Analysis of EEG via Multivariate Empirical Mode Decomposition for Depth of Anesthesia Based on Sample Entropy
Abstract
:1. Introduction
2. Methods
2.1. Approximate Entropy and Sample Entropy
2.2. The Extended EMD Methods
2.3. Statistical Analysis
3. Results
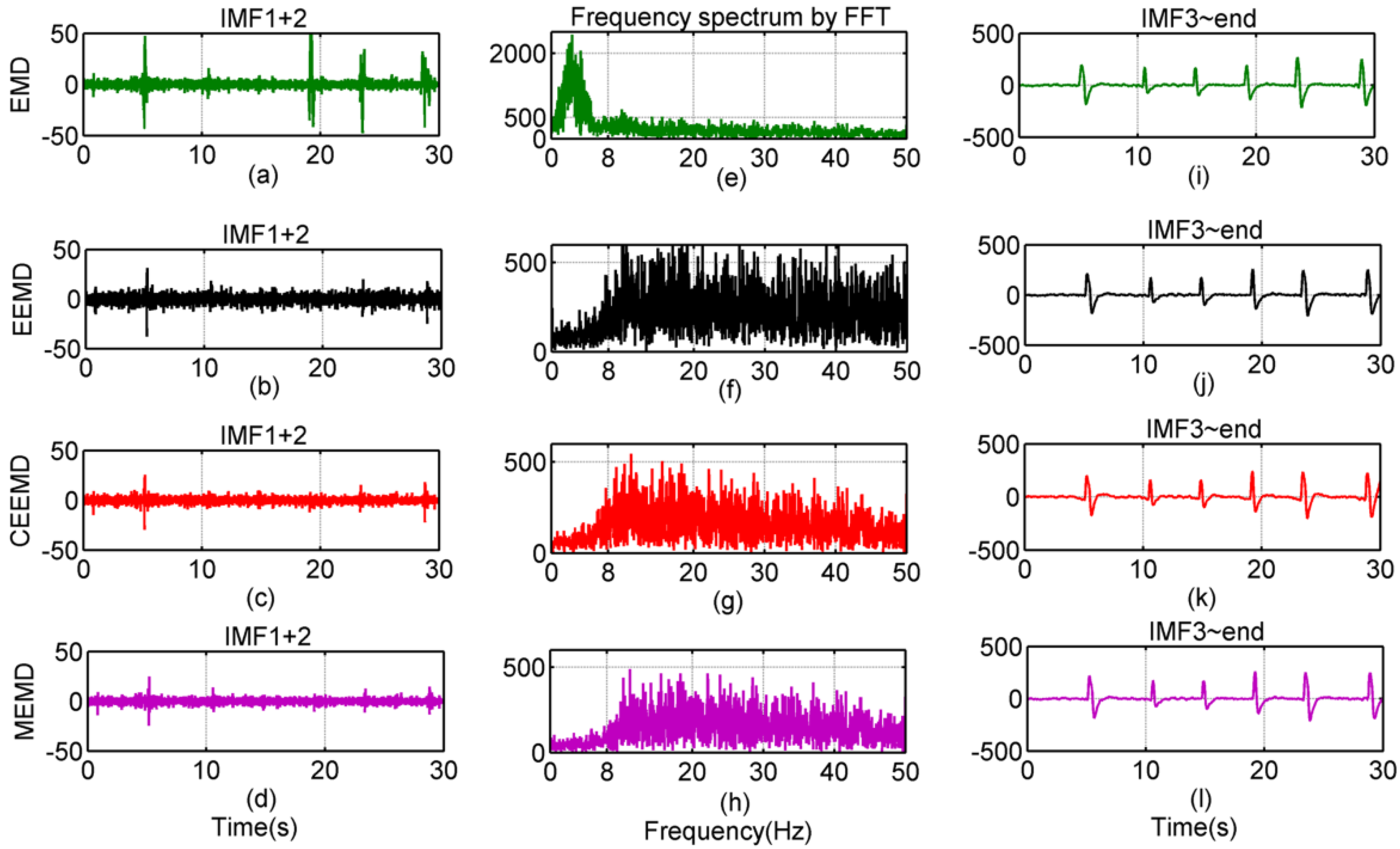
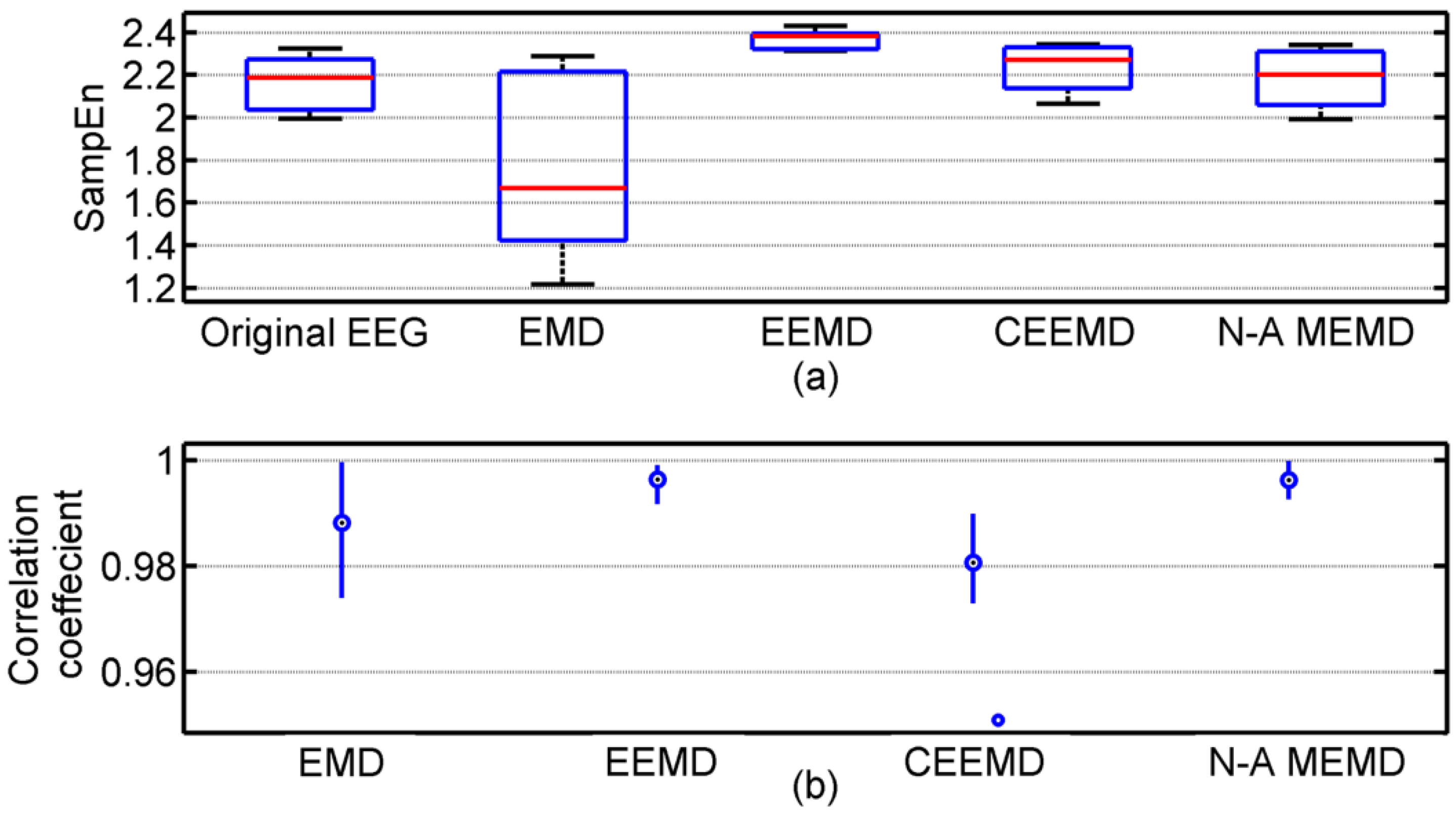
3.1. Results of Comparison among EMD, EEMD, CEEMD and N-A MEMD
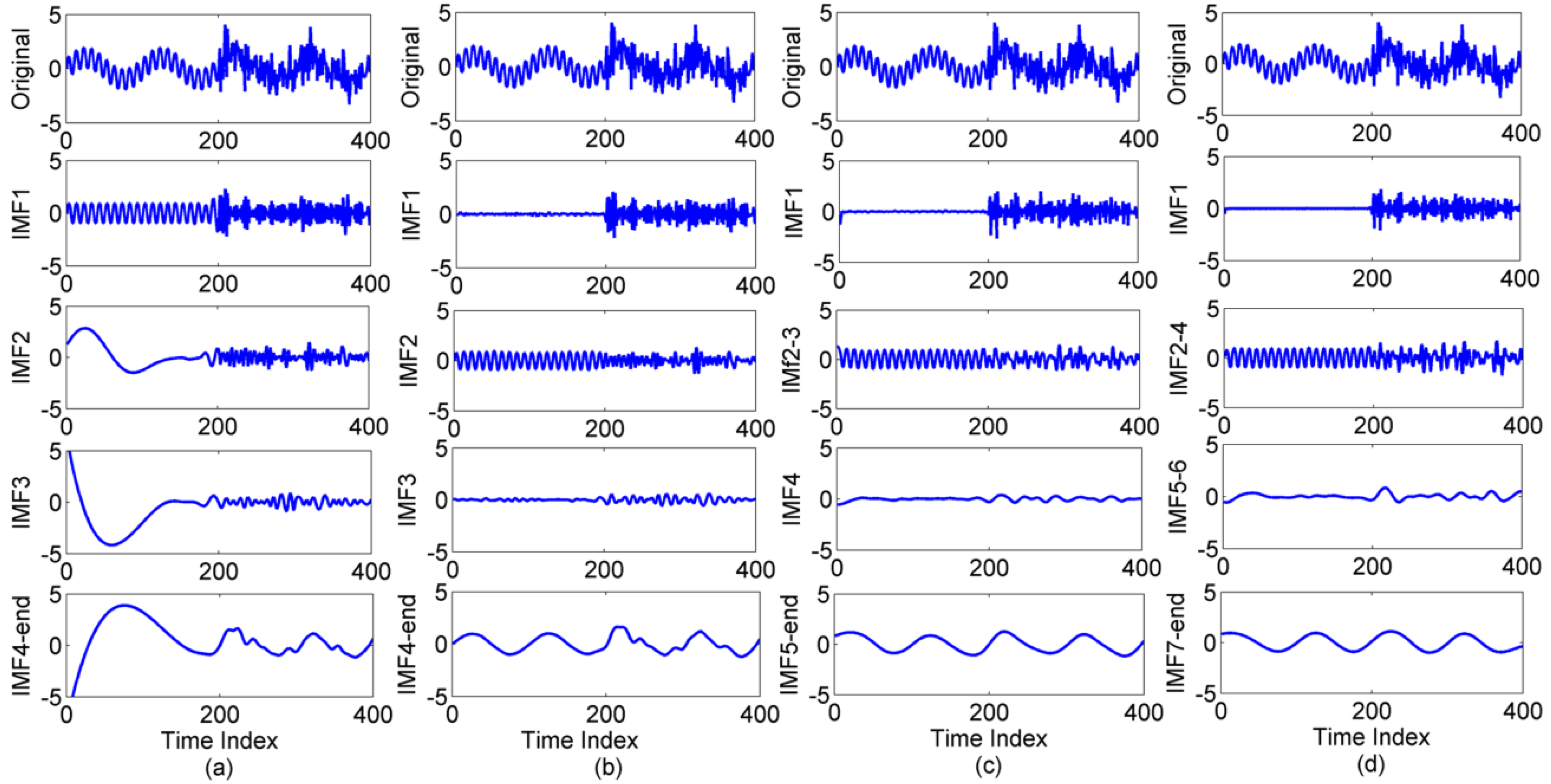
| Methods | 2 Hz | 20 Hz |
|---|---|---|
| EMD | N/A | 0.9846 |
| EEMD | 0.9449 | 0.9907 |
| CEEMD | 0.9627 | 0.9826 |
| N-A MEMD | 0.9674 | 0.9943 |
3.2. Results of the Application in Clinical EEG Recordings

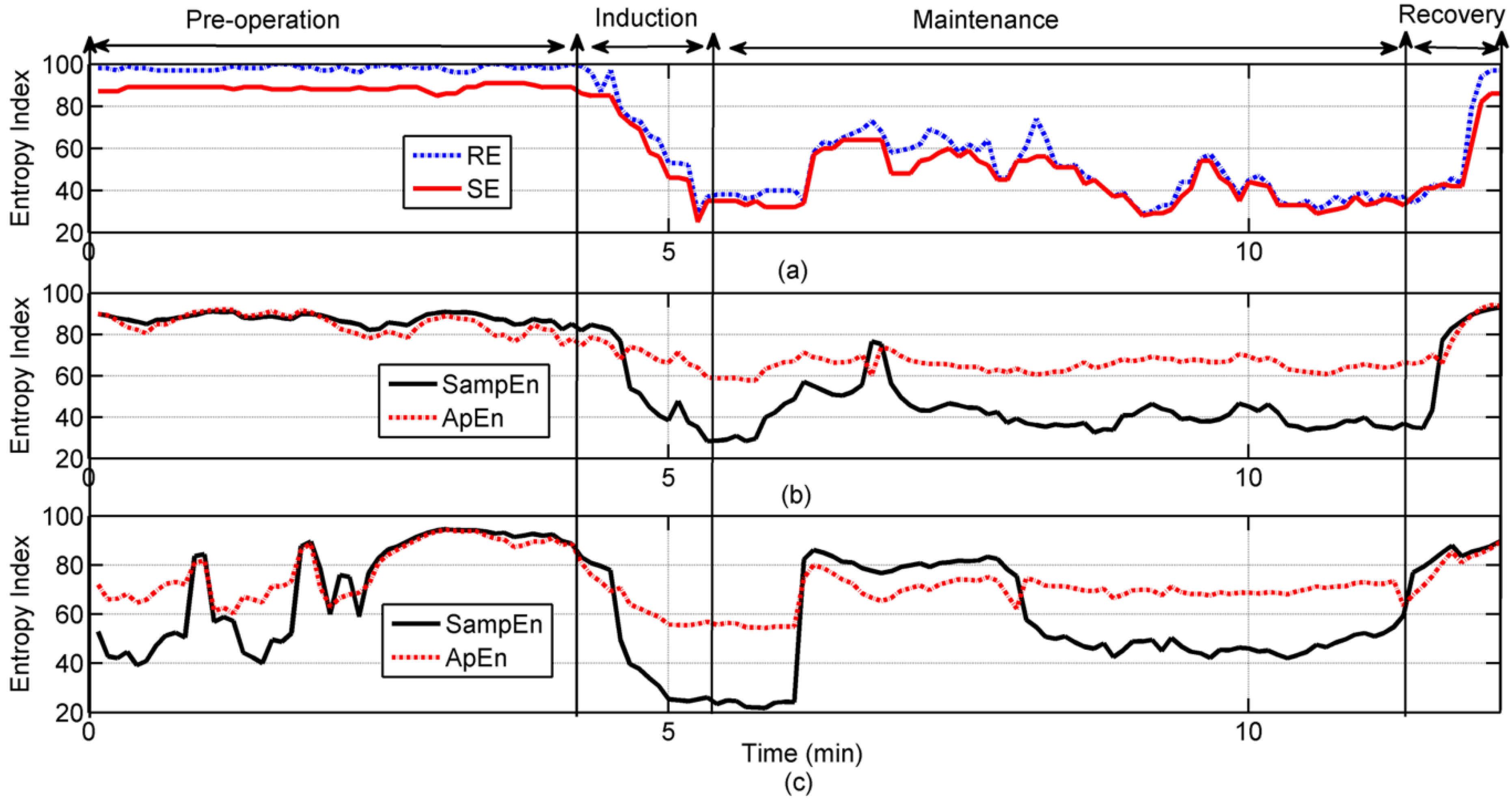
| oEEG | rEEG | |||
|---|---|---|---|---|
| Entropy | SampEn | ApEn | SampEn | ApEn |
| Index | 71.7 ± 20.32 | 77.77 ± 11.0 | 86.53 ± 5.29 | 84.08 ± 5.7 |
| RE | 0.254b | 0.241b | 0.746a | 0.406a |
| 97.16 ± 4.46 | ||||
| SE | 0.246b | 0.236b | 0.740a | 0.370a |
| 87.86 ± 3.09 | ||||
| oEEG | rEEG | |||
|---|---|---|---|---|
| Entropy | SampEn | ApEn | SampEn | ApEn |
| Index | 60. 24 ± 16.8 | 70.48 ± 3.14 | 43.065 ± 8.8 | 65.66 ± 3.14 |
| RE | 0.724a | 0.304b | 0.495a | 0.007c |
| 50.25 ± 13.1 | ||||
| SE | 0.717a | 0.312b | 0.507a | 0.034c |
| 46.4 ± 11.21 | ||||
4. Discussion and Conclusions
Acknowledgments
Conflicts of interest
References
- Billard, V.; Gambus, P.L.; Chamoun, N.; Stanski, D.R.; Shafer, S.L. A comparison of spectral edge, delta power, and bispectral index as EEG measures of alfentanil, propofol, and midazolam drug effect. Clin. Pharmacol. Ther. 1997, 61, 45–58. [Google Scholar] [CrossRef]
- Katoh, T.; Suzuki, A.; Ikeda, K. Electroencephalographic derivatives as a tool for predicting the depth of sedation and anesthesia induced by sevoflurane. Anesthesiology 1998, 88, 642–650. [Google Scholar] [CrossRef] [PubMed]
- Mahon, P.; Greene, B.R.; Greene, C.; Boylan, G.B.; Shorten, G.D. Behaviour of spectral entropy, spectral edge frequency 90%, and alpha and beta power parameters during low-dose propofol infusion. Brit. J. Anaesth. 2008, 101, 213–221. [Google Scholar] [CrossRef] [PubMed]
- De Deyne, C.; Struys, M.; Decruyenaere, J.; Creupelandt, J.; Hoste, E.; Colardyn, F. Use of continuous bispectral EEG monitoring to assess depth of sedation in ICU patients. Intensive Care Med. 1998, 24, 1294–1298. [Google Scholar] [CrossRef] [PubMed]
- Vanluchene, A.L.G.; Vereecke, H.; Thas, O.; Mortier, E.P.; Shafer, S.L.; Struys, M.M. Spectral entropy as an electroencephalographic measure of anesthetic drug effect: a comparison with bispectral index and processed midlatency auditory evoked response. Anesthesiology 2004, 101, 34–42. [Google Scholar] [CrossRef] [PubMed]
- White, P.F.; Tang, J.; Romero, G.F.; Wender, R.H.; Naruse, R.; Sloninsky, A.; Kariger, R. A comparison of state and response entropy versus bispectral index values during the perioperative period. Anesth. Analg. 2006, 102, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Lin, Z.; McCallum, R.W. Artifact reduction in electrogastrogram based on empirical mode decomposition method. Med. Biol. Eng. Comput. 2000, 38, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Sweeney-Reed, C.M.; Nasuto, S.J. A novel approach to the detection of synchronization in EEG based on empirical mode decomposition. J. Comput. Neurosci. 2007, 23, 79–111. [Google Scholar] [CrossRef] [PubMed]
- Huang, N.E.; Shen, Z.; Long, S.R.; Wu, M.C.; Shih, H.H.; Zheng, Q.; Yen, N.C.; Tung, C.C.; Liu, H.H. The empirical mode decomposition and the Hilbert spectrum for nonlinear and non-stationary time series analysis. Proc. R. Soc. Lond. 1998, 454, 903–995. [Google Scholar] [CrossRef]
- Wu, Z.H.; Huang, N.E. Ensemble empirical mode decomposition: A noise assisted data analysis method. Adv. Adaptive Data Anal. 2009, 1, 1–41. [Google Scholar] [CrossRef]
- Yeh, J.R.; Shieh, J.S.; Huang, N.E. Complementary ensemble empirical mode decomposition: A novel noise enhanced data analysis method. Adv. Adaptive Data Anal. 2010, 2, 135–156. [Google Scholar] [CrossRef]
- Rehman, N.; Mandic, D.P. Multivariate empirical mode decomposition. Proc. Royal Society A 2010, 466, 1291–1302. [Google Scholar] [CrossRef]
- Rehman, N.; Mandic, D.P. Filter bank property of multivariate empirical mode decomposition. IEEE Trans. Signal Process. 2010, 59, 2421–2426. [Google Scholar] [CrossRef]
- Wei, Q.; Huang, D.W.; Lu, J.W.; Liu, Q.; Shieh, J.S. Intelligent real time data mining of depth of aneasthesia. In Proceedings of the 13th International Conference on Intelligent System and Control (IASTED 13th), Cambridge, UK, 11–13 July 2011; pp. 35–40.
- Fan, S.Z.; Yeh, J.R.; Chen, B.C.; Shieh, J.S. Comparison of EEG approximate entropy and complexity measures of depth of anaesthesia during inhalational general anaesthesia. J. Med. Bio. Eng. 2011, 31, 359–366. [Google Scholar] [CrossRef]
- Liu, Q.; Wei, Q.; Fan, S.Z.; Lu, C.W.; Lin, T.Y.; Abbod, M.F.; Shieh, J.S. Adaptive computation of multiscale entropy and its application in EEG signals for monitoring depth of anesthesia during surgery. Entropy 2012, 14, 978–992. [Google Scholar] [CrossRef]
- Shalbaf, R.; Behnam, H.; Sleigh, J.; Voss, L. Measuring the effects of sevoflurane on electroencephalogram using sample entropy. Acta Anaesthesiol. Scand. 2012, 56, 880–889. [Google Scholar] [CrossRef] [PubMed]
- Pincus, S.M. Approximate entropy (ApEn) as a measure of system complexity. Proc. Natl. Acad. Sci. 1991, 88, 110–117. [Google Scholar] [CrossRef]
- Richman, J.S.; Moorman, J.R. Physiological time-series analysis using approximate entropy and sample entropy. Am. J. Physiol. Heart Circ. Physiol. 2000, 278, 2039–2049. [Google Scholar]
- Ahmed, M.U.; Li, L.; Cao, J.T.; Mandic, D.P. Multivariate multiscale entropy for brain consciousness analysis. In Proceedings of the 33rd Annual international conference of the IEEE Engineering in Medicine and Biology Society, Boston, MA, USA, 30 August–3 September, 2011; pp. 810–813.
- Ahmed, M.U.; Mandic, D.P. Multivariate multiscale entropy analysis. IEEE Signal Process Lett. 2012, 19, 91–94. [Google Scholar] [CrossRef]
- Chen, D.; Li, D.; Xiong, M.Z.; Bao, H.; Li, X.L. GPGPU-aided ensemble empirical-mode decomposition for EEG analysis during anesthesia. IEEE Trans. Inf. Technol. Biomed. 2010, 14, 1417–1427. [Google Scholar] [CrossRef] [PubMed]
- Olofsen, E.; Sleigh, J.W.; Dahan, A. Permutation entropy of the electroencephalogram: A measure of anaesthetic drug effect. Br. J. Anaesth. 2008, 101, 810–821. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, D.; Liang, Z.; Voss, L.J. Analysis of depth of anesthesia with Hilbert–Huang spectral entropy. Clin. Neurophysiol. 2008, 119, 2465–2475. [Google Scholar] [CrossRef] [PubMed]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Wei, Q.; Liu, Q.; Fan, S.-Z.; Lu, C.-W.; Lin, T.-Y.; Abbod, M.F.; Shieh, J.-S. Analysis of EEG via Multivariate Empirical Mode Decomposition for Depth of Anesthesia Based on Sample Entropy. Entropy 2013, 15, 3458-3470. https://doi.org/10.3390/e15093458
Wei Q, Liu Q, Fan S-Z, Lu C-W, Lin T-Y, Abbod MF, Shieh J-S. Analysis of EEG via Multivariate Empirical Mode Decomposition for Depth of Anesthesia Based on Sample Entropy. Entropy. 2013; 15(9):3458-3470. https://doi.org/10.3390/e15093458
Chicago/Turabian StyleWei, Qin, Quan Liu, Shou-Zhen Fan, Cheng-Wei Lu, Tzu-Yu Lin, Maysam F. Abbod, and Jiann-Shing Shieh. 2013. "Analysis of EEG via Multivariate Empirical Mode Decomposition for Depth of Anesthesia Based on Sample Entropy" Entropy 15, no. 9: 3458-3470. https://doi.org/10.3390/e15093458
APA StyleWei, Q., Liu, Q., Fan, S.-Z., Lu, C.-W., Lin, T.-Y., Abbod, M. F., & Shieh, J.-S. (2013). Analysis of EEG via Multivariate Empirical Mode Decomposition for Depth of Anesthesia Based on Sample Entropy. Entropy, 15(9), 3458-3470. https://doi.org/10.3390/e15093458








