Molecules 2014, 19(2), 1763-1774; https://doi.org/10.3390/molecules19021763 - 3 Feb 2014
Cited by 8 | Viewed by 7166
Abstract
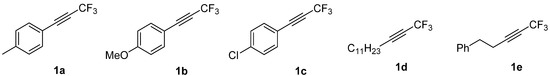
The scope of the Pauson-Khand reaction (PKR) of internal trifluoromethyl alkynes, previously described with norbornadiene, is expanded to norbornene and ethylene. A thorough structural analysis of the resulting PK adducts has been carried out to unveil that α-trifluoromethylcyclopentenones are preferred in all cases,
[...] Read more.
The scope of the Pauson-Khand reaction (PKR) of internal trifluoromethyl alkynes, previously described with norbornadiene, is expanded to norbornene and ethylene. A thorough structural analysis of the resulting PK adducts has been carried out to unveil that α-trifluoromethylcyclopentenones are preferred in all cases, independently of the electronic properties of the alkyne. The regioselectivity observed with norbornadiene and ethylene is higher than in the case of norbornene.
Full article
(This article belongs to the Special Issue Fluorine Chemistry 2016)
►
Show Figures