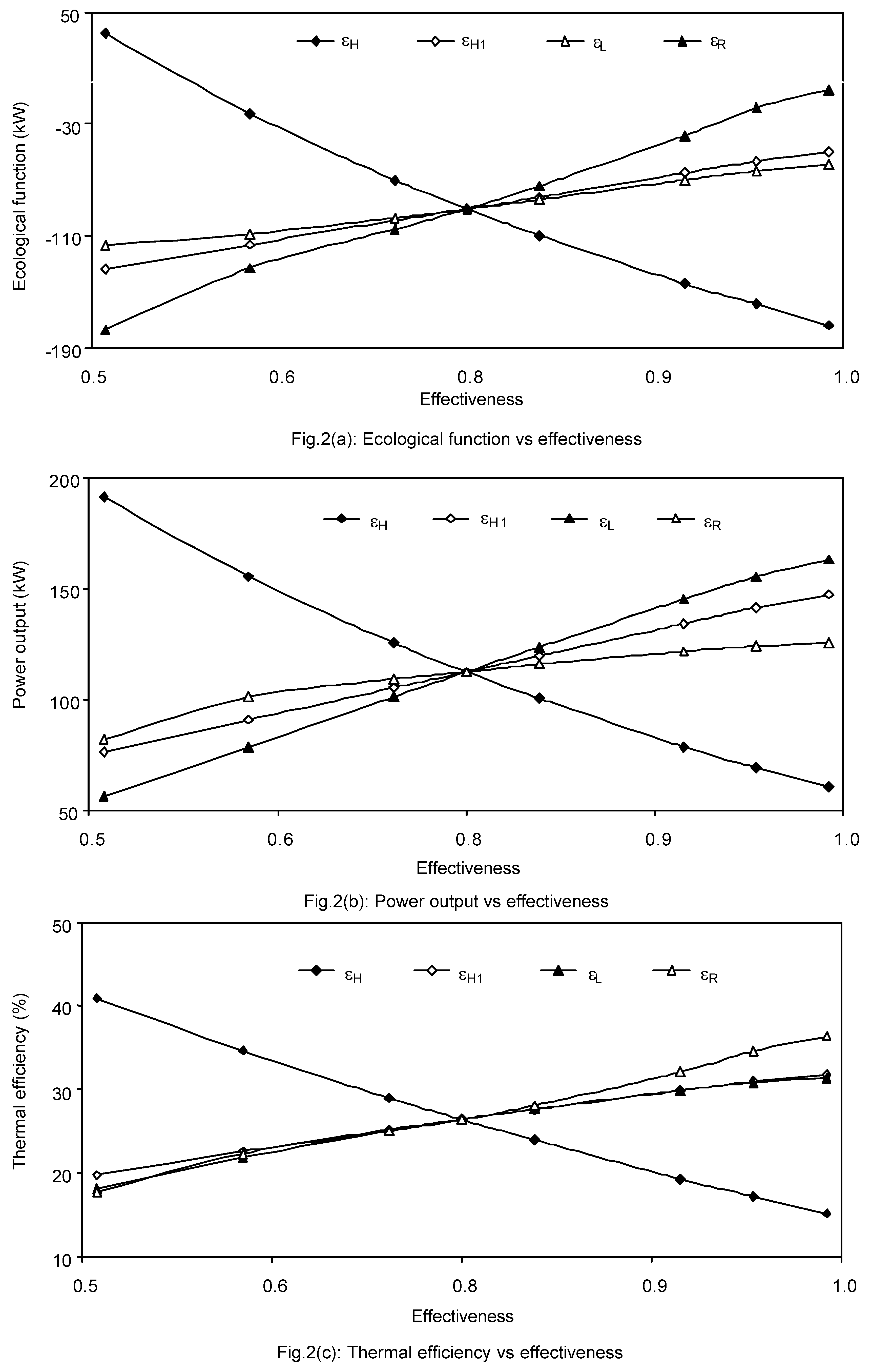
The eff ects of the hot-, regenerative- and the cold-side heat exchangers effectiveness on the maximum ecological function, power output and thermal efficiency are shown in
figures -2(a-c). It is seen from these figures that the maximum ecological function, power output and thermal efficiency increase as the effectiveness on the isothermal-, regenerative- and sink-side heat exchangers increase while all the parameters decrease as the effectiveness on isobaric -side heat exchanger increases. The effect of the isobaric - side effectiveness is found to be more than those of the others side effectiveness on all the performance parameters.
Since, the higher values of (εH, εH1 and εL) decrease the external irreversibility on their respective side heat exchanger by forcing the working fluid to transfer less heat to and from the sink/sources reservoirs by decreasing the heat transfer times and/or the temperature differences. But higher value of εL increases all the parameters than that of the higher value of εH1 while the higher value of εH decreases all the parameters. So it is desirable to have the relation εL > εH1 > εH for the better performance of the cycle and other similar cycles as well.
Again, an irreversible regenerative Brayton cycle with an ideal regenerator is more efficient than that of with a real regenerator but it requires an infinite regenerative time or area, which is not the case in practice. Hence, it would be difficult to conclude new results in the investigation of an irreversible regenerative modified Brayton cycle if the regenerative losses were not considered in the analysis.
Effects of the component efficiencies
The effects of turbine and compressor efficiencies on the maximum ecological function, power output and thermal efficiency are shown in
Fig.3(a-c). It is seen from these figures that the maximum ecological function, power output and thermal efficiency increase as the efficiency of the either component is increased. But the effects of turbine efficiency are more than those of the compressor efficiency on all the performance parameters for the same operating conditions.
Effect of reservoir temperatures
Figure 4 and
Figure 5 show the effects of the different reservoirs temperature on the maximum ecological function, power output and thermal efficiency It is seen from these figures that the maximum ecological function, power output and thermal efficiency increase as the inlet temperature on the isothermal-side reservoir increases while all the parameters decrease as the temperature on the isobaric -side heat reservoir increases. On the other hand, the maximum ecological function increases but the corresponding power output and thermal efficiency decrease as the sink-side reservoir temperature increases. The effects of the isobaric-side reservoir temperature (T
H) are found to be more than those of the isothermal-side inlet temperature (T
H1) and the sink-side temperature (T
L) on all the performance parameters for the same set of operating conditions.
Thus, from these results we can conclude that the temperatures on the isobaric-side and sink-side reservoirs should be low enough and the inlet temperature on the isothermal-side reservoir should be high enough for better performance of this modified Brayton heat engine cycle.
Effect of heat capacitance rates
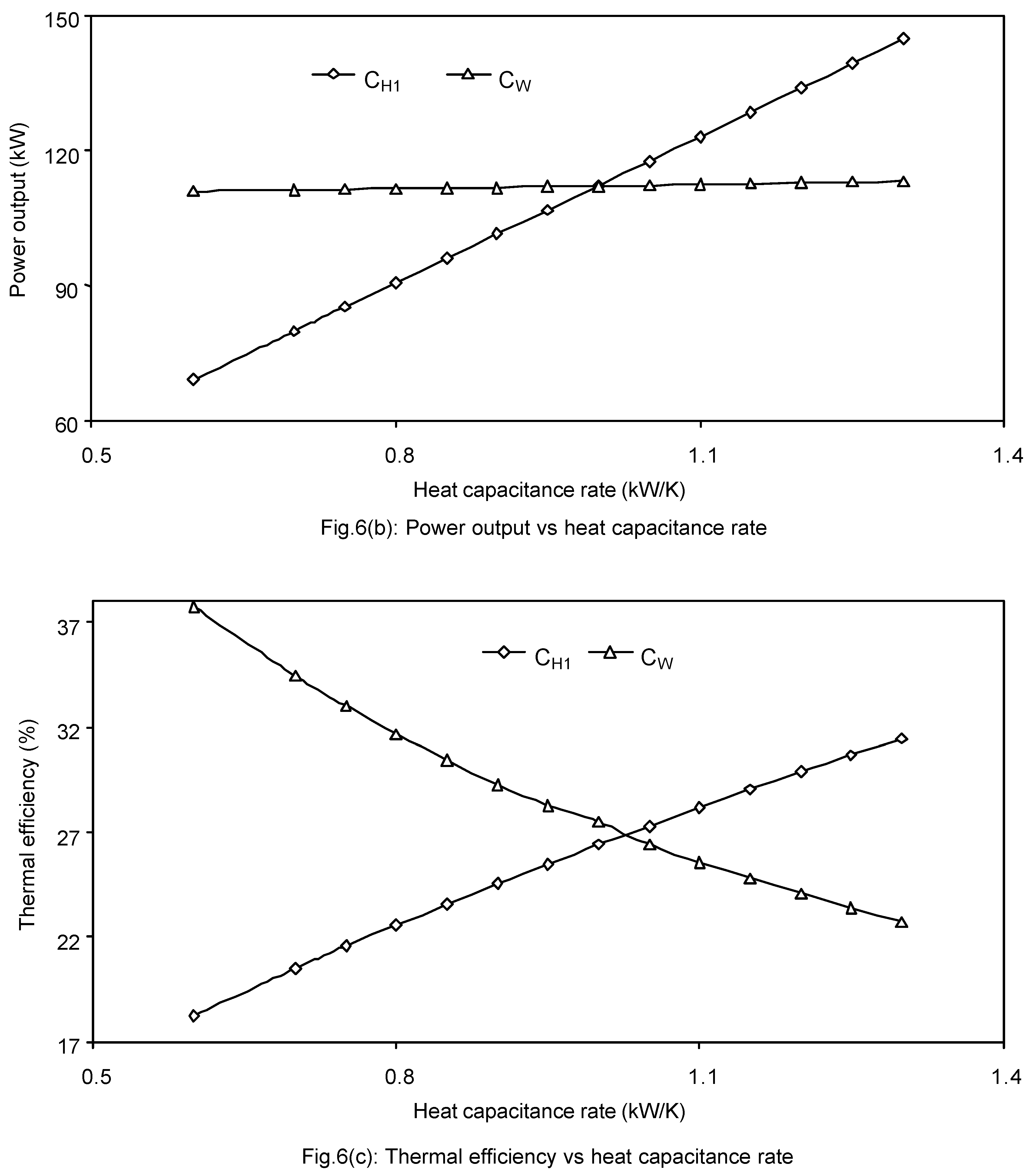
The effects of working fluid and isothermal-side fluid heat capacitance rates on the maximum ecological function, power output and thermal efficiency are shown in
Fig.6(a - c). It is seen from these figures that the maximum ecological function and the corresponding power output and thermal efficiency increase as the heat capacitance rate of the isothermal-side external fluid increases, while the maximum ecological function and thermal efficiency decrease but the power output slightly increases as the heat capacitance rate of the working fluid increases.
Since higher value of heat capacitance rate on the isothermal-side reservoir (CH1) decreases the external irreversibility by forcing the working fluid to transfer heat to and from the cycle by decreasing the heat transfer time and/or the temperature difference between the cycle and the external reservoir. But the higher value of the working heat capacitance rate (CW) increases the external irreversibility on both the sides viz. the cycle absorbs and rejects more heat per unit time which means that a small increment in the power output while a large increment in the heat rejection rate (QL), resulting a loss in the thermal efficiency. Hence, it would be better to have higher CH1 rather than higher CW (i.e. CH1 > CW ) for the better performance of an irreversible regenerative modified Brayton heat engine cycle for a typical set of operating parameters.
Conclusions
An ecological function optimization of an irreversible regenerative Brayton cycle with isothermal heat addition along with the real processes has been carried out using the concept of finite time thermodynamics. The ecological function is maximized with respect to cycle temperatures and the values of the corresponding power output and thermal efficiency are calculated for the optimum operating conditions. The ecological is found to be an increasing function of the components efficiencies, the effectiveness and temperatures on the isothermal-, sink- and the regenerative-side heat exchangers while it is found to be a decreasing function of the working fluid heat capacitance rate and the temperature and effectiveness on the isobaric -side heat exchanger. It is found that the temperature on the isobaric -side heat exchanger should be less than that of the isothermal-side heat exchanger inlet temperature for the better performance of the cycle. Because the heat addition in the former increases the quantity of energy while in the later increases the quality of energy (as there is isothermal heat addition and the heat supplied by the later only increases the kinetic energy rather than the thermal energy unlike the former one). It is also found that the effectiveness on the sink-side heat exchanger and the heat capacitance rate on the isothermal-side heat exchanger should be more than those of the others side effectiveness and temperatures as well as the working fluid heat capacitance rate for the better performance of the cycle. Although an irreversible Brayton cycle with ideal regenerator is more efficient than that of with a real regenerator but it requires an infinite regeneration area or time, which is not the case in practice. Hence, it would be difficult to conclude new results in the investigation of an irreversible regenerative modified Brayton cycle if the regenerative losses were not considered in the analysis. Thus, the present analysis is useful to provide new improvements and modifications for improving the performance in the investigation of an irreversible regenerative modified Brayton heat engine cycle. Some other options like inter-cooling, multistage turbines etc. can also be used for improving the performance of an irreversible regenerative modified Brayton heat engine and other similar cycles too.