A Spectroscopic Study on Secondary Structure and Thermal Unfolding of the Plant Toxin Gelonin Confirms Some Typical Structural Characteristics and Unravels the Sequence of Thermal Unfolding Events
Abstract
:1. Introduction
2. Results and Discussion
2.1. Analysis of Gelonin Secondary Structure
2.2. Thermal Unfolding
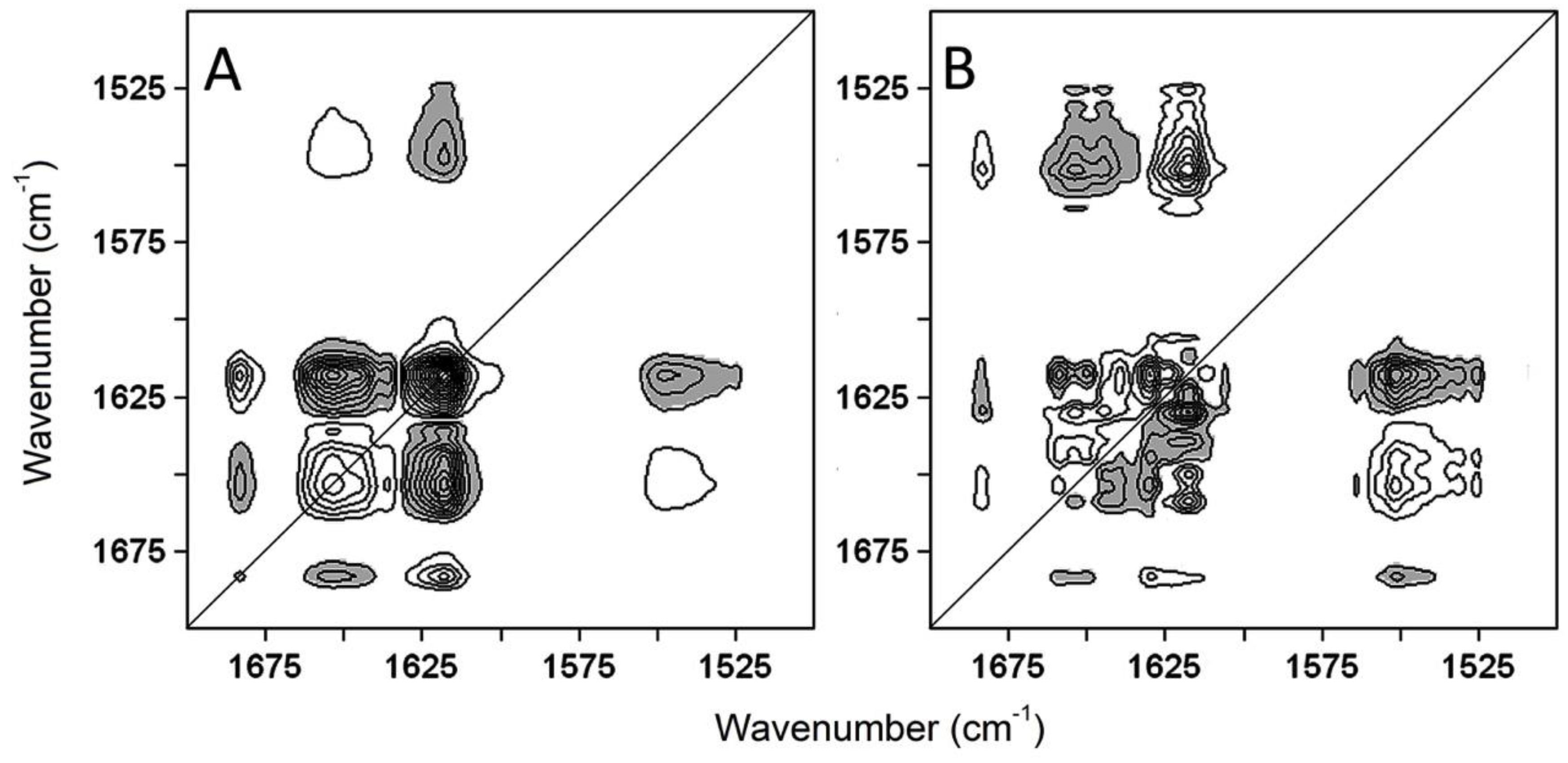
2.3. The Sequence of the Thermal Unfolding Events
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. FTIR Spectra
4.3. Phase Diagram Method
4.4. Two-Dimensional Correlation Spectroscopy (2D-COS)
Author Contributions
Funding
Conflicts of Interest
References
- Mehta, K.; Ocanas, L.; Malavasi, F.; Marks, J.W.; Rosenblum, M.G. Retinoic acid-induced CD38 antigen as a target for immunotoxin-mediated killing of leukemia cells. Mol. Cancer 2004, 3, 345–352. [Google Scholar]
- Rosenblum, M.G.; Marks, J.W.; Cheung, L.H. Comparative cytotoxicity and pharmacokinetics of antimelanoma immunotoxins containing either natural or recombinant gelonin. Cancer Chemother. Pharm. 1999, 44, 343–348. [Google Scholar] [CrossRef] [PubMed]
- Rosenblum, M.G.; Shawver, L.K.; Marks, J.W.; Brink, J.; Cheung, L.; Langton–Webster, B. Recombinant immunotoxins directed against the c–erb–2/HER2/neu oncogene product: in vitro cytotoxicity, pharmacokinetics, and in vivo efficacy studies in xenograft models. Clin. Cancer Res. 1999, 5, 865–874. [Google Scholar] [PubMed]
- Rosenblum, M.G.; Zuckerman, J.E.; Marks, J.W.; Rotbein, J.; Allen, W.R. A gelonin-containing immunotoxin directed against human breast carcinoma. Mol. Biother. 1992, 4, 122–129. [Google Scholar] [PubMed]
- Bolognesi, A.; Polito, L.; Tazzari, P.L.; Lemoli, R.M.; Lubelli, C.; Fogli, M.; Boon, L.; de Boer, M.; Stirpe, F. In vitro anti-tumour activity of anti-CD80 and anti-CD86 immunotoxins containing type 1 ribosome-inactivating proteins. Br. J. Haematol. 2000, 110, 351–361. [Google Scholar] [CrossRef]
- Lee-Huang, S.; Huang, P.L.; Huang, P.L.; Bourinbaiar, A.S.; Chen, H.C.; Kung, H.F. Inhibition of the integrase of human immunodeficiency virus (HIV) type 1 by anti-HIV plant proteins MAP30 and GAP31. Proc. Natl. Acad. Sci. USA 1995, 92, 8818–8822. [Google Scholar] [CrossRef]
- Lee-Huang, S.; Kung, H.F.; Huang, P.L.; Huang, P.L.; Li, B.Q.; Huang, P.; Huang, H.I.; Chen, H.C. A new class of anti-HIV agents: GAP31, DAPs 30 and 32. Febs Lett. 1991, 291, 139–144. [Google Scholar] [CrossRef] [Green Version]
- Li, H.G.; Huang, P.L.; Zhang, D.; Sun, Y.; Chen, H.C.; Zhang, J.; Huang, P.L.; Kong, X.P.; Lee-Huang, S. A new activity of anti-HIV and anti-tumor protein GAP31: DNA adenosine glycosidase-structural and modeling insight into its functions. Biochem. Biophys. Res. Commun. 2010, 391, 340–345. [Google Scholar] [CrossRef]
- Bourinbaiar, A.S.; Lee-Huang, S. The activity of plant-derived antiretroviral proteins MAP30 and GAP31 against herpes simplex virus in vitro. Biochem. Biophys. Res. Commun. 1996, 219, 923–929. [Google Scholar] [CrossRef]
- Sun, Y.; Huang, P.L.; Li, J.J.; Huang, Y.Q.; Zhang, L.; Huang, P.L.; Lee-Huang, S. Anti-HIV agent MAP30 modulates the expression profile of viral and cellular genes for proliferation and apoptosis in AIDS-related lymphoma cells infected with Kaposi’s sarcoma-associated virus. Biochem. Biophys. Res. Commun. 2001, 287, 983–994. [Google Scholar] [CrossRef]
- Lee-Huang, S.; Huang, P.L.; Sun, Y.; Chen, H.C.; Kung, H.F.; Huang, P.L.; Murphy, W.J. Inhibition of MDA-MB-231 human breast tumor xenografts and HER2 expression by anti-tumor agents GAP31 and MAP30. Anticancer. Res. 2000, 20, 653–659. [Google Scholar] [PubMed]
- Schreiber, C.A.; Wan, L.; Sun, Y.; Lu, L.; Krey, L.C.; Lee-Huang, S. The antiviral agents, MAP30 and GAP31, are not toxic to human spermatozoa and may be useful in preventing the sexual transmission of human immunodeficiency virus type 1. Fertil. Steril. 1999, 72, 686–690. [Google Scholar] [CrossRef]
- Bolognesi, A.; Bortolotti, M.; Maiello, S.; Battelli, M.G.; Polito, L. Ribosome-Inactivating Proteins from Plants: A Historical Overview. Molecules 2016, 21, 1627. [Google Scholar] [CrossRef] [PubMed]
- Stirpe, F.; Barbieri, L.; Battelli, M.G.; Soria, M.; Lappi, D.A. Ribosome-inactivating proteins from plants: present status and future prospects. Biotechnology 1992, 10, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Barbieri, L.; Battelli, M.G.; Stirpe, F. Ribosome-inactivating proteins from plants. Biochim. Biophys. Acta. 1993, 1154, 237–282. [Google Scholar] [CrossRef]
- Madan, S.; Ghosh, P.C. Interaction of gelonin with macrophages: effect of lysosomotropic amines. Exp. Cell Res. 1992, 198, 52–58. [Google Scholar] [CrossRef]
- De Virgilio, M.; Lombardi, A.; Caliandro, R.; Fabbrini, M.S. Ribosome-inactivating proteins: from plant defense to tumor attack. Toxins 2010, 2, 2699–2737. [Google Scholar] [CrossRef]
- Lambert, J.M.; Blattler, W.A.; McIntyre, G.D.; Goldmacher, V.S.; Scott, C.F., Jr. Immunotoxins containing single-chain ribosome-inactivating proteins. Cancer Treat. Res. 1988, 37, 175–209. [Google Scholar]
- Endo, Y.; Tsurugi, K. The RNA N-glycosidase activity of ricin A-chain. The characteristics of the enzymatic activity of ricin A-chain with ribosomes and with rRNA. J. Biol. Chem. 1988, 263, 8735–8739. [Google Scholar]
- Stirpe, F.; Bailey, S.; Miller, S.P.; Bodley, J.W. Modification of ribosomal RNA by ribosome-inactivating proteins from plants. Nucleic Acids Res. 1988, 16, 1349–1357. [Google Scholar] [CrossRef] [Green Version]
- FitzGerald, D.J.; Wayne, A.S.; Kreitman, R.J.; Pastan, I. Treatment of hematologic malignancies with immunotoxins and antibody-drug conjugates. Cancer Res. 2011, 71, 6300–6309. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, K.; Nakajima, O.; Morishita, R.; Nagai, R. Targeted anticancer immunotoxins and cytotoxic agents with direct killing moieties. Scientific World J. 2006, 6, 781–790. [Google Scholar] [CrossRef] [PubMed]
- Sonnichsen, B.; De Renzis, S.; Nielsen, E.; Rietdorf, J.; Zerial, M. Distinct membrane domains on endosomes in the recycling pathway visualized by multicolor imaging of Rab4, Rab5, and Rab11. J. Cell Biol. 2000, 149, 901–914. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, D. Why toxins! Semin. Cancer Biol. 1996, 7, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Weyergang, A.; Selbo, P.K.; Berstad, M.E.; Bostad, M.; Berg, K. Photochemical internalization of tumor-targeted protein toxins. Lasers Surg. Med. 2011, 43, 721–733. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, H.; Bachran, C.; Li, T.; Heisler, I.; Durkop, H.; Sutherland, M. A cleavable molecular adapter reduces side effects and concomitantly enhances efficacy in tumor treatment by targeted toxins in mice. J. Control. Release 2007, 117, 342–350. [Google Scholar] [CrossRef] [PubMed]
- Lizzi, A.R.; D’Alessandro, A.M.; Zeolla, N.; Brisdelli, F.; D’Andrea, G.; Pitari, G.; Oratore, A.; Bozzi, A.; Ippoliti, R. The effect of AZT and chloroquine on the activities of ricin and a saporin-transferrin chimeric toxin. Biochem. Pharm. 2005, 70, 560–569. [Google Scholar] [CrossRef] [PubMed]
- Weldon, J.E.; Xiang, L.; Chertov, O.; Margulies, I.; Kreitman, R.J.; FitzGerald, D.J.; Pastan, I. A protease-resistant immunotoxin against CD22 with greatly increased activity against CLL and diminished animal toxicity. Blood 2009, 113, 3792–3800. [Google Scholar] [CrossRef] [Green Version]
- Garay, R.P.; El-Gewely, R.; Armstrong, J.K.; Garratty, G.; Richette, P. Antibodies against polyethylene glycol in healthy subjects and in patients treated with PEG-conjugated agents. Expert Opin. Drug Deliv. 2012, 9, 1319–1323. [Google Scholar] [CrossRef]
- Schellekens, H.; Hennink, W.E.; Brinks, V. The immunogenicity of polyethylene glycol: facts and fiction. Pharm. Res. 2013, 30, 1729–1734. [Google Scholar] [CrossRef]
- Byler, D.M.; Susi, H. Examination of the secondary structure of proteins by deconvolved FTIR spectra. Biopolymers 1986, 25, 469–487. [Google Scholar] [CrossRef] [PubMed]
- Arrondo, J.L.; Muga, A.; Castresana, J.; Goni, F.M. Quantitative studies of the structure of proteins in solution by Fourier-transform infrared spectroscopy. Prog. Biophys. Mol. Biol. 1993, 59, 23–56. [Google Scholar] [CrossRef]
- Roque, A.; Ponte, I.; Arrondo, J.L.; Suau, P. Phosphorylation of the carboxy-terminal domain of histone H1: effects on secondary structure and DNA condensation. Nucleic Acids Res. 2008, 36, 4719–4726. [Google Scholar] [CrossRef] [Green Version]
- Surewicz, W.K.; Mantsch, H.H. New insight into protein secondary structure from resolution-enhanced infrared spectra. Biochim. Biophys. Acta 1988, 952, 115–130. [Google Scholar] [CrossRef]
- Surewicz, W.K.; Mantsch, H.H.; Chapman, D. Determination of protein secondary structure by Fourier transform infrared spectroscopy: a critical assessment. Biochemistry 1993, 32, 389–394. [Google Scholar] [CrossRef] [PubMed]
- Krimm, S.; Bandekar, J. Vibrational spectroscopy and conformation of peptides, polypeptides, and proteins. Adv. Protein Chem. 1986, 38, 181–364. [Google Scholar] [PubMed]
- Kennedy, D.F.; Crisma, M.; Toniolo, C.; Chapman, D. Studies of peptides forming 3(10)- and alpha-helices and beta-bend ribbon structures in organic solution and in model biomembranes by Fourier transform infrared spectroscopy. Biochemistry 1991, 30, 6541–6548. [Google Scholar] [CrossRef]
- Hosur, M.V.; Nair, B.; Satyamurthy, P.; Misquith, S.; Surolia, A.; Kannan, K.K. X-ray structure of gelonin at 1.8 A resolution. J. Mol. Biol. 1995, 250, 368–380. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Qu, Y.; Li, H.; Yuan, J. Truncations of gelonin lead to a reduction in its cytotoxicity. Toxicology 2007, 231, 129–136. [Google Scholar] [CrossRef]
- Pal, B.; Bajpai, P.K. Spectroscopic characterization of gelonin-assignments secondary structure and thermal denaturation. Indian J. Biochem. Biophys. 1998, 35, 166–171. [Google Scholar]
- Jackson, M.; Mantsch, H.H. Beware of proteins in DMSO. Biochim. Biophys. Acta 1991, 1078, 231–235. [Google Scholar] [CrossRef]
- Meersman, F.; Smeller, L.; Heremans, K. Comparative Fourier transform infrared spectroscopy study of cold-, pressure-, and heat-induced unfolding and aggregation of myoglobin. Biophys. J. 2002, 82, 2635–2644. [Google Scholar] [CrossRef]
- D’Auria, S.; Scire, A.; Varriale, A.; Scognamiglio, V.; Staiano, M.; Ausili, A.; Marabotti, A.; Rossi, M.; Tanfani, F. Binding of glutamine to glutamine-binding protein from Escherichia coli induces changes in protein structure and increases protein stability. Proteins 2005, 58, 80–87. [Google Scholar] [CrossRef]
- Fukunaga, Y.; Nishimoto, E.; Otosu, T.; Murakami, Y.; Yamashita, S. The unfolding of alpha-momorcharin proceeds through the compact folded intermediate. J. Biochem. 2008, 144, 457–466. [Google Scholar] [CrossRef] [PubMed]
- Fukunaga, Y.; Nishimoto, E.; Yamashita, K.; Otosu, T.; Yamashita, S. The partially unfolded state of beta-momorcharin characterized with steady-state and time-resolved fluorescence studies. J. Biochem. 2007, 141, 9–18. [Google Scholar] [CrossRef]
- McHugh, C.A.; Tammariello, R.F.; Millard, C.B.; Carra, J.H. Improved stability of a protein vaccine through elimination of a partially unfolded state. Protein Sci. 2004, 13, 2736–2743. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.F.; Sui, S.F. The membrane insertion of trichosanthin is membrane-surface-pH dependent. Biochem. J. 2000, 349, 835–841. [Google Scholar] [CrossRef] [Green Version]
- Santanche, S.; Bellelli, A.; Brunori, M. The unusual stability of saporin, a candidate for the synthesis of immunotoxins. Biochem. Biophys. Res. Commun. 1997, 234, 129–132. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, M.; Scire, A.; Tanfani, F.; Ausili, A. The thermal unfolding of the ribosome-inactivating protein saporin-S6 characterized by infrared spectroscopy. Biochim. Biophys. Acta 2015, 1854, 1357–1364. [Google Scholar] [CrossRef] [PubMed]
- Ptitsyn, O.B. Molten globule and protein folding. Adv. Protein Chem. 1995, 47, 83–229. [Google Scholar] [PubMed]
- Ausili, A.; Scire, A.; Damiani, E.; Zolese, G.; Bertoli, E.; Tanfani, F. Temperature-induced molten globule-like state in human a1-acid glycoprotein: an infrared spectroscopic study. Biochemistry 2005, 44, 15997–16006. [Google Scholar] [CrossRef]
- Noda, I. Two-dimensional infrared spectroscopy. J. Am. Chem. Soc. 1989, 111, 8116–8118. [Google Scholar] [CrossRef]
- Savino, C.; Federici, L.; Ippoliti, R.; Lendaro, E.; Tsernoglou, D. The crystal structure of saporin SO6 from Saponaria officinalis and its interaction with the ribosome. Febs Lett. 2000, 470, 239–243. [Google Scholar] [CrossRef]
- Singh, V.; Kar, S.K. Properties of a ribosome-inactivating protein, gelonin, purified using three different methods. Indian J. Biochem. Biophys. 1992, 29, 31–41. [Google Scholar]
- Ausili, A.; Cobucci-Ponzano, B.; Di Lauro, B.; D’Avino, R.; Perugino, G.; Bertoli, E.; Scire, A.; Rossi, M.; Tanfani, F.; Moracci, M. A comparative infrared spectroscopic study of glycoside hydrolases from extremophilic archaea revealed different molecular mechanisms of adaptation to high temperatures. Proteins 2007, 67, 991–1001. [Google Scholar] [CrossRef]
- Pedone, E.; Saviano, M.; Bartolucci, S.; Rossi, M.; Ausili, A.; Scire, A.; Bertoli, E.; Tanfani, F. Temperature-, SDS-, and pH-induced conformational changes in protein disulfide oxidoreductase from the archaeon Pyrococcus furiosus: a dynamic simulation and fourier transform infrared spectroscopic study. J. Proteome Res. 2005, 4, 1972–1980. [Google Scholar] [CrossRef] [PubMed]
- Scire, A.; Marabotti, A.; Staiano, M.; Briand, L.; Varriale, A.; Bertoli, E.; Tanfani, F.; D’Auria, S. Structure and stability of a rat odorant-binding protein: another brick in the wall. J. Proteome Res. 2009, 8, 4005–4013. [Google Scholar] [CrossRef]
- Scire, A.; Baldassarre, M.; Galeazzi, R.; Tanfani, F. Fibrillation properties of human alpha(1)-acid glycoprotein. Biochimie 2013, 95, 158–166. [Google Scholar] [CrossRef]
- Scire, A.; Tanfani, F.; Bertoli, E.; Furlani, E.; Nadozie, H.O.; Cerutti, H.; Cortelazzo, A.; Bini, L.; Guerranti, R. The belonging of gpMuc, a glycoprotein from Mucuna pruriens seeds, to the Kunitz-type trypsin inhibitor family explains its direct anti-snake venom activity. Phytomedicine 2011, 18, 887–895. [Google Scholar] [CrossRef]
- Scire, A.; Baldassarre, M.; Tanfani, F.; Capraro, J.; Duranti, M.; Scarafoni, A. Interaction of gamma-conglutin from Lupinus albus with model phospholipid membranes: Investigations on structure, thermal stability and oligomerization status. Biochim. Biophys. Acta Proteins Proteom. 2018, 1866, 1242–1248. [Google Scholar] [CrossRef]
- Ausili, A.; Cobucci-Ponzano, B.; Di Lauro, B.; D’Avino, R.; Scire, A.; Rossi, M.; Tanfani, F.; Moracci, M. Structural basis of the destabilization produced by an amino-terminal tag in the beta-glycosidase from the hyperthermophilic archeon Sulfolobus solfataricus. Biochimie 2006, 88, 807–817. [Google Scholar] [CrossRef] [PubMed]
- Cerda-Costa, N.; De la Arada, I.; Aviles, F.X.; Arrondo, J.L.; Villegas, S. Influence of aggregation propensity and stability on amyloid fibril formation as studied by Fourier transform infrared spectroscopy and two-dimensional COS analysis. Biochemistry 2009, 48, 10582–10590. [Google Scholar] [CrossRef] [PubMed]
- Koper, T.; Polit, A.; Sobiecka-Szkatula, A.; Wegrzyn, K.; Scire, A.; Figaj, D.; Kadzinski, L.; Zarzecka, U.; Zurawa-Janicka, D.; Banecki, B.; et al. Analysis of the link between the redox state and enzymatic activity of the HtrA (DegP) protein from Escherichia coli. PLoS ONE 2015, 10, e0117413. [Google Scholar] [CrossRef] [PubMed]
- Kuznetsova, I.M.; Turoverov, K.K.; Uversky, V.N. Use of the phase diagram method to analyze the protein unfolding-refolding reactions: fishing out the “invisible” intermediates. J. Proteome Res. 2004, 3, 485–494. [Google Scholar] [CrossRef] [PubMed]
- Lupidi, G.; Camaioni, E.; Khalife, H.; Avenali, L.; Damiani, E.; Tanfani, F.; Scire, A. Characterization of thymoquinone binding to human alpha(1)-acid glycoprotein. J. Pharm. Sci. 2012, 101, 2564–2573. [Google Scholar] [CrossRef] [PubMed]
- Noda, I. Generalized two-dimensional correlation method applicable to infrared, raman, and other types of spectroscopy. Appl. Spectrosc. 1993, 47, 1329–1336. [Google Scholar] [CrossRef]
- Ausili, A.; Di Lauro, B.; Cobucci-Ponzano, B.; Bertoli, E.; Scire, A.; Rossi, M.; Tanfani, F.; Moracci, M. Two-dimensional IR correlation spectroscopy of mutants of the beta-glycosidase from the hyperthermophilic archaeon Sulfolobus solfataricus identifies the mechanism of quaternary structure stabilization and unravels the sequence of thermal unfolding events. Biochem. J. 2004, 384, 69–78. [Google Scholar] [CrossRef]
- Marabotti, A.; Scire, A.; Staiano, M.; Crescenzo, R.; Aurilia, V.; Tanfani, F.; D’Auria, S. Wild-type and mutant bovine odorant-binding proteins to probe the role of the quaternary structure organization in the protein thermal stability. J. Proteome Res. 2008, 7, 5221–5229. [Google Scholar] [CrossRef]
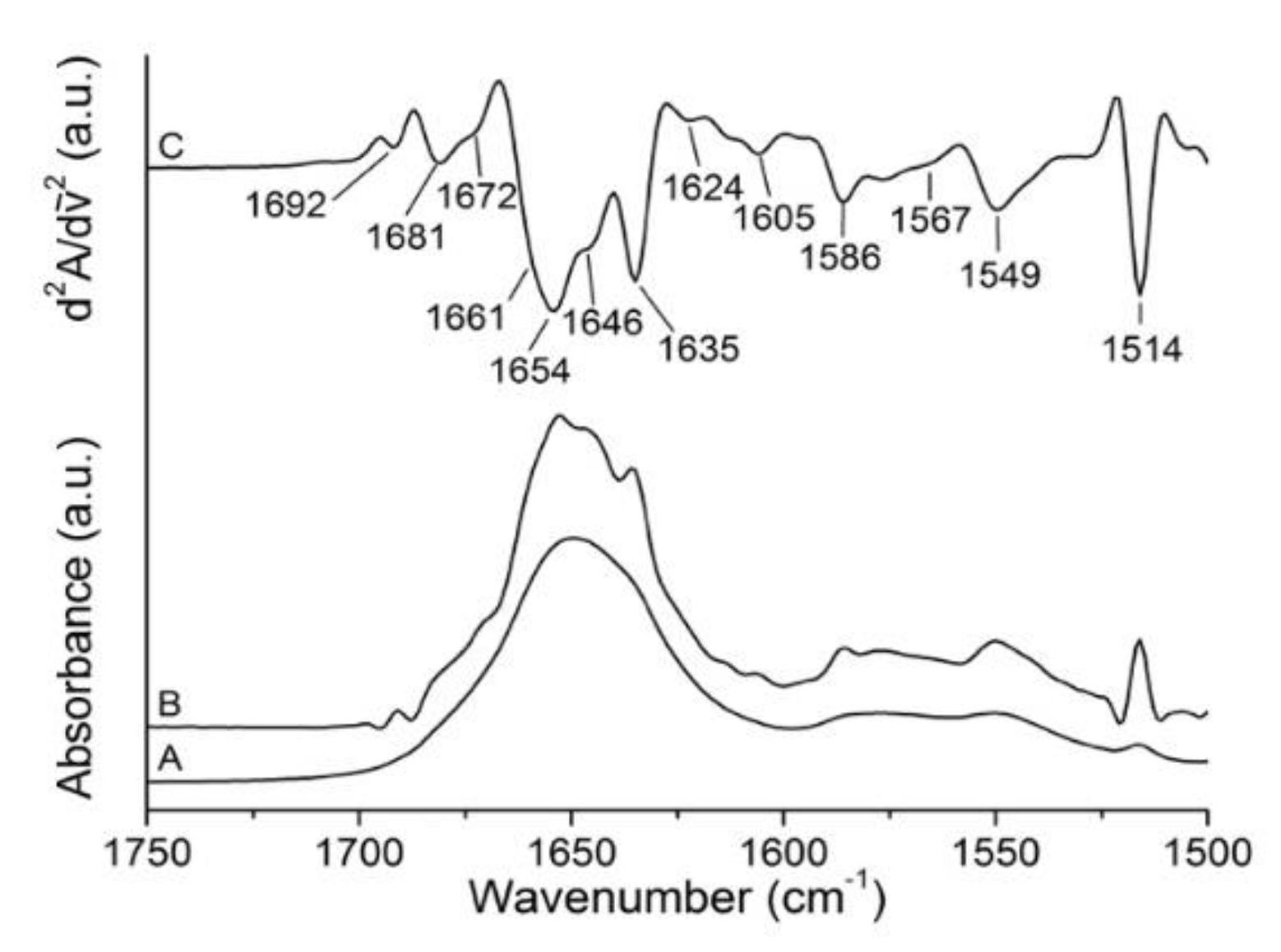
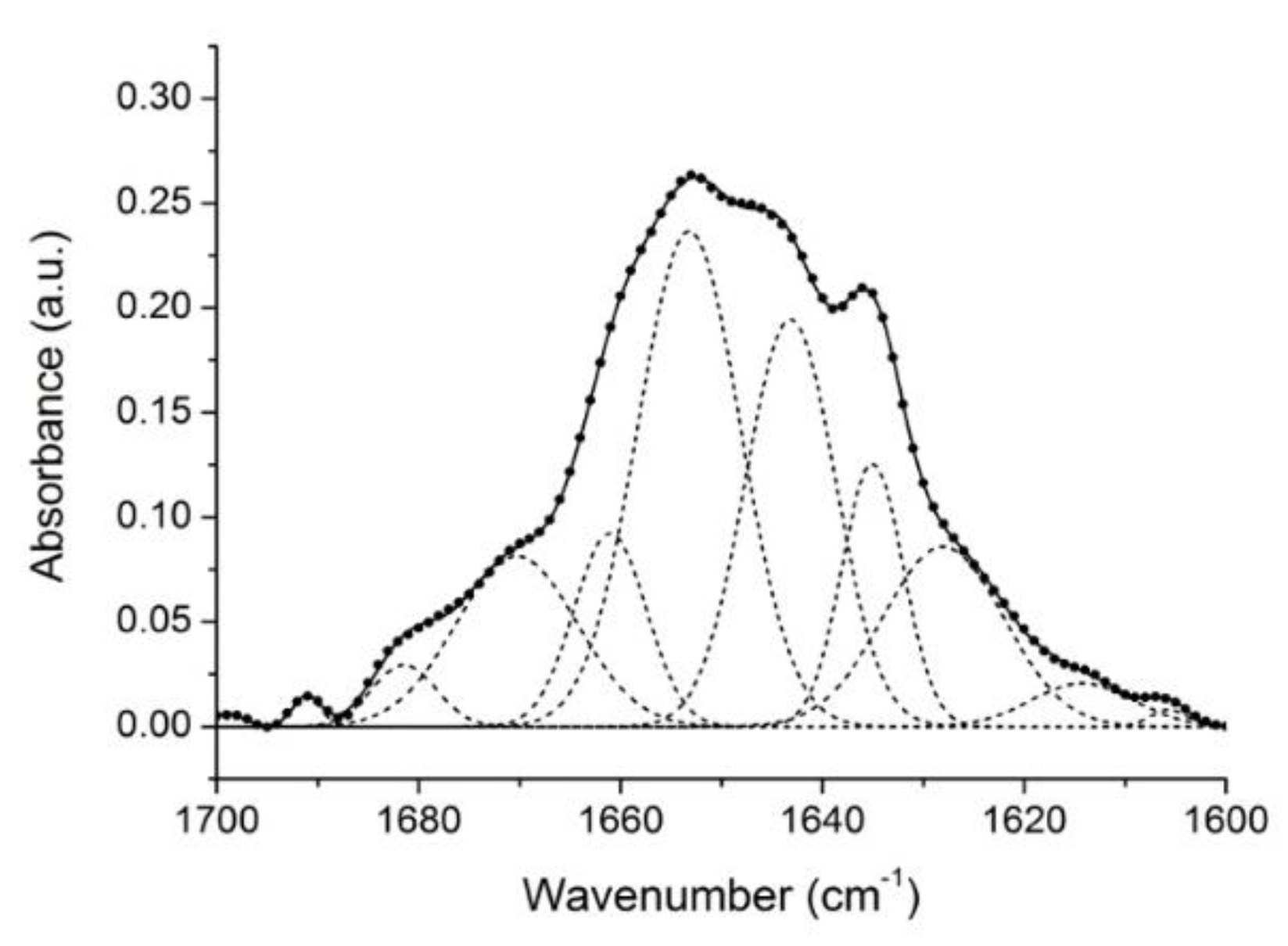

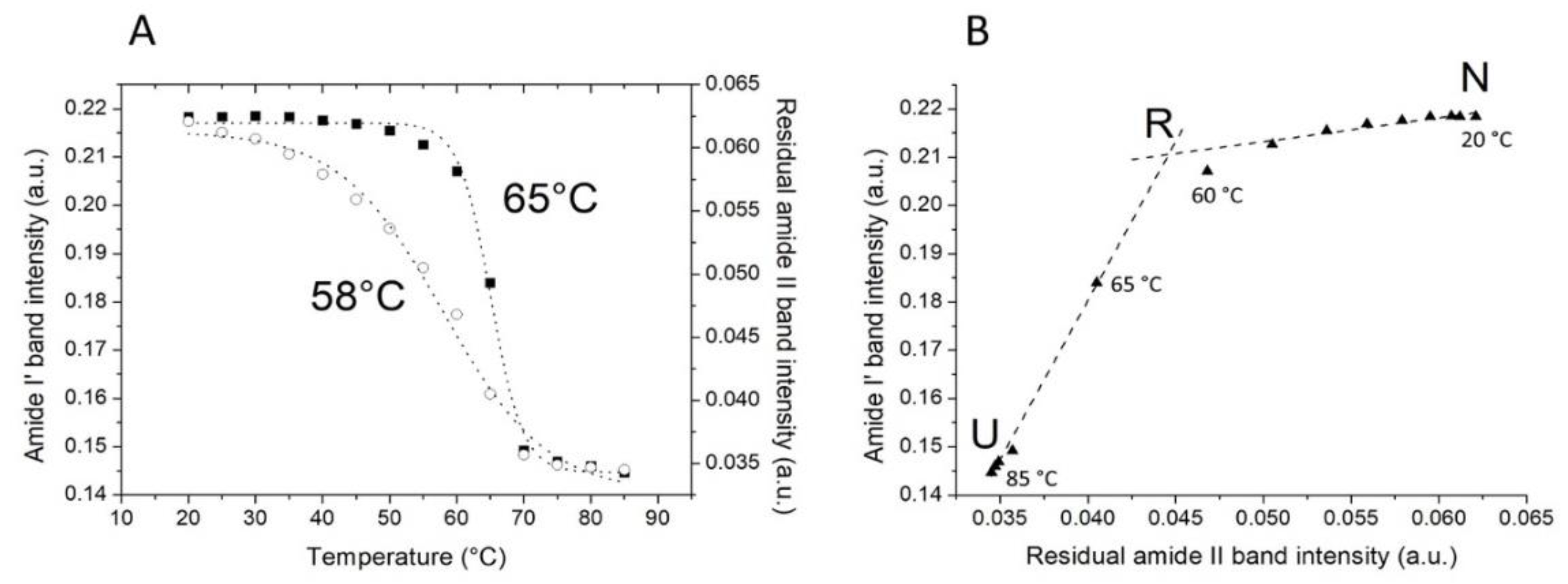
| Band Assignment | Center (cm−1) | Area (%) |
|---|---|---|
| β-sheet (low frequency) | 1624 | 13 |
| β-sheet | 1635 | 10 |
| Unordered | 1646 | 22 |
| α-helix | 1654 | 32 |
| β-turns/310-helix | 1661 | 8 |
| β-turns | 1672 | 12 |
| β-turns/β-sheet (high frequency) | 1681 | 2 |
| β-turns | 1692 | 1 |
| Secondary Structure | This Work | X-ray Data | Circular Dichroism | Raman Spectroscopy |
|---|---|---|---|---|
| α-helix (including 310-helix) | 32–40 | 37 | > 29 | 32 |
| β-sheet | 23–25 | 24 | 20 | |
| Turns | 13–23 | 11 | 26 | |
| Bend | 7 | |||
| Unordered | 22 | 21 | 22 |
| Synchronous Map | Asynchronous Map | |
|---|---|---|
| Auto-Peaks (cm−1) | Cross-Peaks at ν1/ν2 (cm−1) | Cross-Peaks at ν1/ν2 (cm−1) |
| 1683↑ | 1683↑/1618↑ + | 1683↑/1550↓ + a |
| 1654↓ | 1683↑/1654↓ − | 1683↑/1624↓ − a |
| 1618↑ | 1654↓/1550↓ + | 1683↑/1654↓ + a |
| 1654↓/1618↑ − | 1661↓/1550↓ − a | |
| 1654↓/1635↓ + | 1661↓/1618↑ − b | |
| 1635↓/1618↑ − | 1654↓/1550↓ − a | |
| 1618↑/1550↓ − | 1654↓/1618↑ − b | |
| 1654↓/1661↓ − a | ||
| 1654↓/1683↑ − b | ||
| 1624↓/1618↑ + b | ||
| 1618↑/1550↓ + a | ||
| Unfolding Event | FTIR Band Assigment |
|---|---|
| First step (from 20 to 60 °C) | |
| Tertiary structure relaxation | (1550↓) |
| Second step (from 60 to 85 °C) | |
| β-turns/310-helix denaturation | (1661↓) |
| α-helix and β-sheet denaturation | (1654↓, 1624↓, 1635↓) |
| Aggregation and β-turns denaturation | (1618↑, 1672↓) |
| Aggregation | (1683↑) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scirè, A.; Tanfani, F.; Ausili, A. A Spectroscopic Study on Secondary Structure and Thermal Unfolding of the Plant Toxin Gelonin Confirms Some Typical Structural Characteristics and Unravels the Sequence of Thermal Unfolding Events. Toxins 2019, 11, 483. https://doi.org/10.3390/toxins11090483
Scirè A, Tanfani F, Ausili A. A Spectroscopic Study on Secondary Structure and Thermal Unfolding of the Plant Toxin Gelonin Confirms Some Typical Structural Characteristics and Unravels the Sequence of Thermal Unfolding Events. Toxins. 2019; 11(9):483. https://doi.org/10.3390/toxins11090483
Chicago/Turabian StyleScirè, Andrea, Fabio Tanfani, and Alessio Ausili. 2019. "A Spectroscopic Study on Secondary Structure and Thermal Unfolding of the Plant Toxin Gelonin Confirms Some Typical Structural Characteristics and Unravels the Sequence of Thermal Unfolding Events" Toxins 11, no. 9: 483. https://doi.org/10.3390/toxins11090483





