Two Examples of Exergy Optimization Regarding the “Thermo-Frigopump” and Combined Heat and Power Systems
Abstract
:1. Introduction
2. First Example: The “Thermo-Frigopump” TFP
2.1. Thermo-Frigopump Thermodynamical Model
- , heat rate transmitted to the hot sink;
- , heat rate extracted from the cold source;
- , mechanical (electrical) power given to the cycled fluid.
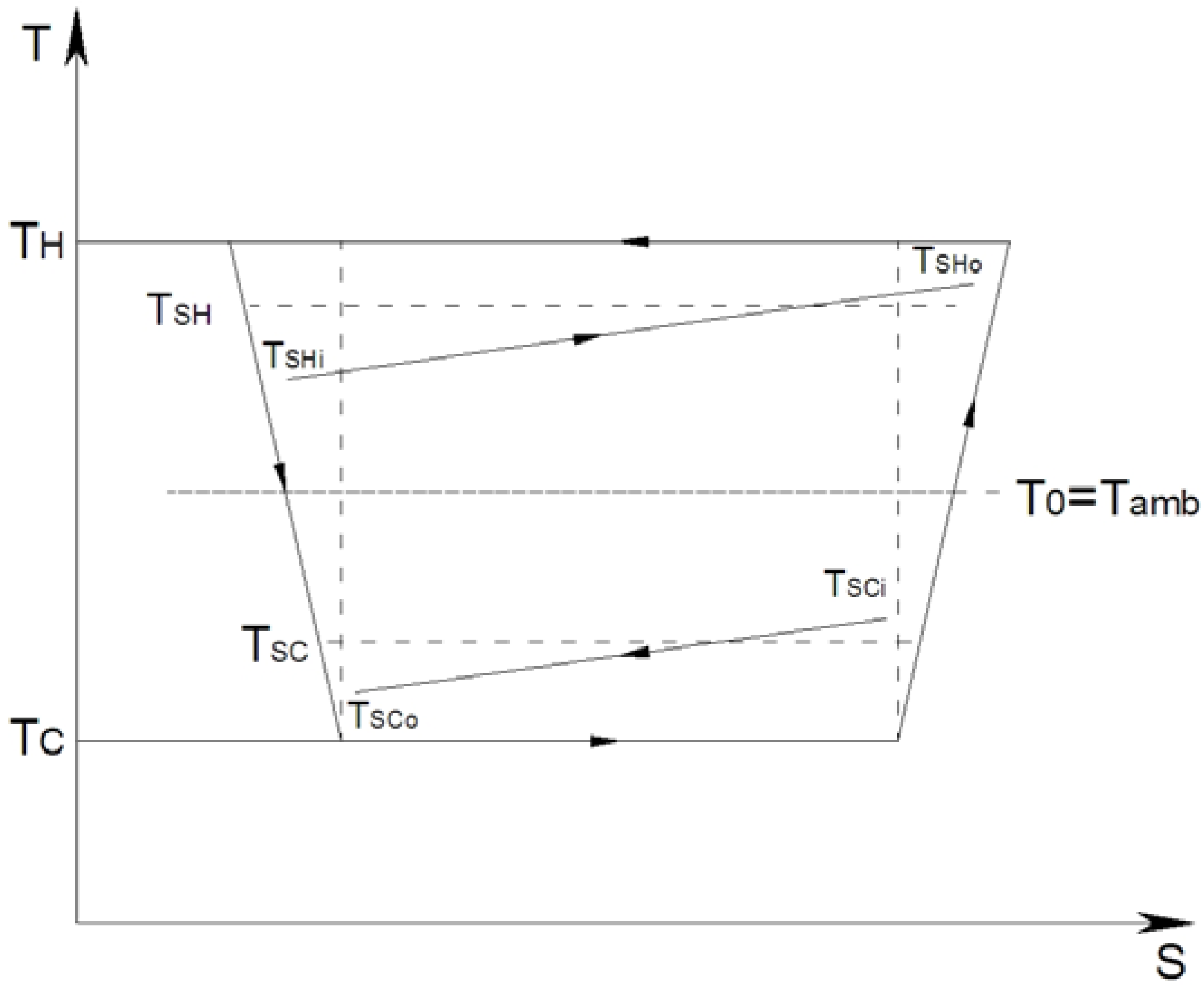
2.2. Efficiency Criterions Regarding Thermo-Frigopump
- , exergy heat rate transfered to the hot sink according to:
- , hot sink entropic temperature
- exergy heat rate transfered to the cold source according to:
- , cold source entropic temperature
2.3. Application to Optimization of a Thermo-Frigopump
2.3.1. Case of the Energy Criterion
2.3.2. Case of the Exergy Criterion
3. Second Example: The CHP System
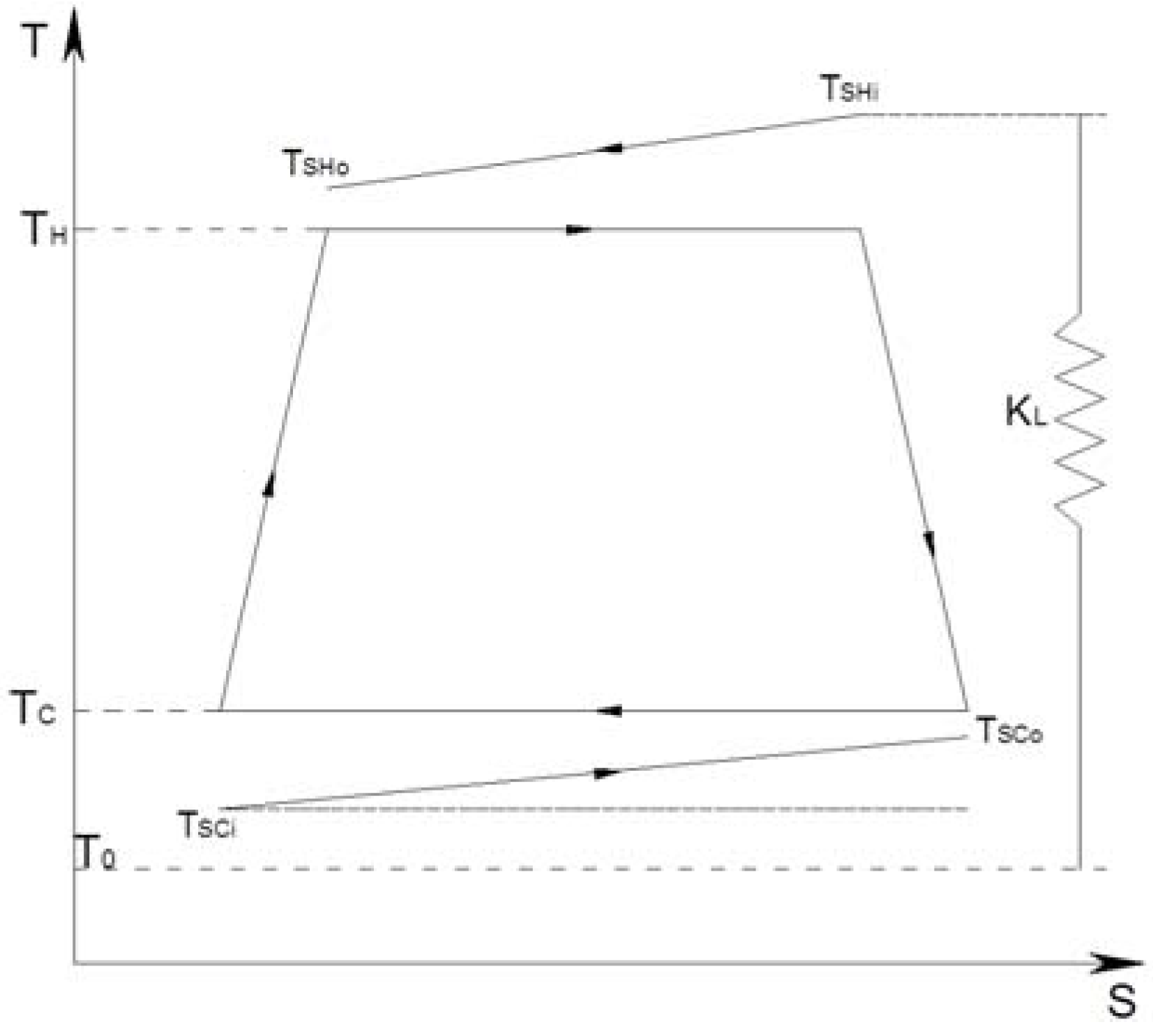
3.1. The CHP Thermodynamical Model
3.2. Efficiency Criterions Regarding CHP System
3.3. Some Results Concerning CHP System Exergetic Optimization
4. Generalization and Conclusions
4.1. Generalization
4.2. Cases with Added Technical Constraint
5. Conclusions
Nomenclature:
heat capacity rate [W K−1] | |
| cp | mass specific heat at constant pressure [W kg−1 K−1] |
exergy rate [W] | |
mass flow rate of the working fluid in the cycle [kg s−1] | |
| I | irreversibility ratio |
| K | heat transfer conductance [W K−1] |
| NTU | number of heat transfer unit |
heat transfer rate [W] | |
entropy rate [W K−1] | |
| T | temperature [K] |
| t | non dimensional temperature |
| X | temperature difference [K] |
mechanical power [W] |
Greek symbols
heat exchanger effectiveness | |
efficiency | |
intermediate variable | |
Carnot factor |
Subscripts and superscripts
| C | related to the working fluid, at the sink |
| c | consumed or Carnot |
| CHP | combined heat and power system |
| ex | exergetic |
| H | related to the working fluid, at the source |
| i | internal |
| L | loss |
| SH | source |
| SC | sink |
| t | total |
| U | useful |
| I | related to first law |
| 0 | ambient or imposed value |
| * | optimal |
References
- Wakui, T.; Yokoyama, R. Optimal sizing of residential gas engine cogeneration system for power interchange operation from energy-saving viewpoint. Energy 2011, 36, 3816–3824. [Google Scholar] [CrossRef]
- Radulescu, M. Combined Electricity and Heat Production Systems with PEMFC or SOFC Fuel Cells and External Vapor Reforming. Ph.D. Thesis, Nancy University, Nancy, France, September 2006. [Google Scholar] [Green Version]
- Milia, D.; Sciubba, E. Exergy-based lumped simulation of complex systems: An interactive analysis tool. Energy 2006, 31, 100–111. [Google Scholar] [CrossRef]
- Descieux, D. Modelling and Exergetic Comparison of Cogeneration Systems. Ph.D. Thesis, Nancy University, Nancy, France, November 2007. [Google Scholar] [Green Version]
- Pehnt, M. Environmental impacts of distributed energy systems—The case of micro cogeneration. Environ. Sci. Policy 2008, 11, 25–37. [Google Scholar] [CrossRef]
- Abusoglu, A.; Kanoglu, M. First and second law analysis of diesel engine powered cogeneration systems. Energy Convers. Manag. 2008, 49, 2026–2031. [Google Scholar] [CrossRef]
- Zhai, H.; Dai, Y.J.; Wu, J.Y.; Wang, R.Z. Energy and exergy analyses on a novel hybrid solar heating, cooling and power generation system for remote areas. Appl. Energy 2009, 86, 1395–1404. [Google Scholar] [CrossRef]
- Abusoglu, A.; Kanoglu, M. Exergoeconomic analysis and optimization of combined heat and power production: A review. Renew. Sustain. Energy Rev. 2009, 13, 2295–2308. [Google Scholar] [CrossRef]
- Aussant, C.D.; Fung, A.S.; Ugursal, V.I.; Taherian, H. Residential application of internal combustion engine based cogeneration in cold climate Canada. Energy Build. 2009, 41, 1288–1298. [Google Scholar] [CrossRef]
- Nieminen, J.; Dincer, I. Comparative exergy analyses of gasoline and hydrogen fuelled ICEs. Int. J. Hydrogen Energy 2010, 35, 5124–5132. [Google Scholar] [CrossRef]
- El-Emam, R.S.; Dincer, I. Energy and exergy analyses of a combined molten carbonate fuel cell—Gas turbine system. Int. J. Hydrogen Energy 2011, 36, 8927–8935. [Google Scholar] [CrossRef]
- Bingöl, E.; Kılkış, B.; Eralp, C. Exergy based performance analysis of high efficiency poly-generation systems for sustainable building applications. Energy Build. 2011, 43, 3074–3081. [Google Scholar] [CrossRef]
- Doluweera, G.H.; Jordaan, S.M.; Moore, M.C.; Keith, D.W.; Bergerson, J.A. Evaluating the role of cogeneration for carbon management in Alberta. Energy Policy 2011, 39, 7963–7974. [Google Scholar] [CrossRef]
- Barelli, L.; Bidini, G.; Gallorini, F.; Ottaviano, A. An energetic–Exergetic comparison between PEMFC and SOFC-based micro-CHP systems. Int. J. Hydrogen Energy 2011, 36, 3206–3214. [Google Scholar] [CrossRef]
- Ji, J.; Liu, K.; Chow, T.T.; Pei, G.; He, H. Thermal Analysis of PV/T Evaporator of A Solar Assisted Heat Pump. Int. J. Energy Res. 2007, 31, 525–545. [Google Scholar] [CrossRef]
- Thilak Raj, N.; Iniyan, S.; Goic, R. A review of renewable energy based cogeneration technologies. Renew. Sustain. Energy Rev. 2011, 15, 3640–3648. [Google Scholar]
- Ibrahim, A.; Othman, M.Y.; Ruslan, M.H.; Mat, S.; Sopian, K. Recent advances in flat plate photovoltaic/thermal (PV/T) solar collectors. Renew. Sustain. Energy Rev. 2011, 15, 352–365. [Google Scholar] [CrossRef]
- Obernberger, I.; Carlsen, H.; Biedermann, F. State of the Art and Future Developments Regarding Small Scale Biomass CHP Systems with a Special Focus on ORC and Stirling Engine Technologies. In Proceedings of the 2003 International Nordic Bioenergy Conference, Jyväskylä, Finland, 2–5 September 2003. [Green Version]
- Uddin, S.N.; Barreto, L. Biomass-fired cogeneration systems with CO2 capture and storage. Renew. Energy 2007, 32, 1006–1019. [Google Scholar] [CrossRef]
- Zhang, X.; Su, S.; Chen, J.; Zhao, Y.; Brandon, N. A new analytical approach to evaluate and optimize the performance of an irreversible solid oxide fuel cell-gas turbine hybrid system. Int. J. Hydrogen Energy 2011, 36, 15304–15312. [Google Scholar] [CrossRef]
- Vuillecard, C.; Hubert, C.E.; Contreau, R.; Mazzenga, A.; Stabat, P.; Adnot, J. Small scale impact of gas technologies on electric load management—μCHP & hybrid heat pump. Energy 2011, 36, 2912–2923. [Google Scholar]
- Lowe, R. Combined heat and power considered as a virtual steam cycle heat pump. Energy Policy 2011, 39, 5528–5534. [Google Scholar] [CrossRef]
- Wang, J.J.; Jing, Y.Y.; Zhang, C.F. Weighting Methodologies in Multicriteria Evaluations of CHP Systems. Int. J. Energy Res. 2009, 33, 1023–1039. [Google Scholar] [CrossRef]
- Chua, K.J.; Yang, W.M.; Wong, T.Z.; Ho, C.A. Integrating renewable energy technologies to support building trigeneration—A multi-criteria analysis. Renew. Energy 2012, 41, 358–367. [Google Scholar] [CrossRef]
- Rubio-Maya, C.; Uche-Marcuello, J.; Martínez-Gracia, A.; Bayod-Rújula, A.A. Design optimization of a polygeneration plant fuelled by natural gas and renewable energy sources. Appl. Energy 2011, 88, 449–457. [Google Scholar] [CrossRef]
- Feidt, M.; Costea, M.; Petre, C.; Petrescu, S. Optimization of Direct Carnot Cycle. Appl. Therm. Eng. 2007, 27, 829–839. [Google Scholar] [CrossRef]
- Feidt, M. Thermodynamics applied to reverse cycle machines, a review. Int. J. Refrig. 2010, 33, 1327–1342. [Google Scholar] [CrossRef]
- Ilmaz, T. Optimization of cogeneration systems under alternative performance criteria. Energy Convers. Manage. 2004, 45, 939–945. [Google Scholar] [CrossRef]
- Erdil, A. Exergy optimization for an irreversible combined cogeneration cycle. J. Energy Inst. 2005, 78, 27–30. [Google Scholar] [CrossRef]
- Atmaca, M.; Gumus, M.; Inan, A.T.; Yilmaz, T. Optimization of irreversible cogeneration systems under alternative performance criteria. Int. J. Thermophys. 2009, 30, 1724–1732. [Google Scholar] [CrossRef]
- Feidt, M.; Costea, M. Energy and exergy analysis and optimization of combined heat and power systems. Energies 2012, 5, 3701–3722. [Google Scholar] [CrossRef]
- Petre, C.; Feidt, M.; Costea, M.; Petrescu, S. A model for study and optimization of real-operating refrigeration machines. Int. J. Energy Res. 2009, 33, 173–179. [Google Scholar] [CrossRef]
- Feidt, M. Reconsideration of criteria and modelling in order to optimize the efficiency of irreversible thermomechanical heat engines. Entropy 2010, 12, 2470–2484. [Google Scholar] [CrossRef]
- Feidt, M. Optimal thermodynamics new upperbounds. Entropy 2009, 11, 529–547. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Feidt, M. Two Examples of Exergy Optimization Regarding the “Thermo-Frigopump” and Combined Heat and Power Systems. Entropy 2013, 15, 544-558. https://doi.org/10.3390/e15020544
Feidt M. Two Examples of Exergy Optimization Regarding the “Thermo-Frigopump” and Combined Heat and Power Systems. Entropy. 2013; 15(2):544-558. https://doi.org/10.3390/e15020544
Chicago/Turabian StyleFeidt, Michel. 2013. "Two Examples of Exergy Optimization Regarding the “Thermo-Frigopump” and Combined Heat and Power Systems" Entropy 15, no. 2: 544-558. https://doi.org/10.3390/e15020544
APA StyleFeidt, M. (2013). Two Examples of Exergy Optimization Regarding the “Thermo-Frigopump” and Combined Heat and Power Systems. Entropy, 15(2), 544-558. https://doi.org/10.3390/e15020544



