Correlation between the Increasing Conductivity of Aqueous Solutions of Cation Chlorides with Time and the “Salting-Out” Properties of the Cations
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Methods
2.2.1. Conductivity Measurements
2.2.2. Analytical Determination of the Impurities
2.3. Course of the Work
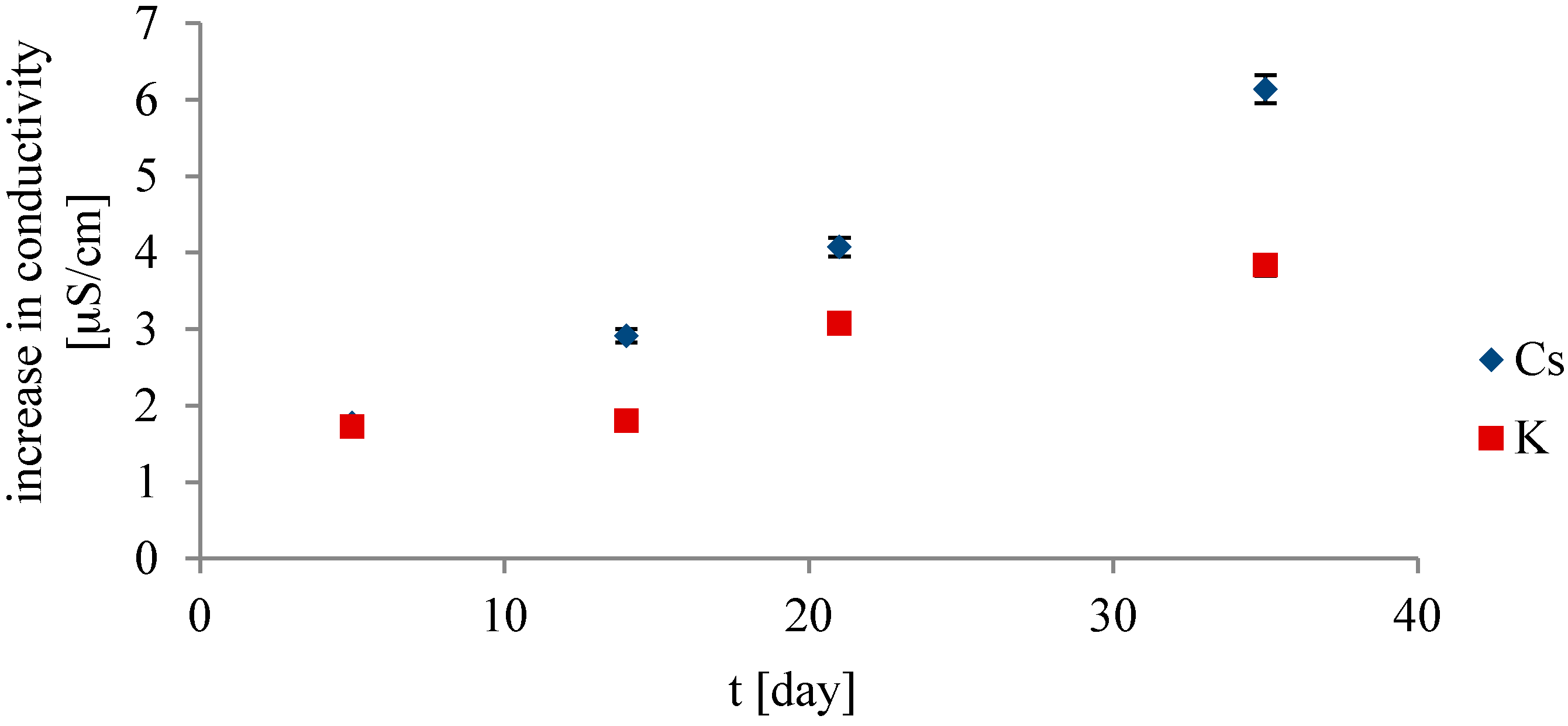
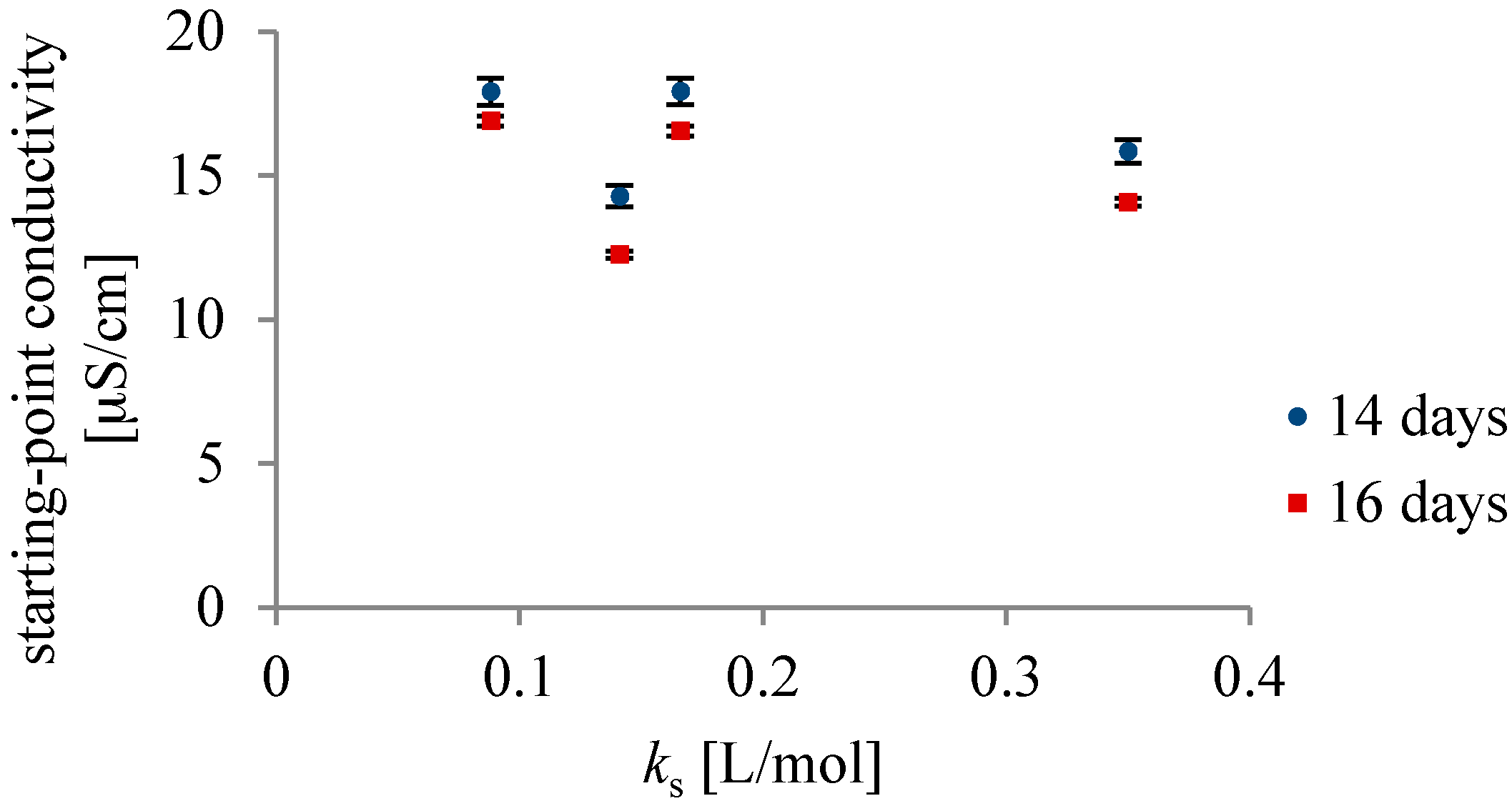
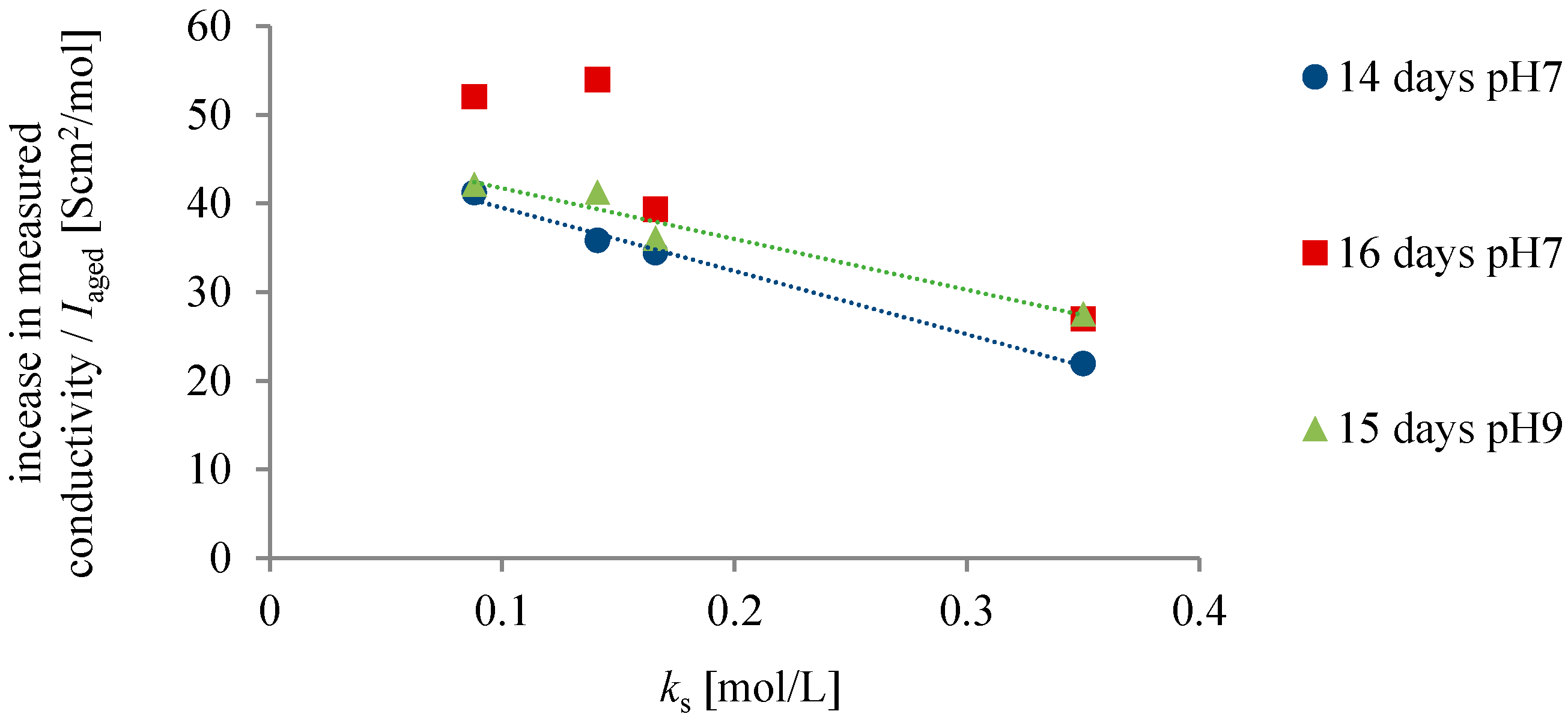
3. Results and Discussion
4. Conclusions
- The highest increase in conductivity at 25 °C with time was measured in chloride solutions of cations with the weakest salting-out effect. A linear correlation between the increase in the conductivity with time and the salting-out effect of the cation was found. The increase in the conductivity values with time cannot be simply attributed to the chemical impurities from leaching out of the glass during ageing or the dissolution of CO2.
- We might ascribe the differences in the increase of the conductivity of cation chloride solutions to the ability of liquid water to spontaneously self-organize into so-called exclusion zones, where the cations with a weak salting-out effect (chaotropes) seem to play a more important role than those that strongly salt-out hydrophobes (kosmotropes).
- The observed increase in the conductivity of long-term-stored solutions of Hofmeister solutes is a thought-provoking finding. The explanation offered, i.e., that the phenomenon is based on the formation of self-organized water forms and the appearance of exclusion zones, should be treated just as one possibility, since there is no direct link between the reported data and the theory.
- In this study the precise time course of the conductivity increase was not studied, and the reported data give no explanation as to why the change would need such a long time to be observed.
- Additionally, the concentration of silica species (mainly sodium silicates) that build up in the vessels during ageing is comparable to that of the salts whose effects are being investigated. Therefore further experiments on the time dependence and with increasing concentration of cation chlorides in order to minimize the influence of the ions that dissolve from glass during ageing are encouraged.
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Baldwin, R.L. How Hofmeister ion interactions affect protein stability. Biophys. J. 1996, 71, 2056–2063. [Google Scholar] [CrossRef]
- Ball, P. Water as an active constituent in cell biology. Chem. Rev. 2008, 108, 74–108. [Google Scholar] [CrossRef] [PubMed]
- Omta, A.W.; Kropman, M.F.; Woutersen, S.; Bakker, H.J. Negligible effect of ions on the hydrogen-bond structure in liquid water. Science 2003, 301, 347–349. [Google Scholar] [CrossRef] [PubMed]
- Paesani, F.; Yoo, S.; Bakker, H.J.; Xantheas, S.S. Nuclear quantum effects in the reorientation of water. J. Phys. Chem. Lett. 2010, 1, 2316–2321. [Google Scholar] [CrossRef]
- Cappa, C.D.; Smith, J.D.; Wilson, K.R.; Messer, B.M.; Gilles, M.K.; Cohen, R.C.; Saykally, R.J. Effects of alkali metal halide salts on the hydrogen bond network of liquid water. J. Phys. Chem. B 2005, 109, 7046–7052. [Google Scholar] [CrossRef] [PubMed]
- Cappa, C.D.; Smith, J.D.; Messer, B.M.; Cohen, R.C.; Saykally, R.J. Effects of cations on the hydrogen bond network of liquid water: New results from X-ray absorption spectroscopy of liquid microjets. J. Phys. Chem. B 2006, 110, 5301–5309. [Google Scholar] [CrossRef] [PubMed]
- Krekeler, C.; Delle Site, L. Solvation of positive ions in water: The dominant role of water-water interaction. J. Phys. Condens. Matter 2007, 19, 192101. [Google Scholar] [CrossRef]
- Chai, B.H.; Zheng, J.M.; Zhao, Q.; Pollack, G.H. Spectroscopic studies of solutes in aqueous solution. J. Phys. Chem. A 2008, 112, 2242–2247. [Google Scholar] [CrossRef] [PubMed]
- Verdel, N.; Jerman, I.; Bukovec, P. The “Autothixotropic” phenomenon of water and its role in proton transfer. Int. J. Mol. Sci. 2011, 12, 7481–7494. [Google Scholar] [CrossRef] [PubMed]
- Verdel, N.; Jerman, I.; Krašovec, R.; Bukovec, P.; Zupančič, M. Possible time-dependent effect of ions and hydrophilic surfaces on the electrical conductivity of aqueous solutions. Int. J. Mol. Sci. 2012, 13, 4048–4068. [Google Scholar] [CrossRef] [PubMed]
- Verdel, N.; Bukovec, P. Possible Further Evidence for the Thixotropic Phenomenon of Water. Entropy 2014, 16, 2146–2160. [Google Scholar] [CrossRef]
- Sedlák, M. Real-time monitoring of the origination of multimacroion domains in a polyelectrolyte solution. J. Chem. Phys. 2005, 122, 151102. [Google Scholar] [CrossRef] [PubMed]
- Sedlák, M. Large-scale supramolecular structure in solutions of low molar mass compounds and mixtures of liquids: I. Light scattering characterization. J. Phys. Chem. B 2006, 110, 4329–4338. [Google Scholar] [CrossRef] [PubMed]
- Sedlák, M. Large-scale supramolecular structure in solutions of low molar mass compounds and mixtures of liquids: II. Kinetics of the formation and long-time stability. J. Phys. Chem. B 2006, 110, 4339–4345. [Google Scholar] [CrossRef] [PubMed]
- Sedlák, M. Large-scale supramolecular structure in solutions of low molar mass compounds and mixtures of liquids. III. Correlation with molecular properties and interactions. J. Phys. Chem. B 2006, 110, 13976–13984. [Google Scholar] [CrossRef] [PubMed]
- Sedlák, M.; Rak, D. Large-scale inhomogeneities in solutions of low molar mass compounds and mixtures of liquids: Supramolecular structures or nanobubbles? J. Phys. Chem. B 2013, 117, 2495–2504. [Google Scholar] [CrossRef] [PubMed]
- Elia, V.; Niccoli, M. Thermodynamics of extremely diluted aqueous solutions. Ann. N. Y. Acad. Sci. 1999, 879, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Elia, V.; Napoli, E.; Niccoli, M.; Marchettini, N.; Tiezzi, E. New physico—Chemical properties of extremely diluted solutions. A conductivity study at 25 °C in relation to ageing. J. Solut. Chem. 2008, 37, 85–96. [Google Scholar] [CrossRef]
- Elia, V.; Elia, L.; Montanino, M.; Napoli, E.; Niccoli, M.; Nonatelli, L. Conductometric studies of the serially diluted and agitated solutions on an anomalous effect that depends on the dilution process. J. Mol. Liq. 2007, 135, 158–165. [Google Scholar] [CrossRef]
- Xantheas, S.S. Computational chemistry: Dances with hydrogen cations. Nature 2009, 457, 673–674. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Zhou, X.; Liu, H. Ab initio simulation on the mechanism of proton transport in water. J. Power Sources 2006, 161, 1420–1427. [Google Scholar] [CrossRef]
- Vybíral, B. The Comprehensive Experimental Research on the Autothixotropy of Water. In Water and the Cell; Pollack, G.H., Cameron, I.L., Wheatley, D.N., Eds.; Springer: New York, NY, USA, 2006; pp. 299–314. [Google Scholar]
- Hribar, B.; Southall, N.T.; Vlachy, V.; Dill, K.A. How ions affect the structure of water. J. Am. Chem. Soc. 2002, 124, 12302–12311. [Google Scholar] [CrossRef] [PubMed]
- MacInnes, D.A. Principles of Electrochemistry; Reinhold Publishing Corporation: New York, NY, USA, 1939. [Google Scholar]
- Zheng, J.M.; Chin, W.C.; Khijniak, E.; Khijniak, E., Jr.; Pollack, G.H. Surfaces and interfacial water: Evidence that hydrophilic surfaces have long-range impact. Adv. Colloid Interface Sci. 2006, 127, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Higashitani, K.; Okuhara, K.; Hatade, S. Effects of magnetic fields on stability of nonmagnetic ultrafine colloidal particles. J. Colloid Interface Sci. 1992, 152, 125–131. [Google Scholar] [CrossRef]
- Higashitani, K.; Iseri, H.; Okuhara, K.; Kage, A.; Hatade, S. Magnetic effects on zeta potential and diffusivity of nonmagnetic colloidal particles. J. Colloid Interface Sci. 1995, 172, 383–388. [Google Scholar] [CrossRef]
- Higashitani, K.; Oshitani, J.; Ohmura, N. Effects of magnetic field on water investigated with fluorescent probes. J. Colloids Surfaces A Physicochem. Eng. Asp. 1996, 109, 167–173. [Google Scholar] [CrossRef]
- Higashitani, K.; Oshitani, J. Magnetic effect on the interface between aqueous solution and solid. J. Surf. Sci. Soc. Jpn. 1999, 20, 764–769. [Google Scholar] [CrossRef]
- Holysz, L.; Szczes, A.; Chibowski, E. Effects of a static magnetic field on water and electrolyte solutions. J. Colloid Interface Sci. 2007, 316, 996–1002. [Google Scholar] [CrossRef] [PubMed]
- Amiri, M.C.; Dadkhah, A.A. On reduction in the surface tension of water due to magnetic treatment. Colloids Surf. A Physicochem. Eng. Asp. 2006, 278, 252–255. [Google Scholar] [CrossRef]
- Deng, B.; Pang, X.F. The variations of optical features of water after action of static magnetic-field. Chin. Sci. Bull. 2007, 52, 3179–3182. [Google Scholar] [CrossRef]
- Guo, Y.Z.; Yin, D.C.; Cao, H.L.; Shi, J.Y.; Zhang, C.Y.; Liu, Y.M.; Huang, H.H.; Liu, Y.; Wang, Y.; Guo, W.H.; et al. Evaporation rate of water as a function of a magnetic field and field gradient. Int. J. Mol. Sci. 2012, 13, 16916–16928. [Google Scholar] [CrossRef] [PubMed]
- Inaba, H.; Saitou, T.; Tozaki, K.; Hayashi, H. Effect of the magnetic field on the melting transition of H2O and D2O measured by a high resolution and supersensitive differential scanning calorimeter. J. Appl. Phys. 2004, 96, 6127–6133. [Google Scholar] [CrossRef]
- Szcześ, A.; Chibowski, E.; Hołysz, L.; Rafalski, P. Effects of static magnetic field on water at kinetic condition. Chem. Eng. Process. 2011, 50, 124–127. [Google Scholar] [CrossRef]
- De Ninno, A.; Congiu Castellano, A. Influence of magnetic fields on the hydration process of amino acids: Vibrational spectroscopy study of L-phenylalanine and L-glutamine. Bioelectromagnetics 2014, 35, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Cai, R.; Yang, H.; He, J.; Zhu, W. The effects of magnetic fields on water molecular hydrogen bonds. J. Mol. Struct. 2009, 938, 15–19. [Google Scholar] [CrossRef]
| Ion | Na | K | Mg | Ca | Si | Al | pH |
|---|---|---|---|---|---|---|---|
| μmol/L | μmol/L | μmol/L | μmol/L | μmol/L | μmol/L | / | |
| Average | 26 | 3.9 | 1.5 | 2.5 | 26.6 | 0.3 | 9 |
| Standard Deviation | / | 0.9 | 0.08 | / | / | 0.04 | / |
| Ion | Na | K | Mg | Ca | Si | Δσm | Δσt | σE | ∆σ/Iaged |
|---|---|---|---|---|---|---|---|---|---|
| μmol/L | μmol/L | μmol/L | μmol/L | μmol/L | μS/cm | μS/cm | μS/cm | S cm2/mol | |
| CsCl | 80.9 | 6.9 | 4.5 | 8.2 | 80 | 7.3 | 6.0 | 1.3 | 41 |
| LiCl | 76.4 | 6.0 | 4.4 | 7.7 | 85 | 6.2 | 5.7 | 0.5 | 36 |
| KCl | 75.9 | 6.9 | 4.1 | 7.0 | 69 | 5.9 | 5.6 | 0.3 | 35 |
| MgCl2 | 72.0 | 9.2 | 4.5 | 5.7 | 65 | 4.8 | 5.4 | −0.6 | 22 |
| Ion | Na | K | Mg | Ca | Si | Δσm | Δσt | σE | ∆σ/Iaged |
|---|---|---|---|---|---|---|---|---|---|
| μmol/L | μmol/L | μmol/L | μmol/L | μmol/L | μS/cm | μS/cm | μS/cm | S cm2/mol | |
| CsCl | 78.8 | 0 | 4.1 | 7.7 | 75 | 8.8 | 5.3 | 3.6 | 52 |
| LiCl | 78.8 | 0 | 4.1 | 7.0 | 85 | 9.1 | 5.2 | 4.0 | 54 |
| KCl | 76.6 | 0 | 4.1 | 7.2 | 79 | 6.6 | 5.1 | 1.5 | 39 |
| MgCl2 | 70.1 | 0 | 4.1 | 5.5 | 60 | 5.7 | 4.6 | 1.1 | 27 |
| Ion | Na | K | Mg | Ca | Si | Al | pH | OH− | Si-ions * |
|---|---|---|---|---|---|---|---|---|---|
| μmol/L | μmol/L | μmol/L | μmol/L | μmol/L | μmol/L | / | μmol/L | μmol/L | |
| CsCl | 69.6 | 10.2 | 4.5 | 7.5 | 83 | 4.3 | 8.9 | 7.9 | 6.6 |
| LiCl | 69.6 | 6.6 | 4.5 | 7.5 | 96 | 4.7 | 8.9 | 7.9 | 7.7 |
| KCl | 74.0 | 8.4 | 4.5 | 7.5 | 93 | 4.7 | 8.9 | 7.9 | 7.4 |
| MgCl2 | 65.3 | 7.2 | 4.5 | 10.0 | 70 | 3.4 | 8.6 | 4.0 | 2.8 |
| Ion | σ0 | σ1 | σ15 | ∆σ * | ∆σt ** | σE | Iaged *** | ∆σ/Iaged |
|---|---|---|---|---|---|---|---|---|
| μS/cm | μS/cm | μS/cm | μS/cm | μS/cm | μS/cm | μmol/L | S cm2/mol | |
| Cs | 15.6 | 20.9 ± 0.2 | 29.1 ± 0.3 | 8.2 | 4.7 | 3.4 | 194 | 42 |
| Li | 11.2 | 16.3 ± 0.2 | 24.4 ± 0.5 | 8.1 | 4.8 | 3.3 | 196 | 41 |
| K | 14.8 | 21.1 ± 0.2 | 28.2 ± 0.3 | 7.1 | 4.7 | 2.3 | 196 | 36 |
| Mg | 12.8 | 17.8 ± 0.1 | 24.3 ± 0.2 | 6.5 | 4.2 | 2.3 | 236 | 28 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Verdel, N.; Bukovec, P. Correlation between the Increasing Conductivity of Aqueous Solutions of Cation Chlorides with Time and the “Salting-Out” Properties of the Cations. Entropy 2016, 18, 66. https://doi.org/10.3390/e18030066
Verdel N, Bukovec P. Correlation between the Increasing Conductivity of Aqueous Solutions of Cation Chlorides with Time and the “Salting-Out” Properties of the Cations. Entropy. 2016; 18(3):66. https://doi.org/10.3390/e18030066
Chicago/Turabian StyleVerdel, Nada, and Peter Bukovec. 2016. "Correlation between the Increasing Conductivity of Aqueous Solutions of Cation Chlorides with Time and the “Salting-Out” Properties of the Cations" Entropy 18, no. 3: 66. https://doi.org/10.3390/e18030066





