Multivariate Conditional Granger Causality Analysis for Lagged Response of Soil Respiration in a Temperate Forest
Abstract
:1. Introduction
3. Methods—Field Experiment
3.1. Site Description
3.2. Soil CO2 Efflux
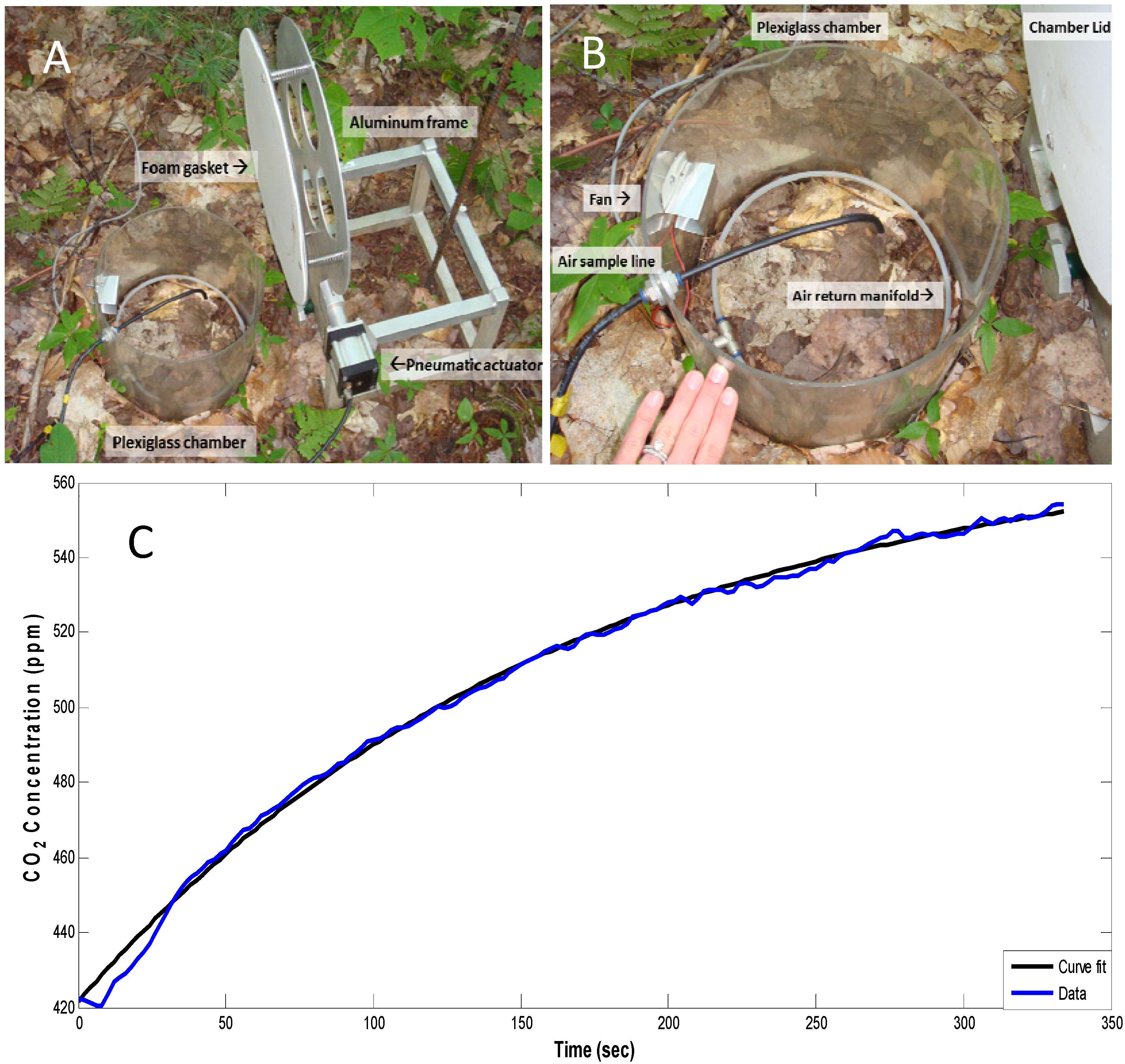
3.3. Other Measurements
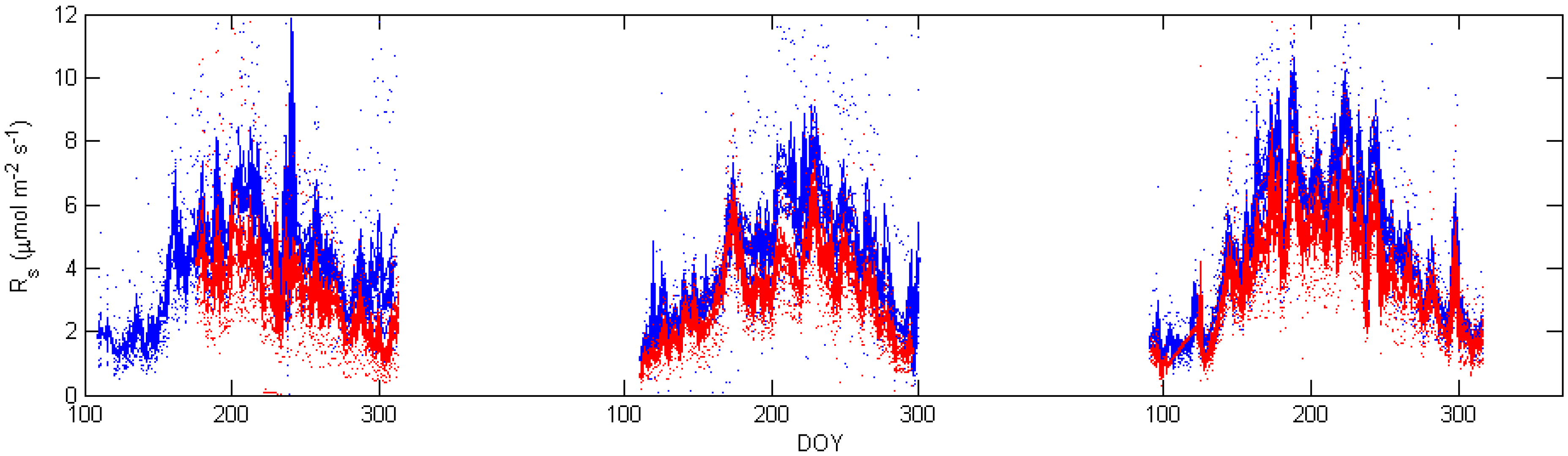
4. Granger Causality


 , and when the multivariate model outperforms the univariate case, (e.g.,
, and when the multivariate model outperforms the univariate case, (e.g.,  ), Y is said to have a causal effect on X (and similarly for the effect of X on Y). This is the statistical interpretation of causality proposed by Granger [11] and is commonly referred to as Granger or G-causality.
), Y is said to have a causal effect on X (and similarly for the effect of X on Y). This is the statistical interpretation of causality proposed by Granger [11] and is commonly referred to as Granger or G-causality. 


 and
and  are the corrected transfer function matrices that separate the pure directional interactions [18]. The rotation matrices P are normalization matrices needed to recast the multivariate systems in the canonical form (with uncorrelated errors).
are the corrected transfer function matrices that separate the pure directional interactions [18]. The rotation matrices P are normalization matrices needed to recast the multivariate systems in the canonical form (with uncorrelated errors). . If Y manifests a causal influence on X at a specific frequency ω then
. If Y manifests a causal influence on X at a specific frequency ω then  .
. 4.1. Estimation of G-Causality
4.2. Direct Connectivity Diagram
- (1)
- Only direct causal interactions are depicted. This implies that if the bivariate interaction is significant, but the multivariate is not, an arrow is not drawn. However, if the bivariate G-causality is not significant, no further action is taken.
- (2)
- If both directional interactions are detected among two nodes, only the greater is retained.
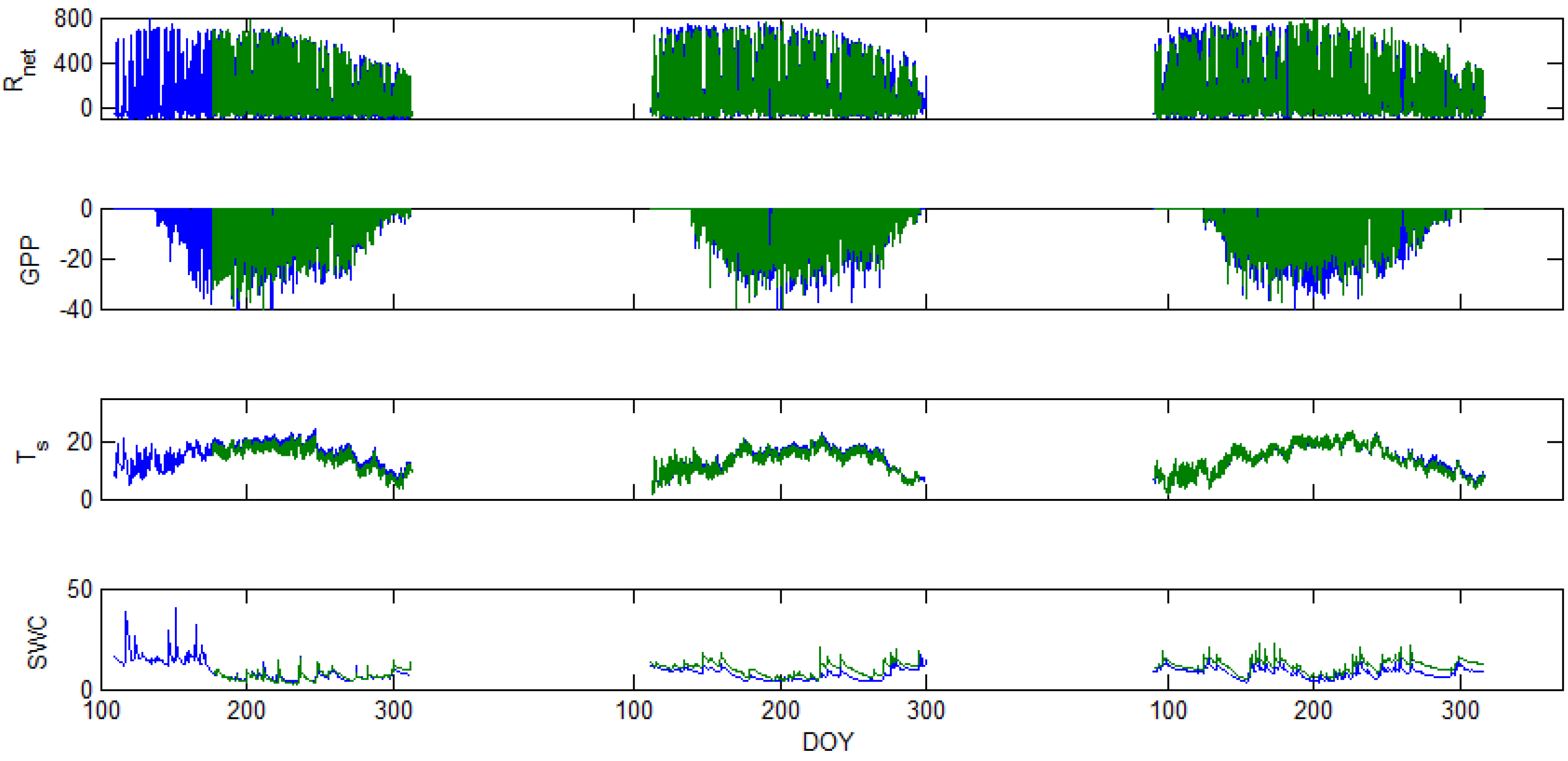
5. Results
 . Confidence intervals obtained upon bootstrapping showed that the differences were not significant (Figure 5).
. Confidence intervals obtained upon bootstrapping showed that the differences were not significant (Figure 5). 
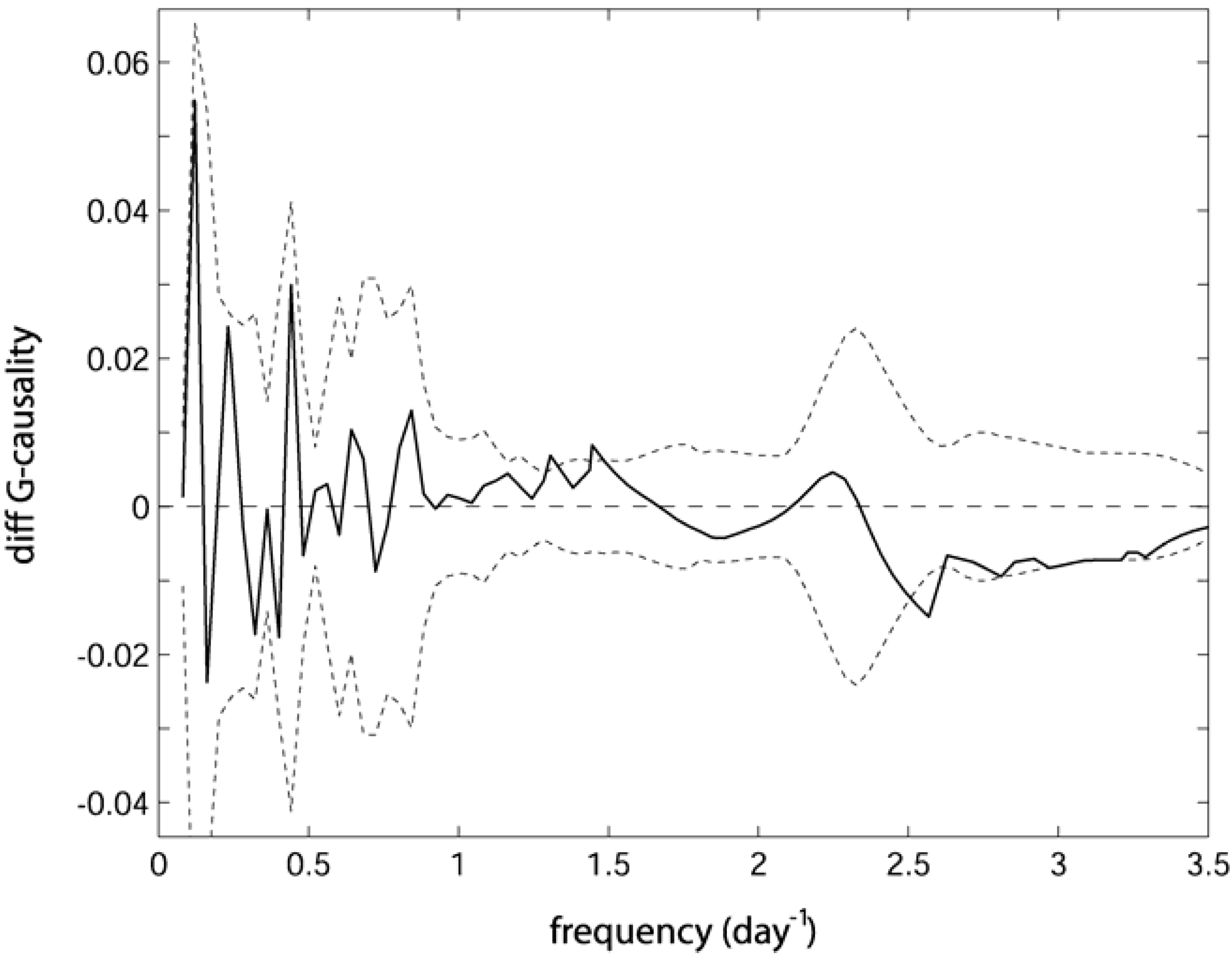
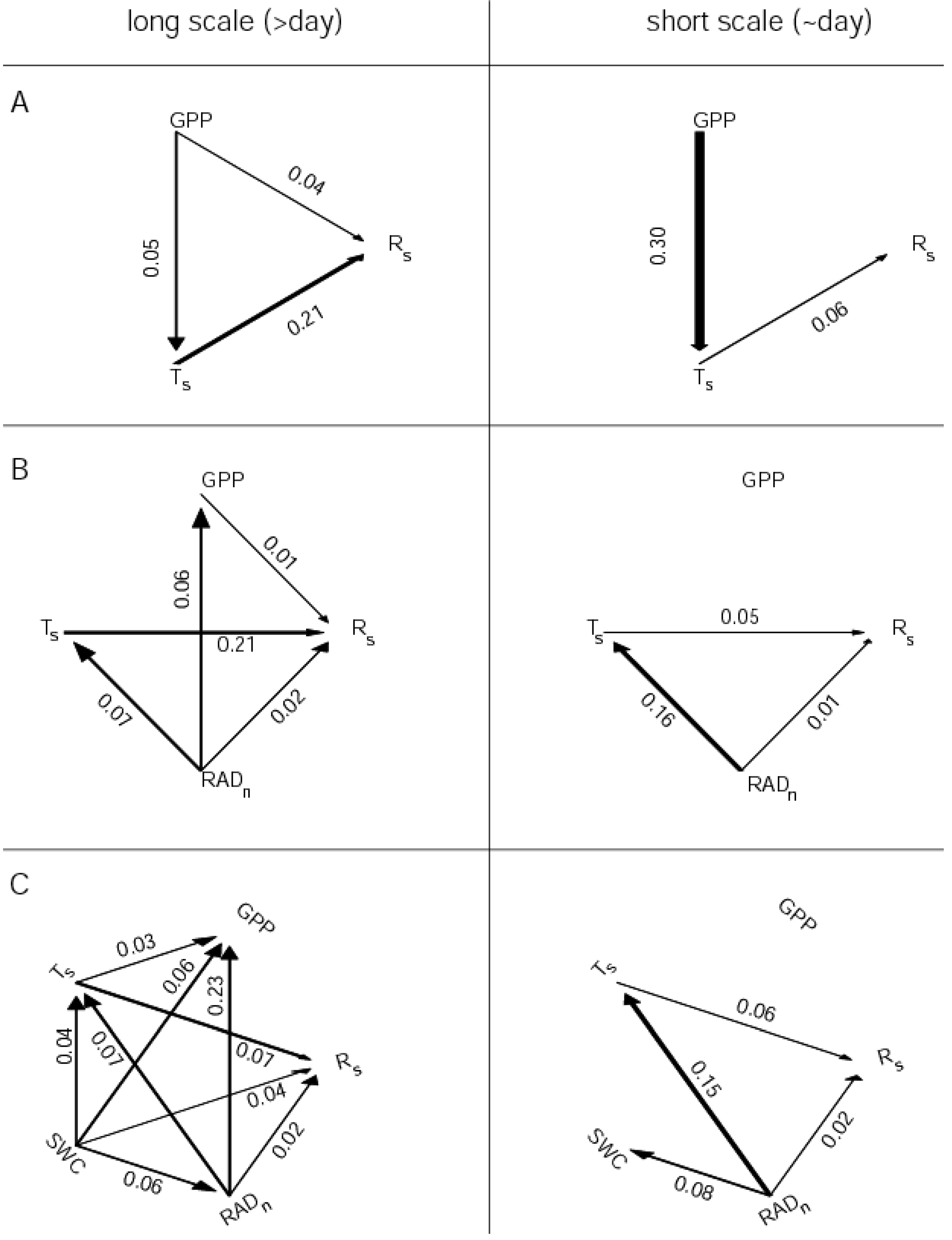
6. Discussion
Acknowledgments
Conflicts of Interest
References
- Melillo, J.M.; Butler, S.; Johnson, J.; Mohan, J.; Steudler, P.; Lux, H.; Burrows, E.; Bowles, F.; Smith, R.; Scott, L.; et al. Soil warming, carbon-nitrogen interactions, and forest carbon budgets. Proc. Natl. Acad. Sci. USA 2011, 108, 9508–9512. [Google Scholar] [CrossRef] [PubMed]
- Bond-Lamberty, B.; Thomson, A. Temperature-associated increases in the global soil respiration record. Nature 2010, 464, 579–582. [Google Scholar] [CrossRef] [PubMed]
- Canadell, J.G.; Le Quéré, C.; Raupach, M.R.; Field, C.B.; Buitenhuis, E.T.; Ciais, P.; Conway, T.J.; Gillett, N.P.; Houghton, R.A.; Marland, G. Contributions to accelerating atmospheric CO2 growth from economic activity, carbon intensity, and efficiency of natural sinks. Proc. Natl. Acad. Sci. USA 2007, 104, 18866–70. [Google Scholar] [CrossRef] [PubMed]
- Fierer, N.; Strickland, M.S.; Liptzin, D.; Bradford, M.A.; Cleveland, C.C. Global patterns in belowground communities. Ecol. Lett. 2009, 12, 1238–1249. [Google Scholar] [CrossRef] [PubMed]
- Brutsaert, W. Evaporation into the Atmosphere: Theory, History, and Applications; Springer: Dordrecht, The Netherlands and Boston, MA, USA, 1982; p. 316. [Google Scholar]
- Shipley, B. Cause and Correlation in Biology; Cambridge University Press: Cambridge, UK, 2000; p. 317. [Google Scholar]
- Wiener, N. The Theory of Prediction. In Modern Mathematics for Engineers; Beckenbach, E.F., Ed.; McGraw-Hill: New York, NY, USA, 1956; Volume 1, pp. 165–183. [Google Scholar]
- Barnett, L.; Barrett, A.; Seth, A. Granger causality and transfer entropy are equivalent for Gaussian variables. Phys. Rev. Lett. 2009, 103, 238701. [Google Scholar] [CrossRef] [PubMed]
- Hlavácková-Schindler, K. Equivalence of Granger causality and transfer entropy: A generalization. Appl. Math. Sci. 2011, 5, 3637–3648. [Google Scholar]
- Granger, C.W.J. Some recent developments in a concept of causality. J. Econom. 1988, 39, 199–211. [Google Scholar] [CrossRef]
- Granger, C.W.J. Investigating causal relations by econometric models and cross-spectral methods. Econometrica 1969, 37, 424–438. [Google Scholar] [CrossRef]
- Salvucci, G.D.; Saleem, J.A.; Kaufmann, R. Investigating soil moisture feedbacks on precipitation with tests of Granger causality. Adv Water Resour. 2002, 25, 1305–1312. [Google Scholar] [CrossRef]
- Kaufmann, R.K.; Paletta, L.F.; Tian, H.Q.; Myneni, R.B.; D’Arrigo, R.D. The power of monitoring stations and a CO2 fertilization effect: Evidence from causal relationships between NDVI and carbon dioxide. Earth Interact. 2008, 12, 1–23. [Google Scholar]
- Smirnov, D.A.; Mokhov, I.I. From Granger causality to long-term causality: Application to climatic data. Phys. Rev. E 2009, 80, 016208. [Google Scholar] [CrossRef]
- Detto, M.; Molini, A.; Katul, G.; Stoy, P.; Palmroth, S.; Baldocchi, D. Causality and persistence in ecological systems: A nonparametric spectral Granger causality approach. Am. Nat. 2012, 179, 524–535. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Ruddell, B.L. Information driven ecohydrologic self-organization. Entropy 2010, 12, 2085–2096. [Google Scholar] [CrossRef]
- Ruddell, B.L.; Kumar, P. Ecohydrologic process networks: 2. Analysis and characterization. Water Resour. Res. 2009, 45. [Google Scholar] [CrossRef]
- Geweke, J. Measurement of linear dependence and feedback between multiple time series. J. Am. Stat. Assoc. 1982, 77, 304–313. [Google Scholar] [CrossRef]
- Dhamala, M.; Rangarajan, G.; Ding, M. Estimating Granger causality from Fourier and wavelet transforms of time series data. Phys. Rev. Lett. 2008, 100, 1–4. [Google Scholar] [CrossRef]
- Baldocchi, D.; Falge, E.; Wilson, K. A spectral analysis of biosphere–atmosphere trace gas flux densities and meteorological variables across hour to multi-year time scales. Agricul. For. Meteorol. 2001, 107, 1–27. [Google Scholar] [CrossRef]
- Katul, G.; Lai, C.; Schäfer, K. Multiscale analysis of vegetation surface fluxes: From seconds to years. Adv. Water Resour. 2001, 24, 1119–1132. [Google Scholar] [CrossRef]
- Hatala, J.A.; Detto, M.; Baldocchi, D.D. Gross ecosystem photosynthesis causes a diurnal pattern in methane emission from rice. Geophys. Res. Lett. 2012, 39. [Google Scholar] [CrossRef]
- Kilburn, P.D. Effects of logging and fire on xerophytic forests in northern Michigan. Bull. Torrey Bot. Club 1960, 87, 402–405. [Google Scholar] [CrossRef]
- Nave, L.E.; Gough, C.M.; Maurer, K.D.; Bohrer, G.; Hardiman, B.S.; Le Moine, J.; Munoz, A.B.; Nadelhoffer, K.J.; Sparks, J.P.; Strahm, B.D.; et al. Disturbance and the resilience of coupled carbon and nitrogen cycling in a north temperate forest. J. Geophys. Res. Biogeosci. 2011, 116. [Google Scholar] [CrossRef]
- Stoy, P.C.; Palmroth, S.; Oishi, A.C.; Siqueira, M.B.S.; Juang, J.-Y.; Novick, K.A.; Ward, E.J.; Katul, G.G.; Oren, R. Are ecosystem carbon inputs and outputs coupled at short time scales? A case study from adjacent pine and hardwood forests using impulse-response analysis. Plant Cell Environ. 2007, 30, 700–710. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.; Phillips, C.; Schmidt, A.; Irvine, J.; Law, B. High-frequency analysis of the complex linkage between soil CO2 fluxes, photosynthesis and environmental variables. Tree Physiol. 2012, 32, 49–64. [Google Scholar] [CrossRef] [PubMed]
- Bouma, T.; Nielsen, K.; Eissenstat, D.; Lynch, J. Estimating respiration of roots in soil: interactions with soil CO2, soil temperature and soil water content. Plant Soil 1997, 195, 221–232. [Google Scholar] [CrossRef]
- Nave, L.; Vogel, C. Contribution of atmospheric nitrogen deposition to net primary productivity in a northern hardwood forest. Can. J. For. Res. 2009, 39, 1108–1118. [Google Scholar] [CrossRef]
- MatLab, version 7.6.0 R2008a; software for technical computation, Mathworks: Natick, MA, USA, 2008.
- Curtis, P.S.; Vogel, C.S.; Gough, C.M.; Schmid, H.P.; Su, H.-B.; Bovard, B.D. Respiratory carbon losses and the carbon-use efficiency of a northern hardwood forest, 1999-2003. New Phytol. 2005, 167, 437–455. [Google Scholar] [CrossRef] [PubMed]
- Lee, X.J.; Finnigan, J.; Paw U, K.T. Coordinate Systems and Flux Bias Error. In Handbook of Micrometeorology, a Guide for Surface Flux Measurement and Analysis; Lee, X., Massman, W., Law, B., Eds.; Kluwer Academic: Dordrecht, The Netherlands, 2004; pp. 33–66. [Google Scholar]
- Massman, W.J. A simple method for estimating frequency response corrections for eddy covariance systems. Agricul. For. Meteorol. 2000, 104, 185–198. [Google Scholar] [CrossRef]
- Schotanus, P.; Nieuwstadt, F.T.M.; Bruin, H.A.R. Temperature measurement with a sonic anemometer and its application to heat and moisture fluxes. Bound.-Layer Meteorol. 1983, 26, 81–93. [Google Scholar] [CrossRef]
- Liu, H.; Peters, G.; Foken, T. New equations for sonic temperature variance and buoyancy heat flux with an omnidirectional sonic anemometer. Bound.-Layer Meteorol. 2001, 100, 459–468. [Google Scholar] [CrossRef]
- Kaimal, J.C.; Gaynor, J.E. Another look at sonic thermometry. Bound.-Layer Meteorol. 1991, 56, 401–410. [Google Scholar] [CrossRef]
- Munger, J.W.; Loescher, H.W. Guidelines For Making Eddy Covariance Flux Measurements, 2009. Available online: http://public.ornl.gov/ameriflux/measurement_standards_020209.doc (accessed on 5 October 2013).
- Webb, E.K.; Pearman, G.I.; Leuning, R. Correction of flux measurements for density effects due to heat and water vapour transfer. Quart. J. Roy. Meteorol. Soc. 1980, 106, 85–100. [Google Scholar] [CrossRef]
- Detto, M.; Katul, G.G. Simplified expressions for adjusting higher-order turbulent statistics obtained from open path gas analyzers. Bound.-Layer Meteorol. 2007, 122, 205–216. [Google Scholar] [CrossRef]
- Garrity, S.R.; Bohrer, G.; Maurer, K.D.; Mueller, K.L.; Vogel, C.S.; Curtis, P.S. A comparison of multiple phenology data sources for estimating seasonal transitions in deciduous forest carbon exchange. Agricul. For. Meteorol. 2011, 151, 1741–1752. [Google Scholar] [CrossRef]
- Reichstein, M.; Falge, E.; Baldocchi, D.; Papale, D.; Aubinet, M.; Berbigier, P.; Bernhofer, C.; Buchmann, N.; Gilmanov, T.; Granier, A.; et al. On the separation of net ecosystem exchange into assimilation and ecosystem respiration: review and improved algorithm. Glob. Change Biol. 2005, 11, 1424–1439. [Google Scholar] [CrossRef]
- Papale, D.; Reichstein, M.; Aubinet, M.; Canfora, E.; Bernhofer, C.; Kutsch, W.; Longdoz, B.; Rambal, S.; Valentini, R.; Vesala, T.; et al. Towards a standardized processing of net ecosystem exchange measured with eddy covariance technique: Algorithms and uncertainty estimation. Biogeosciences 2006, 3, 571–583. [Google Scholar] [CrossRef]
- Schmid, H.P. Ecosystem-atmosphere exchange of carbon dioxide over a mixed hardwood forest in northern lower Michigan. J. Geophys. Res. 2003, 108. [Google Scholar] [CrossRef]
- Lasslop, G.; Migliavacca, M.; Bohrer, G.; Reichstein, M.; Bahn, M.; Ibrom, A.; Jacobs, C.; Kolari, P.; Papale, D.; Vesala, T.; et al. On the choice of the driving temperature for eddy-covariance carbon dioxide flux partitioning. Biogeosciences 2012, 9, 5243–5259. [Google Scholar] [CrossRef]
- Papale, D.; Valentini, R. A new assessment of European forests carbon exchanges by eddy fluxes and artificial neural network spatialization. Glob. Change Biol. 2003, 9, 525–535. [Google Scholar] [CrossRef]
- Detto, M.; Montaldo, N.; Albertson, J.D.; Mancini, M.; Katul, G. Soil moisture and vegetation controls on evapotranspiration in a heterogeneous Mediterranean ecosystem on Sardinia, Italy. Water Resour. Res. 2006, 42. [Google Scholar] [CrossRef]
- Chen, B.; Black, T.A.; Coops, N.C.; Hilker, T.; Trofymow, J.A.; Morgenstern, K. Assessing tower flux footprint climatology and scaling between remotely sensed and eddy covariance measurements. Bound.-Layer Meteorol. 2008, 130, 137–167. [Google Scholar] [CrossRef]
- Masani, P. Recent Trends in Multivariate Prediction Theory. In Multivariate Analysis, Proceedings of International Symposium on Multivariate Analysis, Dayton, OH, USA, 1965; Academic Press: New York, NY, USA, 1966; pp. 351–382. [Google Scholar]
- Chen, Y.; Bressler, S.L.; Ding, M. Frequency decomposition of conditional Granger causality and application to multivariate neural field potential data. J. Neurosci. Methods 2006, 150, 228–37. [Google Scholar] [CrossRef] [PubMed]
- Hlavackova-Schindler, K.; Paluš, M.; Vejmelkab, M.; Bhattacharya, J. Causality detection based on information-theoretic approaches in time series analysis. Phys. Rep. 2007, 441, 1–46. [Google Scholar] [CrossRef]
- Masani, P. Wiener’s contribution to generalized harmonic analysis prediction theory and filter theory. Bull. Am. Math. Soc. 1966, 72, 73–125. [Google Scholar] [CrossRef]
- Wilson, G. The factorization of matricial spectral densities. SIAM J. Appl. Math. 1972, 23, 420–426. [Google Scholar] [CrossRef]
- Schreiber, T.; Schmitz, A. Surrogate time series. Physica D 2000, 142, 346–382. [Google Scholar] [CrossRef]
- Hibbard, K.A.; Law, B.E.; Reichstein, M.; Sulzman, J. An analysis of soil respiration across northern hemisphere temperate ecosystems. Biogeochemistry 2005, 73, 29–70. [Google Scholar] [CrossRef]
- Levy-Varon, J.H.; Schuster, W.S.F.; Griffin, K.L. The autotrophic contribution to soil respiration in a northern temperate deciduous forest and its response to stand disturbance. Oecologia 2012, 169, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Ryan, M.G.; Law, B.E. Interpreting, measuring, and modeling soil respiration. Biogeochemistry 2005, 73, 3–27. [Google Scholar] [CrossRef]
- Elberling, B. Seasonal trends of soil CO2 dynamics in a soil subject to freezing. J. Hydrol. 2003, 276, 159–175. [Google Scholar] [CrossRef]
- Lin, Z.; Zhang, R.; Tang, J.; Zhang, J. Effects of high soil water content and temperature on soil respiration. Soil Sci. 2011, 176, 150–155. [Google Scholar] [CrossRef]
- Braswell, B.; Sacks, W. Estimating diurnal to annual ecosystem parameters by synthesis of a carbon flux model with eddy covariance net ecosystem exchange observations. Glob. Change Biol. 2005, 11, 335–355. [Google Scholar] [CrossRef]
- Kodama, N.; Barnard, R.L.; Salmon, Y.; Weston, C.; Ferrio, J.P.; Holst, J.; Werner, R.A.; Saurer, M.; Rennenberg, H.; Buchmann, N.; Gessler, A. Temporal dynamics of the carbon isotope composition in a Pinus sylvestris stand: From newly assimilated organic carbon to respired carbon dioxide. Oecologia 2008, 156, 737–750. [Google Scholar] [CrossRef] [PubMed]
- Kuzyakov, Y.; Gavrichkova, O. Review: Time lag between photosynthesis and carbon dioxide efflux from soil: A review of mechanisms and controls. Glob. Change Biol. 2010, 16, 3386–3406. [Google Scholar] [CrossRef]
- Werner, C.; Gessler, A. Diel variations in the carbon isotope composition of respired CO2 and associated carbon sources: A review of dynamics and mechanisms. Biogeosciences 2011, 8, 2437–2459. [Google Scholar] [CrossRef]
- Hardiman, B.S.; Bohrer, G.; Gough, C.M.; Vogel, C.S.; Curtis, P.S. The role of canopy structural complexity in wood net primary production of a maturing northern deciduous forest. Ecology 2011, 92, 1818–1827. [Google Scholar] [CrossRef] [PubMed]
- Bohrer, G.; Katul, G.; Walko, R.; Avissar, R. Exploring the effects of microscale structural heterogeneity of forest canopies using large-eddy simulations. Bound.-Layer Meteorol. 2009, 132, 351–382. [Google Scholar] [CrossRef]
- Maurer, K.D.; Hardiman, B.S.; Vogel, C.S.; Bohrer, G. Canopy-structure effects on surface roughness parameters: Observations in a Great Lakes mixed-deciduous forest. Agricul. For. Meteorol. 2013, 177, 24–34. [Google Scholar] [CrossRef]
- Gough, C.M.; Hardiman, B.S.; Nave, L.; Bohrer, G.; Maurer, K.D.; Vogel, C.S.; Nadelhoffer, K.J.; Curtis, P.S. Sustained carbon uptake and storage following moderate disturbance in a Great Lakes forest. Ecol. Appl. 2013, 23, 1202–1215. [Google Scholar] [CrossRef] [PubMed]
- Högberg, P.; Nordgren, A.; Buchmann, N.; Taylor, A.F.; Ekblad, A.; Högberg, M.N.; Nyberg, G.; Ottosson-Löfvenius, M.; Read, D.J. Large-scale forest girdling shows that current photosynthesis drives soil respiration. Nature 2001, 411, 789–92. [Google Scholar] [CrossRef] [PubMed]
- Betson, N.R.; Göttlicher, S.G.; Hall, M.; Wallin, G.; Richter, A.; Högberg, P. No diurnal variation in rate or carbon isotope composition of soil respiration in a boreal forest. Tree Physiol. 2007, 27, 749–56. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.W.; Baldocchi, D.D.; Xu, L. Tree photosynthesis modulates soil respiration on a diurnal time scale. Glob. Change Biol. 2005, 11, 1298–1304. [Google Scholar] [CrossRef]
- Mencuccini, M.; Hölttä, T. The significance of phloem transport for the speed with which canopy photosynthesis and belowground respiration are linked. New Phytol. 2010, 185, 189–203. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Zhang, Y.; Lin, Y.; Zhu, W.; Fu, S. Changes in belowground carbon in Acacia crassicarpa and Eucalyptus urophylla plantations after tree girdling. Plant Soil 2010, 326, 123–135. [Google Scholar] [CrossRef]
- Edwards, N.T.; Ross-Todd, B.M. Effects of stem girdling on biogeochemical cycles within mixed deciduous forest in Eastern Tennessee. 1. Soil solition chamistry, soil respiration, litterfall and root biomass studies. Oecologia 1979, 40, 247–257. [Google Scholar] [CrossRef]
- Morehouse, K.; Johns, T.; Kaye, J.; Kaye, A. Carbon and nitrogen cycling immediately following bark beetle outbreaks in southwestern ponderosa pine forests. For. Ecol. Manag. 2008, 255, 2698–2708. [Google Scholar] [CrossRef]
- Hicke, J.A.; Allen, C.D.; Desai, A.R.; Dietze, M.C.; Hall, R.J.; Hogg, E.H.; Kashian, D.M.; Moore, D.; Raffa, K.F.; Sturrock, R.N.; et al. Effects of biotic disturbances on forest carbon cycling in the United States and Canada. Glob. Change Biol. 2012, 18, 7–34. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Detto, M.; Bohrer, G.; Nietz, J.G.; Maurer, K.D.; Vogel, C.S.; Gough, C.M.; Curtis, P.S. Multivariate Conditional Granger Causality Analysis for Lagged Response of Soil Respiration in a Temperate Forest. Entropy 2013, 15, 4266-4284. https://doi.org/10.3390/e15104266
Detto M, Bohrer G, Nietz JG, Maurer KD, Vogel CS, Gough CM, Curtis PS. Multivariate Conditional Granger Causality Analysis for Lagged Response of Soil Respiration in a Temperate Forest. Entropy. 2013; 15(10):4266-4284. https://doi.org/10.3390/e15104266
Chicago/Turabian StyleDetto, Matteo, Gil Bohrer, Jennifer Goedhart Nietz, Kyle D. Maurer, Chris S. Vogel, Chris M. Gough, and Peter S. Curtis. 2013. "Multivariate Conditional Granger Causality Analysis for Lagged Response of Soil Respiration in a Temperate Forest" Entropy 15, no. 10: 4266-4284. https://doi.org/10.3390/e15104266





