Synthesis and Surface Thermodynamic Functions of CaMoO4 Nanocakes
Abstract
:1. Introduction
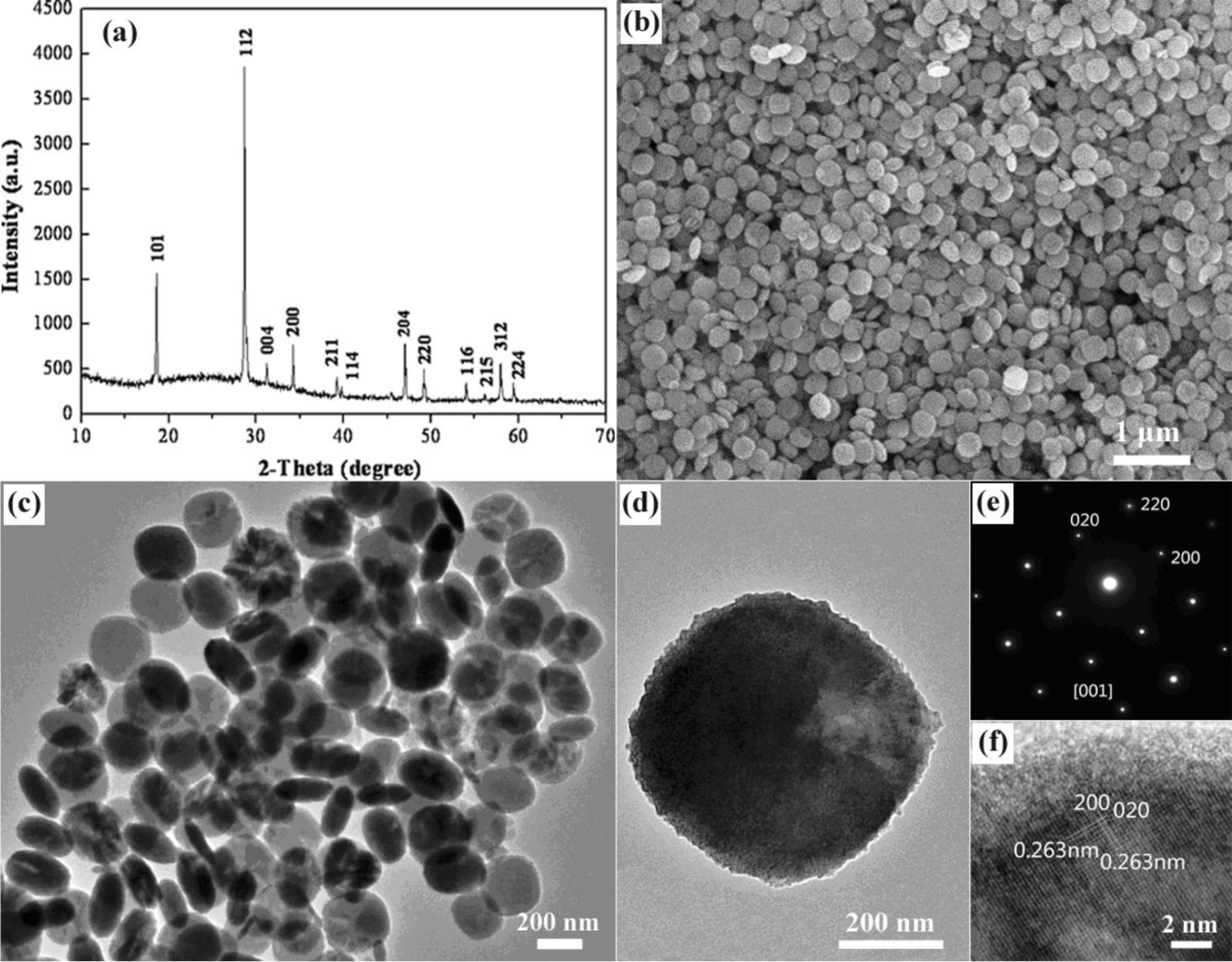
2. Results and Discussion
3. Experimental Section
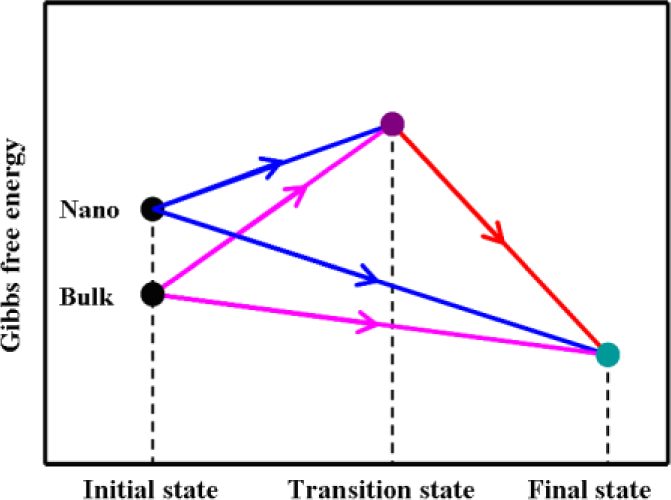
4. Methodology
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hill, T.L. Perspective: Nanothermodynamics. Nano Lett. 2001, 1, 111–112. [Google Scholar]
- Hill, T.L. Extension of Nanothermodynamics to Include a One-dimensional Surface Excess. Nano Lett. 2001, 1, 159–160. [Google Scholar]
- Hill, T.L. A Different Approach to Nanothermodynamics. Nano Lett. 2001, 1, 273–275. [Google Scholar]
- Ball, P.; Garwin, L. Science at the Atomic Scale. Nature 1992, 355, 761–765. [Google Scholar]
- Xue, Y.Q.; Gao, B.J.; Gao, J.F. The Theory of Thermodynamics for Chemical Reactions in Dispersed Heterogeneous Systems. J. Colloid Interf. Sci. 1997, 191, 81–85. [Google Scholar]
- Du, J.P.; Wang, H.Y.; Zhao, R.H. Size-dependent Thermodynamic Properties and Equilibrium Constant of Chemical Reaction in Nanosystem: An Experimental Study. J. Chem. Thermodyn. 2013, 65, 29–33. [Google Scholar]
- Kuang, Q.; Wang, X.; Jiang, Z.; Xie, Z.; Zheng, L. High-energy-surface Engineered Metal Oxide Micro-and Nanocrystallites and their Applications. Acc. Chem. Res. 2013, 47, 308–318. [Google Scholar]
- Hu, L.H.; Peng, Q.; Li, Y. Selective Synthesis of Co3O4 Nanocrystal with Different Shape and Crystal Plane Effect on Catalytic Property for Methane Combustion. J. Am. Chem. Soc. 2008, 130, 16136–16137. [Google Scholar]
- Zhou, Z.Y.; Tian, N.; Li, J.T.; Broadwell, I.; Sun, S.G. Nanomaterials of High Surface Energy with Exceptional Properties in Catalysis and Energy Storage. Chem. Soc. Rev. 2011, 40, 4167–4185. [Google Scholar]
- Wen, Y.Z.; Xue, Y.Q.; Cui, Z.X.; Wang, Y. Thermodynamics of Nanoadsorption from Solution: Theoretical and Experimental Research. J. Chem. Thermodyn. 2015, 80, 112–118. [Google Scholar]
- Cui, Z.X.; Zhao, M.Z.; Lai, W.P.; Xue, Y.Q. Thermodynamics of Size Effect on Phase Transition Temperatures of Dispersed Phases. J. Phys. Chem. C 2011, 115, 22796–22803. [Google Scholar]
- Yang, Y.F.; Xue, Y.Q.; Cui, Z.X.; Zhao, M.Z. Effect of Particle Size on Electrode Potential and Thermodynamics of Nanoparticles Electrode in Theory and Experiment. Electrochim. Acta. 2014, 136, 565–571. [Google Scholar]
- Jiang, J.Y.; Huang, Z.Y.; Mi, Y.; Li, Y.F.; Yuan, A.Q. Development and Prospects for Thermodynamics of Nanomaterials. Prog. Chem. 2010, 22, 1058–1067. [Google Scholar]
- Gong, Q.; Qian, X.F.; Ma, X.D.; Zhu, Z.K. Large-scale Fabrication of Novel Hierarchical 3D CaMoO4 and SrMoO4 Mesocrystals via a Microemulsion-mediated Route. Cryst. Growth Des. 2006, 6, 1821–1825. [Google Scholar]
- Singh, B.P.; Parchur, A.K.; Ningthoujam, R.S.; Ansari, A.A.; Singha, P.; Rai, S.B. Influence of Gd3+ co-doping on structural property of CaMoO4:Eu nanoparticles. Dalton T. 2014, 43, 4770–4778. [Google Scholar]
- Kwan, S.; Kim, F.; Akana, J.; Yang, P.D. Synthesis and assembly of BaWO4 nanorods. Chem. Commun. 2001, 5, 447–448. [Google Scholar]
- Tanaka, K.; Miyajima, T.; Shirai, N.; Zhang, Q.; Nakata, R. Laser photochemical ablation of CdWO4 studied with the time-of-flight mass spectrometric technique. J. Appl. Phys. 1995, 77, 6581–6587. [Google Scholar]
- Sen, A.; Pramanik, P. Low-temperature Synthesis of Nano-sized Metal Molybdate Powders. Mater. Lett. 2001, 50, 287–294. [Google Scholar]
- Choi, G.K.; Cho, S.Y.; An, J.S.; Hong, K.S. Microwave dielectric properties and sintering behaviors of scheelite compound CaMoO4. J. Eur. Ceram. Soc. 2006, 26, 2011–2015. [Google Scholar]
- Sharma, N.; Shaju, K.M.; Rao, G.V.S.; Chowdari, B.V.R.; Dong, Z.L.; White, T.J. Carbon-coated nanophase CaMoO4 as anode material for Li ion batteries. Chem. Mater. 2004, 16, 504–512. [Google Scholar]
- Fan, G.C.; Huang, Z.Y.; Jiang, J.Y.; Sun, L. Standard molar enthalpy of formation of the ZnO nanosheets. J. Therm. Anal. Calorim. 2012, 110, 1471–1474. [Google Scholar]
- Du, J.P.; Zhao, R.H.; Xue, Y.Q. Effects of Sizes of Nano-copper Oxide on the Equilibrium Constant and Thermodynamic Properties for the Reaction in Nanosystem. J. Chem. Thermodyn. 2012, 45, 48–52. [Google Scholar]
- Fu, X.C.; Shen, W.X.; Yao, T.Y.; Hou, W.H. Physical chemistry, 5th ed; Higher Education Press: Beijing, China, 2006. [Google Scholar]
- Fan, G.C.; Sun, L.; Huang, Z.Y.; Jiang, J.Y.; Li, Y.F. Thermodynamic Functions of the Grain-like ZnO Nanostructures. Mater. Lett. 2011, 65, 2783–2785. [Google Scholar]
- Fan, G.C.; Jiang, J.Y.; Li, Y.F.; Huang, Z.Y. Thermodynamic Functions of the ZnO Nanoweeds. Mater. Chem. Phys. 2011, 130, 839–842. [Google Scholar]
- Gao, S.L.; Chen, S.P.; Hu, R.Z.; Li, H.Y.; Shi, Q.Z. Derivation and Application of Thermodynamic Equations. Chin. J. Inorg. Chem. 2002, 18, 362–366. [Google Scholar]
| Reaction system | No. | m/mg | Q/mJ | ΔrHmθ/kJ·mor−1 | lnk |
|---|---|---|---|---|---|
| Bulk CaMoO4 | 1 | 5.109 | −756.75 | −29.627 | −6.913 |
| 2 | 5.114 | −757.61 | −29.632 | −6.919 | |
| 3 | 5.089 | −753.38 | −29.611 | −6.887 | |
| 4 | 5.094 | −754.27 | −29.617 | −6.895 | |
| 5 | 5.099 | −755.43 | −29.621 | −6.901 | |
| Average value | −29.622±0.008 | −6.903±0.013 | |||
| Nano CaMoO4 | 1 | 5.167 | −1273.75 | −49.308 | −6.659 |
| 2 | 5.157 | −1270.66 | −49.284 | −6.647 | |
| 3 | 5.171 | −1274.91 | −49.315 | −6.665 | |
| 4 | 5.163 | −1272.42 | −49.295 | −6.654 | |
| 5 | 5.156 | −1270.34 | −49.280 | −6.641 | |
| Average value | −49.296±0.015 | −6.653±0.010 | |||
© 2015 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Fan, G.; Huang, Z. Synthesis and Surface Thermodynamic Functions of CaMoO4 Nanocakes. Entropy 2015, 17, 2741-2748. https://doi.org/10.3390/e17052741
Li X, Fan G, Huang Z. Synthesis and Surface Thermodynamic Functions of CaMoO4 Nanocakes. Entropy. 2015; 17(5):2741-2748. https://doi.org/10.3390/e17052741
Chicago/Turabian StyleLi, Xingxing, Gaochao Fan, and Zaiyin Huang. 2015. "Synthesis and Surface Thermodynamic Functions of CaMoO4 Nanocakes" Entropy 17, no. 5: 2741-2748. https://doi.org/10.3390/e17052741





