2. The Organism and the Environment
In the following I shall introduce a simplification. Since the energetic cost of bacterial chemotaxis is relative low, this allows us to ideally split the whole process in a pure informational part in which we can avoid thermodynamic considerations, and a metabolic step (in which the bacterium feeds) in which those considerations are relevant. It is true that recent studies show that there is also an energy taxis, through which cells do not navigate toward the greatest concentration(s) of effectors but seek positions where metabolic rates are optimized [
3,
4]. However, the fact that there is here a correlation between the strength of a chemostimulus as an attractant and its efficiency as a growth substrate suggests that this behavior is particularly relevant not very far away from the energy source, where the metabolic step as such is accomplished.
A generic principle that describes how organisms deal with environmental information has been proposed in [
5,
6,
7,
8]. Although originally formulated to describe how the brain deals with sensory information, the principle can be easily generalized to describe any exchanges of an orgasm with its environment ([
9], Chapters 7 and 8). This (free energy minimisation) principle provides the starting point for our formulation: Consider the environmental input
i as it is encoded by the receptors of a bacterium; the internal state
s describing the state of the organism; an action parameter
a describing the bacterium’s action (reaction), and an unknown or hidden environmental state
k that generates environmental input (for example, the relative concentration of attractants or repellents). Given these variables, we can posit a function
F expressing the mean logarithmic difference between the probability of both the input
i and the external state
k, conditional on the action
a, on the one hand, and a probability distribution over the external state
k, on the other (the derivation of these equalities can be found in
appendix for the interested reader):
The prime on the distribution
has the purpose to show that this is another kind probability (dealing with other sets of events) relative to
. The internal parameter
s expresses both the organism’s response to the external parameter
k and the internal contribution of the genetic system [
Figure 1]. Then, parameter
s is not determined by the exterior only but is a sort of encoding of the behavior taking into account also an internal component. Moreover,
displays a semi-column, because the probability distribution is over the external states
k, while the internal states
s play the role of sufficient statistics that parameterize this distribution. The quantity
expresses the probability of changes in the parameters
k and
i given the final choice of an action
a (therefore it implements a feedback loop). The functional
F is known as the analogue of free energy, were the expressions
denotes a statistical average over the distribution
and lg is the binary logarithm. The first term in the last equality is the analogue of (Gibbs) energy and the second term is the negative entropy of the distribution over external states. In other words, the free-energy function is the Gibbs energy function minus the entropy of
. Generally, we can express the (Shannon) entropy of a probability distribution, say
, as
In information theory, the expression
is called
surprisal and denotes the unexpectedness of any given event (under some action
a). In other words, it represents the mismatch between what the organism expects given its action and what actually happens. Free energy can also be expressed as surprisal plus a Kullback–Leibler divergence or relative entropy. This divergence measures the difference in entropy between the distribution parameterized by the internal states and the (true) probability distribution over external states, given the current inputs (see
Appendix A):
We could also formulate Equation (1) as (see
Appendix B)
whose second term shows the entropic distance computed on the two distributions
p and
as functions of the same parameter
k. Crucially the cross entropy or divergence in Equations (3) and (4) can never be less than zero. This means that when the divergence is zero, free energy becomes surprisal. Technically this means that the function
F is, by construction, an upper bound on surprisal. What is important here is that the bacterium is able to minimize its surprisal by minimizing the function
F [
10]. The time average of the free energy is always greater than the time average of surprisal. This is important because, under ergodic assumptions, the time average of surprisal is equal to the entropy of the inputs:
almost surely. This is crucial because it means that we can explain how action resists the natural tendency of systems towards disorder (cf., the second law of thermodynamics) and places an upper bound on the entropy of the inputs
and, implicitly, the external states causing those inputs. Therefore, minimizing the function
F makes the bacterium able to reduce the gap between the expected causes of its inputs (following its action
a) and actual causes (expressed in the current input
i). This provides a mechanism for maintaining a homoeostasis.
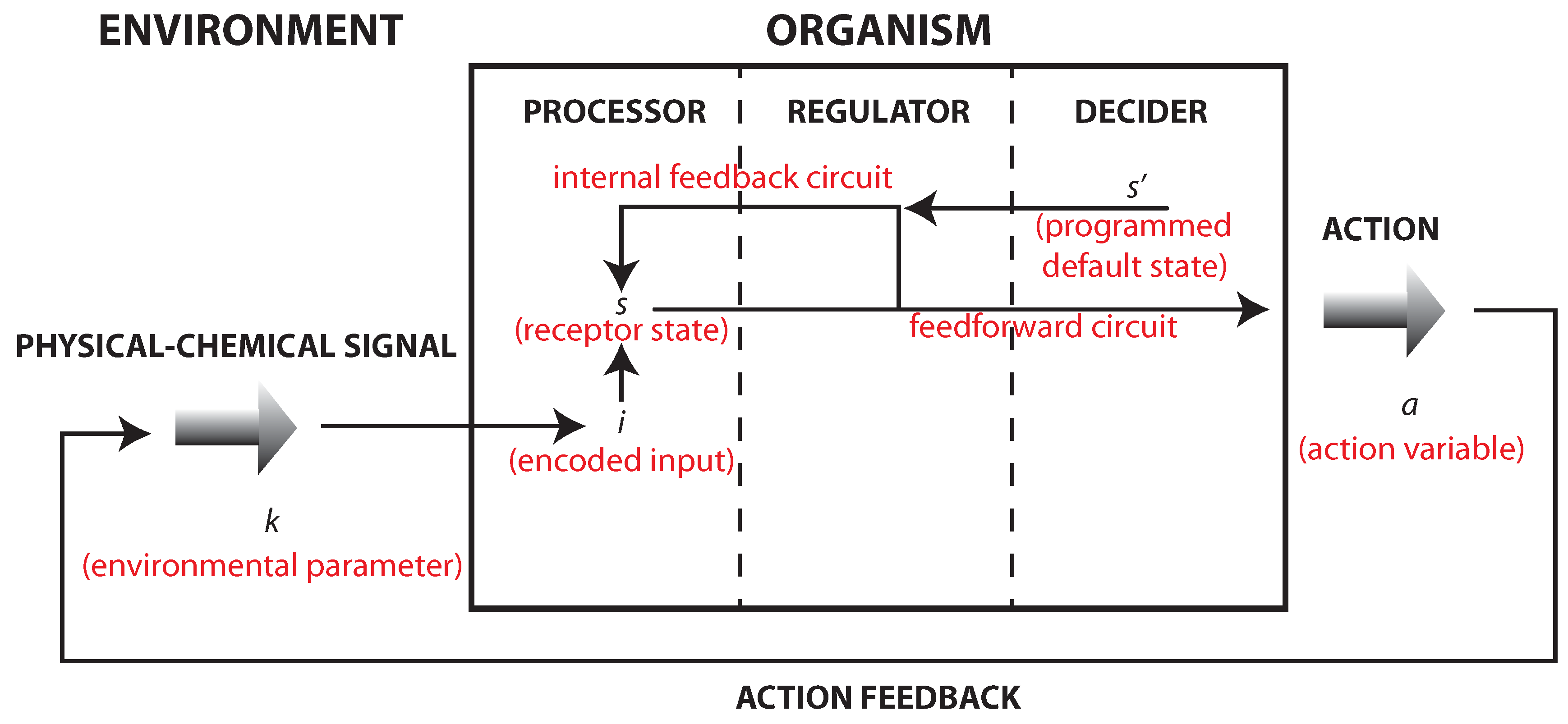
Figure 1.
A reformulation of Friston’s model that accounts for the studies of bacterial chemotaxis that is also quite in accordance with the model presented in [
11] (see also [
9], Chapters 2 and 8). The action
a on the environment will change environmental states. Even a simple motion away from a certain signal source will induce a change external states and consequence input. This establishes an internal–external feedback circuit (the action
a changes the external state
k that determines the input
i). Internally, there are two kinds of processes: (i) information transmission processes (from
s to
a) displaying a feedforward activity; which (ii) are coupled to internal feedback circuit (from
to
s).
Figure 1.
A reformulation of Friston’s model that accounts for the studies of bacterial chemotaxis that is also quite in accordance with the model presented in [
11] (see also [
9], Chapters 2 and 8). The action
a on the environment will change environmental states. Even a simple motion away from a certain signal source will induce a change external states and consequence input. This establishes an internal–external feedback circuit (the action
a changes the external state
k that determines the input
i). Internally, there are two kinds of processes: (i) information transmission processes (from
s to
a) displaying a feedforward activity; which (ii) are coupled to internal feedback circuit (from
to
s).
Note that the physical motion of the bacterium—although representing a kind of random walk—violates the principle of detailed balance, according to which there are as many transitions per time unit from a position
x to a position
as from from
to
x ([
12], pp. 77 and 89). Indeed, it is this sort of directed motion that integrates random fluctuations in pursuit of a biological goal, as we shall see below (this can obviously also described using statistical methods and computer simulations [
13]). However, before turning to goals and homoeostatic set points, we consider another profound implication of minimizing the function
F.
Action minimizes
F by changing the input so that it reduces surprisal; but what about the internal states? From the second equality in Equation (1), it can be seen that the only way internal states can minimize free energy is by reducing the divergence between the probability distribution over external states, parameterized by internal states and the true (posterior) distribution over external states. In other words, the internal states come to parameterize (approximately) the true distribution over external states causing inputs. In this sense, they represent or codify the hidden external causes of inputs in their environment. This is entirely consistent with the good regulator hypothesis that states any self-organizing homoeostatic system
must have a model of the environment in which it is immersed [
11,
14]. In the current formulation, this model is probabilistic, such that hidden environmental states are inferred—in a Bayesian sense—when internal states minimize free energy.
Although we can account for homoeostasis and a resistance to disorder using free-energy function minimization, we have not explained why bacteria act to increase their sensed levels of attractants and avoid repellents. This speaks to some internal or intrinsic preferences that can be considered in terms of the set points of their homoeostatic behavior. I shall associate these preferred states with a subset of internal states denoted by
. As noted above, internal states can be regarded as representations of hidden external states. In this interpretation, the subset
play the role of default representations that are endowed by natural selection and define the external states preferred by the organism. In brief, these internal states determine what is surprising and enable action to minimize surprisal or prediction error to realize these default or preferred states. In more detail: The existence of any (bi) partition of internal states means that all organisms have two internal processes for dealing with environmental information [
Figure 1]: a feedforward mapping from the internal state
s to the action
a and a second process that provides constraints on
s, provided by the (default or homeostatic) state
of the system. In other words, the internal states
s representing external states are compared with the expected homeostatic state
to reduce the gap (prediction error) between
s and
. This is an important aspect of minimizing surprisal; in the sense that surprisal is not just the difference between the predicted input and the input encountered—but it also includes the difference between the predictions
before and after seeing input. In Bayesian statistics, these homoeostatic predictions are known as prior expectations or beliefs. This means that genetically endowed prior beliefs, entailed by the internal states and configuration of the organism (denoted by
) specify what is innately surprising and enable action to counter unpredicted deviations from expected states. In the context of chemotaxis, one might imagine that the bacterium has prior beliefs that it should occupy regimes with high concentrations of chemical attractants. Action is then enslaved, through minimization of the function
F, to search out these regimes.
In short, action will take into account both contributions from the informational evidence afforded by environment and the homoeostatic (prior) states that constitute the organism. As a consequence, the organism will tend to restore its homeostatic state and undertake appropriate actions for reducing any gap between inferred and expected external states. As we shall see next, s can be regarded as an estimate of the external state of the environment, while the apparent goal of the system is to maintain these hidden states close to its prior expectations (), in the presence of environmental fluctuations.
I have largely cast my treatment purely in terms of information theory. However, it is also possible to talk about the quantities explicitly in terms of Bayesian inference [
15]. In this context, cells that minimize the free-energy function implicitly minimize surprisal. Surprisal is also known as negative Bayesian model evidence in statistics. This means that minimizing the function
F is equivalent to maximizing the Bayesian evidence for an organism’s model of its external milieu. In this context, we can associate the internal states (
) with Bayesian representations or sufficient statistics of the posterior probability
over the external states of the environment. In other words, the internal states encode or represent the external states
k. Crucially, we can interpret
as prior expectations or beliefs about external states. In other words, the cell is equipped with prior beliefs
about the external states it should encounter; where
places constraints on the posterior representations
s that are trying to explain the input. We can thus understand the homeostasis afforded by minimization of
F in terms of action fulfilling posterior beliefs that are constrained by prior expectations, due to the cells internal structure and states. In this sense, the cell is literally representing or recognizing (in a Bayesian sense) the causes of its input and is acting to sample those inputs that it expects to encounter.
These expectations are set by the internal states , which provide prior constraints on expectations and therefore active sampling of the chemical sensorium. By analogy with free energy minimization in the brain, we can therefore associate chemotaxis with the dual processes of action and perception. Action corresponds to movement that minimizes the surprisal of sensory inputs, while perception corresponds to changes in internal states that try to explain sensory inputs. Both of these processes are minimizing free energy.
In the most elementary case, that of bacteria, such dual processes have been well studied: the feedforward process is based on phosphorylation, while the feedback process is based on methylation–demethylation (as well as the action of the protein CheZ that dephosphorylates the protein CheY responsible for the motor output). However, I assume that these dual processes are present—in some form—in all organisms.
3. Signals and Signs
Pursuing the dual process notion of the previous section, we can split self-organization into two main parts (
Figure 2):
The former entails the endogenous, programmed, default-state constraints associated with the organism: it is the way in which the organism tries to assimilate the external environment by selectively sampling predicted inputs. The latter represents the changes in the bacterium’s state induced by the environment: this is the way in which the organism accommodates the external environment and its inherent fluctuations. Action only makes sense only when it is useful to reach a goal (maximal concentration of sugars for feeding) at a later time. Therefore, this goal needs to be independent of changes in the external states and therefore needs to be genetically programmed [
16]. Little is known about the genetic (expression–repression) factors involved in chemotaxis, but see the pioneering study [
17]. This also justifies the necessity of an endogenous component in chemotaxis. However, even in the absence of a true epigenetic process, the genetic component only represents a part of the explanation for bacterial behavior, which belies its ability to react to unexpected environmental fluctuations. This illustrates that the evolutionary stable outcome of natural selection is precisely the combination of these two components that constitute the minimal level of complexity that is necessary for survival. This can be understood in two senses: it is the level under which the system is most statistically inefficient and—on the other hand—this minimal level is selected because it is more metabolically efficient. In other words, one can assume that natural selection drives representational capacity—inherent in a cells intracellular processing—towards a low level of complexity. Mathematically, this tendency to find simple or parsimonious mechanisms to suppress the variability (and surprisal) of inputs (that underwrites homeostasis) can be seen as a natural consequence of minimizing free energy at an evolutionary timescale. In informational terms, this can be expressed formally using Equation (4):
The second term is the divergence between the (parameterized) posterior density
and the prior probability over hidden external states parameterized by
. In statistics this is known as complexity—which means that minimizing the function
F minimizes the complexity of internal representations of hidden external states [
18]. Roughly speaking, the divergence or complexity in Equation (6) is proportional to the number of internal states used to encode or represent the causes of the inputs.
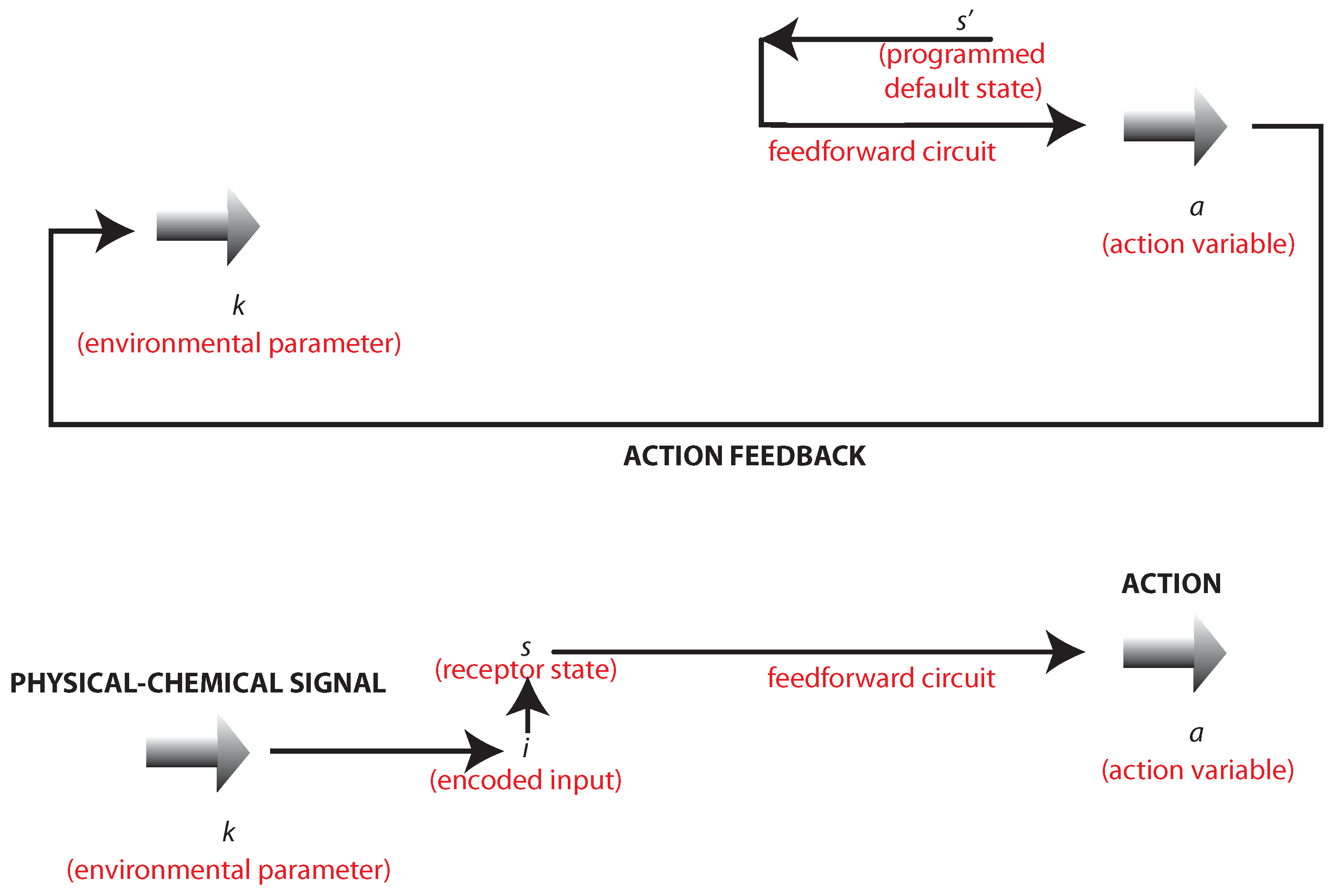
Figure 2.
This diagram illustrates the bidirectional or circular causality discussed in the main text. Top: the action a of the organism on the environment resulting from a programmed goal: the maintenance of the default state . Bottom: the action a of the organism as induced by the codification of the physical-chemical signal.
Figure 2.
This diagram illustrates the bidirectional or circular causality discussed in the main text. Top: the action a of the organism on the environment resulting from a programmed goal: the maintenance of the default state . Bottom: the action a of the organism as induced by the codification of the physical-chemical signal.
In the case of the bacterium
E. coli, the goal to reach the maximal concentration of nutrients is attained by swimming in one direction (with a corresponding action
a) in presence of a low activity of the CheA—determining counterclockwise rotation of the flagella (for details on the molecular machinery of receptors see [
19]). Conversely, if there is high concentration of repellents, the external signal is codified by the receptors in such a way to determine a high–level of the CheA (the induced internal state
s), which gives rise to clockwise rotation of the flagella (due to the action of the final protein CheY that is phosphorylated). This changes the direction of swimming, through tumbling and the bacterium remains in the vicinity of high nutrient concentrations, until these concentrations fall. Once tumbling occurs, the dual process of perception and action can start again—as external states change and are registered by receptive input. Note that we can consider the genetically programmed effect (prior expectations) as inducing a feedback contribution to the action—and we can consider the presence of attractants (inducing changes in the internal state
s) as a source of environmental contribution to action [
20]. Since the latter depends on action through consequent changes in external states, we have something very similar to the action–perception cycle, with a recurrent emission of optimal action and adjustments to internal states that inform the action and are informed by the consequences of action.
Therefore, in themselves, the two processes (from the interior to the exterior and from the exterior to the interior) can be understood as pure information–transmission (or information–processing) dynamics. In this sense, we can say that information–transmission (perception or recognizing external states) is first-order, while chemotaxis is second-order: resting on both perception and action. Such a second-order process can be considered as a controlled exchange of signs [
21,
22]. In fact, the input
i can be called an informative factor, since it informs the organism about the state of the world outside. It therefore stands for the physical-chemical signal. The action determines or “points to" the external signal (for instance, the concentration of repellents as expressed by the external states
k); like an
index used to introduce some kind of modification. In this setting,
is the ideal default state that the organism tends to maintain, and therefore constrains or informs the induced state
s—enabling action to fulfill the constrained or biased estimate of external states. The default states could be called an
icon: that is, the internal structure that represents the states that the organism expects to occupy (or to become) in the external world. In other words, the input
i is informative precisely because it can be placed in relation to the external signal but, in turn, it can play the role of something standing for the external signal, because there is an indexical action towards the signal and an icon, under which not only this action is generated but also
i is evaluated (through the induced state
s). Therefore, the state
s becomes in turn both the representation of the external signal (through
i) and also the representation of the gap relative to the programmed state (through comparison with
). Furthermore, action could be understood—to a certain extent—as a representation or reflection of this gap, although its function is pragmatic (
i.e., to change the organisms relationship with the external environment; e.g., by moving). I shall deal with this issue below. Obviously, an “evaluation" of the state
s or the input
i in bacteria and in lower eukaryotes only exists in chemical reactions induced by different concentration levels of certain molecules. However, we should not conflate the mechanism—through which this process is implemented—with the informational-cybernetic process itself. Indeed it is only through essential needs encoded by
that the organism has “chosen" to sample specific physical-chemical signals in an environment, where the possible signals are potentially infinite. One could say that such a “choice" is a result of natural selection, enabling the organism to select and deal with specific information, and this is what really matters in the end (as it enables survival).
4. Information Control
The processes sketched above are chemical and their induction can be due to mechanical forces, but the biological and survival-related significance is informational, given the crucial importance of the “comparison" between the current state
s and the default state
. In particular, the significance of the whole process is that of an information-control mechanism:
Information control is any procedure through which a system (i) ascertains the functional relevance of a certain signal (where, as said, a signal can be understood as any modification of a physical or chemical medium); and (ii) tends to re-establish a certain steady or default state when certain operations of functional–vital relevance are impaired or perturbed.
Figure 3.
Top: on the left the instructional semiotic process, on the right the representational one. They correspond roughly to the top and bottom parts of
Figure 2, respectively. Bottom: the whole semiotic circle of information control that corresponds to
Figure 1.
Figure 3.
Top: on the left the instructional semiotic process, on the right the representational one. They correspond roughly to the top and bottom parts of
Figure 2, respectively. Bottom: the whole semiotic circle of information control that corresponds to
Figure 1.
Therefore, we deal here with the pursuit of a goal—due to a programmed behavior—through continuous error correction [
23]. One can summarize the dual processes above with the help of two semiotic triangles (exhibiting a cyclical nature) as shown in
Figure 3. The triangle on the left shows the process going from the icon
—through
a—to the external parameter
k while the second triangle shows the mapping from
k—through
i—to
a. The first process can also be called an
instructional one, since its source is ultimately in the codification of the genetic material giving rise to a set of instructions—executed through the whole machinery of the RNA until proteins (for instance the CheZ) are formed and the appropriate action performed. Conversely, the second process, going from the exterior to the interior can be called
representational,
i.e., the constitution of a model [
11,
14]. Here, we have the external signal that is codified at the surface of the organism (membrane) where specific receptors produce the input
i that leads to action
a. Obviously, we do not need to suppose that we have (in bacteria) the complexity required for an elaborate representation (which is likely to require a central nervous system). Nevertheless, a general representational function cannot be denied to the humble bacterium since, as mentioned, it is able to monitor the environment and to translate the external situation using an internal model implicit in its intracellular chemistry. Taking into account the previous considerations, action has two different aspects; according to the process involved: In the instructional process,
a represents the assimilation (active sampling) while in the representational process is a represents the accommodation (perceptual adjustment).
In the instructional process, a represents the assimilation whilst
In the representational process a represents the accommodation.
The two processes can be summarized as in the triangle at the bottom of
Figure 3. Obviously, the external parameter
k is hidden in the internal terms
i and
a. I emphasize that it is the connection between these two processes (instructional and representational)—which, considered separately, are nothing but pure information transmission—that ensures any detection of an external signal, once that it has been codified and compared with the icon, becomes a sign of whether the direction of swimming is good or bad (consistent with prior beliefs). Therefore, it indicates whether the organism is approaching or receding from the nutrients necessary for self-maintenance. It may be helpful to refer to the schematic overview of the combined process in
Figure 4.
Figure 4.
The instructional information bridges between genetic information (the icon) and a given functionality (the referent) necessary for survival, while information acquisition from the exterior allows the organism to be informed about the (induced) changes of the environment (the referent) due to appropriate functional steps (the icon). Through regulation, these functionalities couple back to the genetic system allowing for expression or repression. Ensuing distributed circuit therefore displays information control. Adapted from [
9].
Figure 4.
The instructional information bridges between genetic information (the icon) and a given functionality (the referent) necessary for survival, while information acquisition from the exterior allows the organism to be informed about the (induced) changes of the environment (the referent) due to appropriate functional steps (the icon). Through regulation, these functionalities couple back to the genetic system allowing for expression or repression. Ensuing distributed circuit therefore displays information control. Adapted from [
9].
5. Informational and Metabolic Steps
It is important to stress that the significance of the endogenous process going from the interior to the exterior is to ensure the maintenance of the internal order against environmental perturbations. This can only be accomplished with appropriate and consequently controlled metabolic exchanges with the environment. Therefore, the mechanism of information control is coupled to, and ultimately serves the metabolic self-maintenance of the organism that constitutes a cybernetic system. From an informational point of view, the imperative is to minimize the informational free energy
F. If
F is minimized (and implicitly surprisal), the organism will seek out nutrients, which in turn means it can maintain a thermodynamic exchange with the environment that is favorable to the maintenance of internal order. Such maintenance of order is necessary to physically encode prior beliefs or referents, thus enabling information control. So, the information-control and thermodynamic (or entropic) aspects constitute a single cybernetic, self-organizing process with circular causality [
Figure 5]—a circular causality that we can separate into two processes:
One can consider the dual (action–perception) process as a part of the thermodynamic exchange of the organism. At this level, genetic systems are able to induce the motor–metabolic system to perform an action that has a relative low energetic cost and that is far from thermodynamic equilibrium. Therefore, the sensory-selective system exhibits order parameters;
i.e., parameters that are able to drive a system far from equilibrium ([
12], pp. 191–200). Note that the time constants of action are much shorter than the genetic process.
Conversely, from the point of view of metabolic–thermodynamic processes, it is the action–perception system (the gate proteins) that is controlled by the genetic system (this now provides the order parameters).
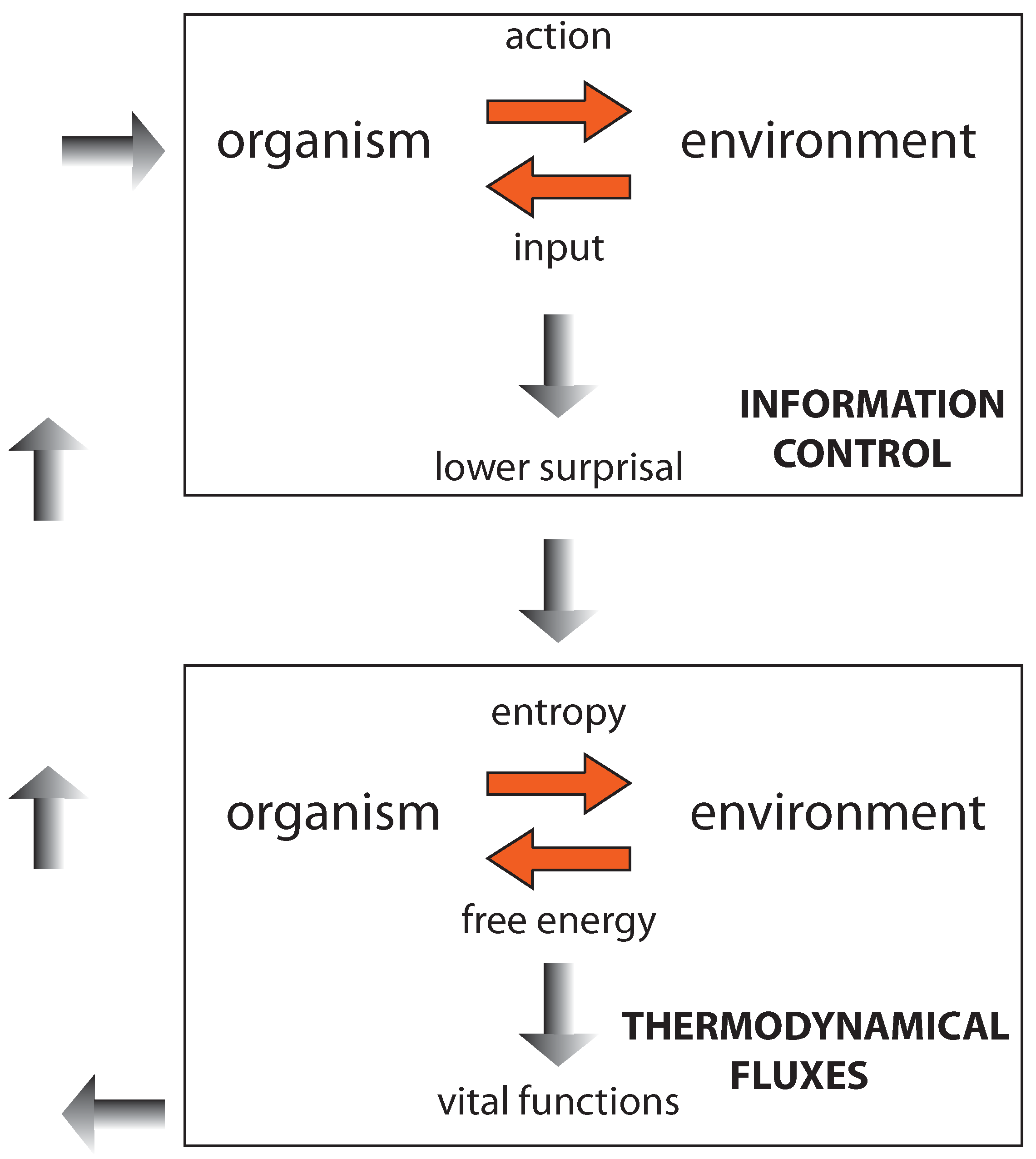
Figure 5.
The figure should be read first from the top to bottom (top-down, following the gray-shadowed arrows in the middle): The information-control step leads—through appropriate actions—to lower the surprisal, enabling the organism to approach and consume nutrients. Then, the second (thermodynamicit) step (represented at the bottom) starts, through which appropriate metabolic exchanges with the environment are deployed and controlled. This enables the organism to maintain its order and preserve its configuration (encoding its prior beliefs) so that it can engage in a further information-control step—to find new energy sources or, alternatively, to reproduce through binary fission. Here, we go from the bottom towards the top (bottom-up, following the gray arrows on the left).
Figure 5.
The figure should be read first from the top to bottom (top-down, following the gray-shadowed arrows in the middle): The information-control step leads—through appropriate actions—to lower the surprisal, enabling the organism to approach and consume nutrients. Then, the second (thermodynamicit) step (represented at the bottom) starts, through which appropriate metabolic exchanges with the environment are deployed and controlled. This enables the organism to maintain its order and preserve its configuration (encoding its prior beliefs) so that it can engage in a further information-control step—to find new energy sources or, alternatively, to reproduce through binary fission. Here, we go from the bottom towards the top (bottom-up, following the gray arrows on the left).
It is worth noting that the metabolic level itself can be rather complex—even in bacteria, so that many different kinds of action and control can be involved ([
9], Ch. 11). Again, a good example is provided by
E. coli. I shall follow here the somehow particular but, for our purposes, instructive model of Jacob and Monod [
24,
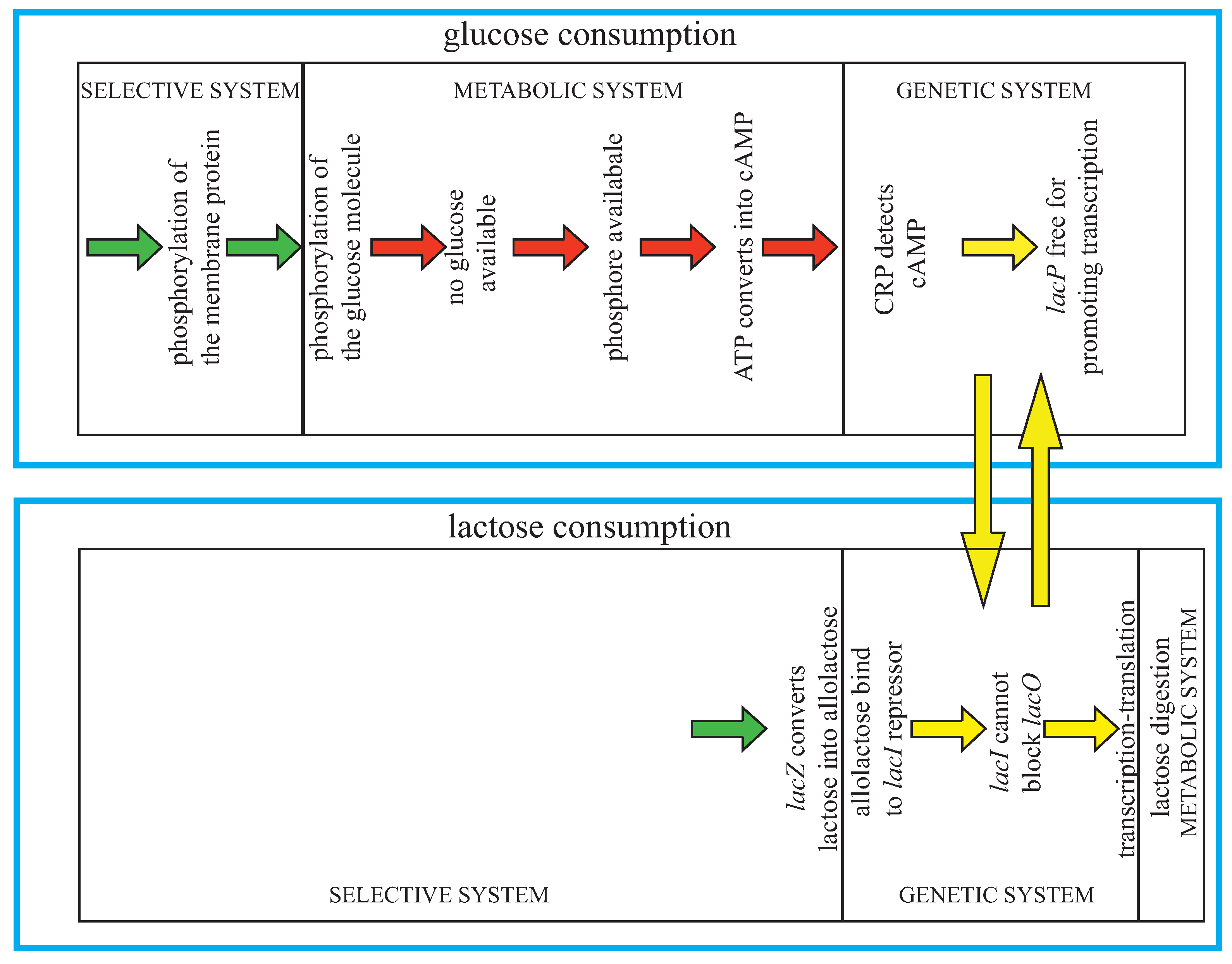
25]. As is well known, the bacterium can discriminate between glucose and lactose—with a mixture of the two sugars, it shows a preference for glucose, since it first consumes the glucose and only then digests lactose. When glucose is available, a membrane-associated protein (which becomes phosphorylated) is involved in transporting glucose into the cell and passes the phosphate group to the glucose. When glucose is consumed, the protein remains phosphorylated—since there is nothing to receive the phosphate group, and is consequently able to convert ATP (adenosine triphosphate) into cAMP (cyclic adenosine monophosphate), thus raising the cellular concentration of cAMP. The cell uses the phosphorylated transport protein and a high cAMP concentration as a signal indicating that glucose is no longer available. Namely, the cAMP concentration is read by the cAMP Receptor Protein (CRP), which binds to the CRP site in lac, only in the presence of abundant cAMP. This then stabilizes the contact between lacP (the −10 and −35 regions of the canonical 70 promoters) and RNA polymerase and therefore signals that the lac operon (lacO) is ready for transcription. In the absence of lactose, however, transcription events are rare, since the lac repressor molecules bind to two of the operator sites (O1 and O2) and create a loop in the DNA, blocking the access to the promoter lacP. When lactose is available the protein
β-galactosidase, coded by lacZ, converts some of these sugar molecules to a related sugar called allolactose, which can bind to the lac repressor, inducing a change in the shape of the repressor that makes it unable to bind lacO and so freeing lacP for transcription [
Figure 6].
Figure 6.
Jacob and Monod’s cybernetic model of
lac operon. Adapted from [
9].
Figure 6.
Jacob and Monod’s cybernetic model of
lac operon. Adapted from [
9].
In summary, Jacob and Monod discovered that in the absence of lactose, the repressor gene codes for a protein that binds to the promoter of the gene coding for the enzyme that is able to digest lactose, thus preventing its transcription. When the E. coli is in a solution of lactose, this substance is allowed to enter the cell to bind the repressor proteins inhibiting the transcription of the genes encoding the lactose metabolizing enzyme. This shows that a molecule activates an expression that is necessary for its own metabolism, a beautiful example of feedback and circular causality. Moreover, note that in order to digest lactose, both control conditions must be satisfied: a negative one (due to concentration) and a positive one (due to the presence of lactose) involving the transformation of lactose molecules into allolactose.