Pharmaceutics 2023, 15(4), 1193; https://doi.org/10.3390/pharmaceutics15041193 - 9 Apr 2023
Cited by 5 | Viewed by 4795
Abstract
Pain is the most common symptom that dentists are confronted with, whether acute (pulpitis, acute periodontitis, post-surgery, etc.) or chronic diseases, such as periodontitis, muscle pain, temporomandibular joint (TMJ) disorders, burning mouth syndrome (BMS), oral lichen planus (OLP) and others. The success of
[...] Read more.
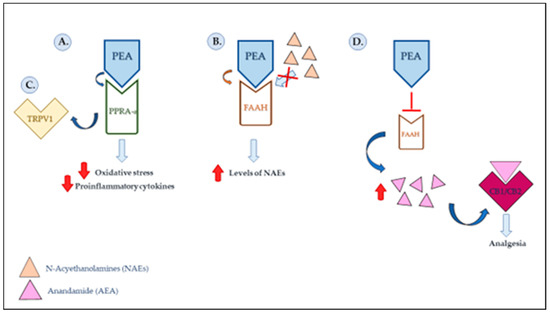
Pain is the most common symptom that dentists are confronted with, whether acute (pulpitis, acute periodontitis, post-surgery, etc.) or chronic diseases, such as periodontitis, muscle pain, temporomandibular joint (TMJ) disorders, burning mouth syndrome (BMS), oral lichen planus (OLP) and others. The success of therapy depends on the reduction in and management of pain through specific drugs, hence the need to analyze new pain medications with specific activity, which are suitable for long-term use, with a low risk of side effects and interactions with other drugs, and capable of leading to a reduction in orofacial pain. Palmitoylethanolamide (PEA) is a bioactive lipid mediator, which is synthesized in all tissues of the body as a protective pro-homeostatic response to tissue damage and has aroused considerable interest in the dental field due to its anti-inflammatory, analgesic, antimicrobial, antipyretic, antiepileptic, immunomodulatory and neuroprotective activities. It has been observed that PEA could play a role in the management of the pain of orofacial origin, including BMS, OLP, periodontal disease, tongue a la carte and temporomandibular disorders (TMDs), as well as in the treatment of postoperative pain. However, actual clinical data on the use of PEA in the clinical management of patients with orofacial pain are still lacking. Therefore, the main objective of the present study is to provide an overview of orofacial pain in its many manifestations and an updated analysis of the molecular pain-relieving and anti-inflammatory properties of PEA to understand its beneficial effects in the management of patients with orofacial pain, both neuropathic and nociceptive in nature. The aim is also to direct research toward the testing and use of other natural agents that have already been shown to have anti-inflammatory, antioxidant and pain-relieving actions and could offer important support in the treatment of orofacial pain.
Full article
(This article belongs to the Special Issue Drug Delivery and Pharmacokinetics in Oral Medicine and Dental Infection)
►
Show Figures