Modifications of Polymeric Membranes Used in Guided Tissue and Bone Regeneration
Abstract
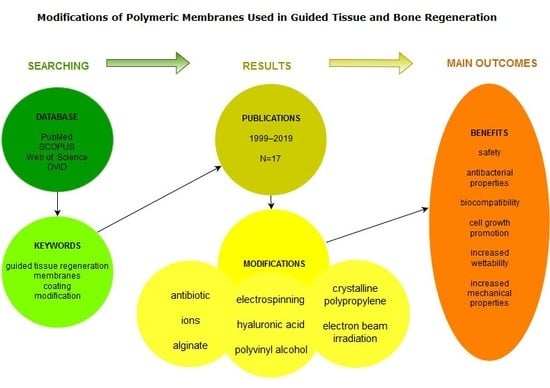
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
4.1. Antibiotic Coating
4.2. Ion Modification
4.3. Other Modifications
4.3.1. Alginate Coating
4.3.2. Hyaluronic Acid Coating
4.3.3. Polyvinyl Alcohol Coating
4.3.4. Crystalline Polypropylene Coating
4.3.5. Electron Beam Irradiation
4.3.6. Electrospinning
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chiapasco, M.; Zaniboni, M. Clinical outcomes of GBR procedures to correct peri-implant dehiscences and fenestrations: A systematic review. Clin. Oral Implants Res. 2009, 20 (Suppl. 4), 113–123. [Google Scholar] [CrossRef] [PubMed]
- Rakhmatia, Y.D.; Ayukawa, Y.; Furuhashi, A.; Koyano, K. Current barrier membranes: Titanium mesh and other membranes for guided bone regeneration in dental applications. J. Prosthodont. Res. 2013, 57, 3–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hämmerle, C.H.F.; Jung, R.E. Bone augmentation by means of barrier membranes. Periodontology 2000 2003, 33, 36–53. [Google Scholar] [CrossRef] [PubMed]
- Dimitriou, R.; Mataliotakis, G.I.; Calori, G.M.; Giannoudis, P.V. The role of barrier membranes for guided bone regeneration and restoration of large bone defects: Current experimental and clinical evidence. BMC Med. 2012, 10, 81. [Google Scholar] [CrossRef] [PubMed]
- Murphy, K.G.; Gunsolley, J.C. Guided Tissue Regeneration for the Treatment of Periodontal Intrabony and Furcation Defects. A Systematic Review. Ann. Periodontol. 2003, 8, 266–302. [Google Scholar] [CrossRef] [PubMed]
- Melcher, A.H. On the Repair Potential of Periodontal Tissues. J. Periodontol. 1976, 47, 256–260. [Google Scholar] [CrossRef]
- Iviglia, G.; Kargozar, S.; Baino, F.; Iviglia, G.; Kargozar, S.; Baino, F. Biomaterials, Current Strategies, and Novel Nano-Technological Approaches for Periodontal Regeneration. J. Funct. Biomater. 2019, 10, 3. [Google Scholar] [CrossRef]
- Muzzarelli, R.A. Chitins and chitosans for the repair of wounded skin, nerve, cartilage and bone. Carbohydr. Polym. 2009, 76, 167–182. [Google Scholar] [CrossRef]
- Elgali, I.; Turri, A.; Xia, W.; Norlindh, B.; Johansson, A.; Dahlin, C.; Thomsen, P.; Omar, O. Guided bone regeneration using resorbable membrane and different bone substitutes: Early histological and molecular events. Acta Biomater. 2016, 29, 409–423. [Google Scholar] [CrossRef] [Green Version]
- Darby, I. Periodontal materials. Aust. Dent. J. 2011, 56, 107–118. [Google Scholar] [CrossRef] [Green Version]
- Aurer, A.; Jorgie-Srdjak, K. Membranes for Periodontal Regeneration. Acta Stomatol. Croat. 2005, 39, 107–112. [Google Scholar]
- Liu, J.; Kerns, D.G. Mechanisms of guided bone regeneration: A review. Open Dent. J. 2014, 8, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Hämmerle, C.H.; Karring, T. Guided bone regeneration at oral implant sites. Periodontology 2000 1998, 17, 151–175. [Google Scholar] [CrossRef]
- Evans, G.H.; Yukna, R.A.; Cambre, K.M.; Gardiner, D.L. Clinical regeneration with guided tissue barriers. Curr. Opin. Periodontol. 1997, 4, 75–81. [Google Scholar] [PubMed]
- Bergsma, J.E.; Rozema, F.R.; Bos, R.R.; Boering, G.; de Bruijn, W.C.; Pennings, A.J. In vivo degradation and biocompatibility study of in vitro pre-degraded as-polymerized polyactide particles. Biomaterials 1995, 16, 267–274. [Google Scholar] [CrossRef]
- Shue, L.; Yufeng, Z.; Mony, U. Biomaterials for periodontal regeneration. Biomatter 2012, 2, 271–277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patrulea, V.; Ostafe, V.; Borchard, G.; Jordan, O. Chitosan as a starting material for wound healing applications. Eur. J. Pharm. Biopharm. 2015, 97, 417–426. [Google Scholar] [CrossRef] [Green Version]
- Wieckiewicz, M.; Boening, K.; Grychowska, N.; Paradowska-Stolarz, A. Clinical Application of Chitosan in Dental Specialities. Mini-Rev. Med. Chem. 2017, 17, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Elgali, I.; Omar, O.; Dahlin, C.; Thomsen, P. Guided bone regeneration: Materials and biological mechanisms revisited. Eur. J. Oral Sci. 2017, 125, 315–337. [Google Scholar] [CrossRef]
- Jin, S.-H.; Kweon, H.; Park, J.-B.; Kim, C.-H. The effects of tetracycline-loaded silk fibroin membrane on proliferation and osteogenic potential of mesenchymal stem cells. J. Surg. Res. 2014, 192, e1–e9. [Google Scholar] [CrossRef]
- Kütan, E.; Duygu-Çapar, G.; Özçakir-Tomruk, C.; Dilek, O.C.; Özen, F.; Erdoğan, Ö.; Özdemir, I.; Korachi, M.; Gürel, A. Efficacy of doxycycline release collagen membrane on surgically created and contaminated defects in rat tibiae: A histopathological and microbiological study. Arch. Oral Biol. 2016, 63, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Lian, M.; Sun, B.; Qiao, Z.; Zhao, K.; Zhou, X.; Zhang, Q.; Zou, D.; He, C.; Zhang, X. Bi-layered electrospun nanofibrous membrane with osteogenic and antibacterial properties for guided bone regeneration. Colloids Surf. B Biointerfaces 2019, 176, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Adayi, A.; Liu, Z.; Li, M.; Wu, M.; Xiao, L.; Sun, Y.; Cai, Q.; Yang, X.; Zhang, X.; et al. Asymmetric Collagen/chitosan Membrane Containing Minocycline-loaded Chitosan Nanoparticles for Guided Bone Regeneration. Sci. Rep. 2016, 6, 31822. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zarkesh, N.; Nowzari, H.; Morrison, J.L.; Slots, J. Tetracycline-Coated Polytetrafluoroethylene Barrier Membranes in the Treatment of Intraosseous Periodontal Lesions. J. Periodontol. 1999, 70, 1008–1016. [Google Scholar] [CrossRef]
- Zohar, R.; Nemcovsky, C.E.; Kebudi, E.; Artzi, Z.; Tal, H.; Moses, O. Tetracycline impregnation delays collagen membrane degradation in vivo. J. Periodontol. 2004, 75, 1096–1101. [Google Scholar] [CrossRef]
- Castillo-Dalí, G.; Castillo-Oyagüe, R.; Batista-Cruzado, A.; López-Santos, C.; Rodríguez-González-Elipe, A.; Saffar, J.-L.; Lynch, C.-D.; Gutiérrez-Pérez, J.-L.; Torres-Lagares, D. Reliability of new poly (lactic-co-glycolic acid) membranes treated with oxygen plasma plus silicon dioxide layers for pre-prosthetic guided bone regeneration processes. Med. Oral Patol. Oral Cir. Bucal 2017, 22, e242–e250. [Google Scholar] [CrossRef]
- Jin, S.; Li, J.; Wang, J.; Jiang, J.; Zuo, Y.; Li, Y.; Yang, F. Electrospun silver ion-loaded calcium phosphate/chitosan antibacterial composite fibrous membranes for guided bone regeneration. Int. J. Nanomed. 2018, 13, 4591–4605. [Google Scholar] [CrossRef] [PubMed]
- Castillo-Dalí, G.; Castillo-Oyagüe, R.; Terriza, A.; Saffar, J.L.; Batista, A.; Barranco, A.; Cabezas-Talavero, J.; Lynch, C.D.; Barouk, B.; Llorens, A.; et al. In vivo comparative model of oxygen plasma and nanocomposite particles on PLGA membranes for guided bone regeneration processes to be applied in pre-prosthetic surgery: A pilot study. J. Dent. 2014, 42, 1446–1457. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, Q.; Huang, C.; Mo, A.; Li, J.; Zuo, Y. Biological properties of an anti-bacterial membrane for guided bone regeneration: An experimental study in rats. Clin. Oral Implants Res. 2010, 21, 321–327. [Google Scholar] [CrossRef]
- Ye, J.; Yao, Q.; Anchun, M.; Nie, J.; Cui Ye, W.; Liu, W.; Chen, X. Effects of an antibacterial membrane on osteoblast-like cells in vitro. Int. J. Nanomed. 2011, 6, 1853. [Google Scholar] [CrossRef]
- Chen, T.W.; Chang, S.J.; Niu, G.C.-C.; Hsu, Y.T.; Kuo, S.M. Alginate-coated chitosan membrane for guided tissue regeneration. J. Appl. Polym. Sci. 2006, 102, 4528–4534. [Google Scholar] [CrossRef]
- Silva, E.C.; Omonte, S.V.; Martins, A.G.V.; de Castro, H.H.O.; Gomes, H.E.; Zenóbio, É.G.; de Oliveira, P.A.D.; Horta, M.C.R.; Souza, P.E.A. Hyaluronic acid on collagen membranes: An experimental study in rats. Arch. Oral Biol. 2017, 73, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Bilgi, E.; Bayir, E.; Sendemir-Urkmez, A.; Hames, E.E. Optimization of bacterial cellulose production by Gluconacetobacter xylinus using carob and haricot bean. Int. J. Biol. Macromol. 2016, 90, 2–10. [Google Scholar] [CrossRef]
- Zhuang, P.-Y.; Li, Y.-L.; Fan, L.; Lin, J.; Hu, Q.-L. Modification of chitosan membrane with poly(vinyl alcohol) and biocompatibility evaluation. Int. J. Biol. Macromol. 2012, 50, 658–663. [Google Scholar] [CrossRef] [PubMed]
- Qasim, S.B.; Najeeb, S.; Delaine-Smith, R.M.; Rawlinson, A.; Ur Rehman, I. Potential of electrospun chitosan fibers as a surface layer in functionally graded GTR membrane for periodontal regeneration. Dent. Mater. 2017, 33, 71–83. [Google Scholar] [CrossRef] [Green Version]
- Franco, J.A.; Kentish, S.E.; Perera, J.M.; Stevens, G.W. Fabrication of a superhydrophobic polypropylene membrane by deposition of a porous crystalline polypropylene coating. J. Membr. Sci. 2008, 318, 107–113. [Google Scholar] [CrossRef]
- Nowzari, H.; MacDonald, E.S.; Flynn, J.; London, R.M.; Morrison, J.L.; Slots, J. The Dynamics of Microbial Colonization of Barrier Membranes for Guided Tissue Regeneration. J. Periodontol. 1996, 67, 694–702. [Google Scholar] [CrossRef]
- Nowzari, H.; London, R.; Slots, J. The importance of periodontal pathogens in guided periodontal tissue regeneration and guided bone regeneration. Compend. Contin. Educ. Dent. 1995, 16, 1042–1044. [Google Scholar]
- Sivolella, S.; Stellini, E.; Brunello, G.; Gardin, C.; Ferroni, L.; Bressan, E.; Zavan, B. Silver Nanoparticles in Alveolar Bone Surgery Devices. J. Nanomater. 2012, 2012, 1–12. [Google Scholar] [CrossRef]
- Lee, S.-W.; Kim, S.-G. Membranes for the Guided Bone Regeneration. Maxillofac. Plast. Reconstr. Surg. 2014, 36, 239–246. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [PubMed]
- Pandolfi, V.; Pereira, U.; Dufresne, M.; Legallais, C. Alginate-Based Cell Microencapsulation for Tissue Engineering and Regenerative Medicine. Curr. Pharm. Des. 2017, 23, 3833–3844. [Google Scholar] [CrossRef] [PubMed]
- Song, J.E.; Kim, A.R.; Lee, C.J.; Tripathy, N.; Yoon, K.H.; Lee, D.; Khang, G. Effects of purified alginate sponge on the regeneration of chondrocytes: In vitro and in vivo. J. Biomater. Sci. Polym. Ed. 2015, 26, 181–195. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Jiang, F.; Ye, S.; Wu, Y.; Zhu, K.; Wang, D. Bioactive apatite incorporated alginate microspheres with sustained drug-delivery for bone regeneration application. Mater. Sci. Eng. C 2016, 62, 779–786. [Google Scholar] [CrossRef] [PubMed]
- Stagnaro, P.; Schizzi, I.; Utzeri, R.; Marsano, E.; Castellano, M. Alginate-polymethacrylate hybrid hydrogels for potential osteochondral tissue regeneration. Carbohydr. Polym. 2018, 185, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Lequeux, I.; Ducasse, E.; Jouenne, T.; Thebault, P. Addition of antimicrobial properties to hyaluronic acid by grafting of antimicrobial peptide. Eur. Polym. J. 2014, 51, 182–190. [Google Scholar] [CrossRef]
- Wu, S.; Deng, L.; Hsia, H.; Xu, K.; He, Y.; Huang, Q.; Peng, Y.; Zhou, Z.; Peng, C. Evaluation of gelatin-hyaluronic acid composite hydrogels for accelerating wound healing. J. Biomater. Appl. 2017, 31, 1380–1390. [Google Scholar] [CrossRef]
- Mohan, N.; Mohanan, P.; Sabareeswaran, A.; Nair, P. Chitosan-hyaluronic acid hydrogel for cartilage repair. Int. J. Biol. Macromol. 2017, 104, 1936–1945. [Google Scholar] [CrossRef]
- Litwiniuk, M.; Krejner, A.; Speyrer, M.S.; Gauto, A.R.; Grzela, T. Hyaluronic Acid in Inflammation and Tissue Regeneration. Wounds Compend. Clin. Res. Pract. 2016, 28, 78–88. [Google Scholar]
- Halachmi, S.; Ben Amitai, D.; Lapidoth, M. Treatment of acne scars with hyaluronic acid: An improved approach. J. Drugs Dermatol. 2013, 12, e121–e123. [Google Scholar]
- Erbil, H.Y.; Demirel, A.L.; Avci, Y.; Mert, O. Transformation of a Simple Plastic into a Superhydrophobic Surface. Science 2003, 299, 1377–1380. [Google Scholar] [CrossRef] [PubMed]
- Gu, H.; Li, G.; Liu, C.; Yuan, F.; Han, F.; Zhang, L.; Wu, S. Considerable knock-on displacement of metal atoms under a low energy electron beam. Sci. Rep. 2017, 7, 184. [Google Scholar] [CrossRef] [PubMed]
- Niebuhr, S.E.; Dickson, J.S. Destruction of Bacillus anthracis strain Sterne 34F2 spores in postal envelopes by exposure to electron beam irradiation. Lett. Appl. Microbiol. 2003, 37, 17–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gentile, P.; Chiono, V.; Tonda-Turo, C.; Ferreira, A.M.; Ciardelli, G. Polymeric membranes for guided bone regeneration. Biotechnol. J. 2011, 6, 1187–1197. [Google Scholar] [CrossRef] [PubMed]
- Bottino, M.C.; Thomas, V.; Schmidt, G.; Vohra, Y.K.; Chu, T.-M.G.; Kowolik, M.J.; Janowski, G.M. Recent advances in the development of GTR/GBR membranes for periodontal regeneration—A materials perspective. Dent. Mater. 2012, 28, 703–721. [Google Scholar] [CrossRef] [PubMed]
- Hughes, F.J.; Ghuman, M.; Talal, A. Periodontal regeneration: A challenge for the tissue engineer? Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2010, 224, 1345–1358. [Google Scholar] [CrossRef]
| Author, year [ref.] | Type of Membrane | Morphology of Membrane | Membrane Material | Modification | Additional Properties |
|---|---|---|---|---|---|
| Jin, et al., 2014 [20] | Resorbable membrane | Compact | Silk fibroin solution casted on a polystyrene dish | Impregnation with tetracycline | Increase in proliferation, osteogenic potential of gingiva-derived mesenchymal stem cells |
| Kütan, et al., 2016 [21] | Resorbable membrane | Not reported | Collagen | Impregnation with doxycycline | Inhibition of bacterial growth |
| Lian, et al., 2019 [22] | Resorbable bi-layered composite membrane | Porous | Poly(lactic-co-glycolic) acid | Poly(lactic-co-glycolic) acid nanofibres loaded with doxycycline and dexamethasone | Inhibition of bacterial growth |
| Ma, et al., 2016 [23] | Resorbable asymmetric membrane | Porous | Collagen, chitosan | Minocycline-loaded chitosan nanoparticles | Inhibition of bacterial growth, promotion of osteoblast and fibroblast growth, promotion of angiogenesis |
| Zarkesh, et al., 1999 [24] | Non-resorbable membrane | Porous | Polytetrafluoroethylene | Impregnation with tetracycline | Reduced colonisation of membranes with periodontal pathogens |
| Zohar, et al., 2004 [25] | Resorbable membrane | Not reported | Collagen | Impregnation with tetracycline | Slowing membrane degradation |
| Castillo-Dali, et al., 2017 [26] | Resorbable membrane | Not reported | Poly(lactic-co-glycolic) acid | Incorporation of bioactive layers of SiO2 onto poly(lactic-co-glycolic) acid membranes modified with PO2 | Enhance bone regeneration, stimulation of adhesion of osteogenic mediators and cells, stimulation of new bone formation, mineralisation enhancement of osteosynthetic activity |
| Jin, et al., 2018 [27] | Resorbable membrane | Porous | Chitosan | Electrospun silver ion-loaded calcium phosphate subsequently crosslinked with vanillin | Inhibition of bacterial growth, increased biocompatibility |
| Castillo-Dali, et al., 2014 [28] | Bilayer resorbable membrane | Not reported | Poly(lactic-co-glycolic) acid | Poly(lactic-co-glycolic) acid being treated with oxygen plasma (PO2) and/or being functionalised with silicon dioxide (SiO2) or titanium dioxide (TiO2) nanoparticles | Enhanced osteosynthetic activity, enhanced bone regeneration |
| Zhang, et al., 2010 [29] | Resorbable composite membrane | Porous | Polyamide nanocomposite membrane | Silver-hydroxyapatite/titania | Increased biocompatibility, increased antibacterial properties, induced inflammatory response, enhanced bone regeneration |
| Ye, et al., 2011 [30] | Resorbable composite membrane | Porous | Polyamide nanocomposite membrane | Silver–hydroxyapatite/titania | Increased adhesion, increased proliferation of osteoblast-like -cells |
| Chen, et al., 2006 [31] | Resorbable membrane | Not reported | Chitosan | Alginate coating | Increased wettability, increased stiffness, increased tear strength, increased resistance to fibroblast cell adhering |
| Silva, et al., 2017 [32] | Biodegradable bovine and porcine membrane | Porous | Collagen | Impregnation with hyaluronic acid | No modifying effect on guided bone regeneration |
| Bilgi, et al., 2016 [33] | Non- resorbable membrane | Not reported | Bacterial cellulose | Electron beam irradiation | Acceleration of degradation, reduction of mechanical properties |
| Zhuang, et al., 2012 [34] | Resorbable membrane | Not reported | Chitosan | Synthesise with poly(vinyl alcohol) | Increased tensile strength in wet conditions |
| Qasim, et al., 2017 [35] | Resorbable membrane | Not reported | Chitosan | Electrospinning | Increased proliferation of osteoblastic cells, enhanced tissue regeneration for all fibre types, randomly oriented fibre promote osteoblastic cell proliferation, aligned fibres promote ligament growth |
| Franco, et al., 2008 [36] | Non-resorbable, synthetic polymer | Porous | Polypropylene, polytetrafluoroethylene | Porous crystalline polypropylene coating | Enhanced separation of repaired bone and soft tissue |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Florjanski, W.; Orzeszek, S.; Olchowy, A.; Grychowska, N.; Wieckiewicz, W.; Malysa, A.; Smardz, J.; Wieckiewicz, M. Modifications of Polymeric Membranes Used in Guided Tissue and Bone Regeneration. Polymers 2019, 11, 782. https://doi.org/10.3390/polym11050782
Florjanski W, Orzeszek S, Olchowy A, Grychowska N, Wieckiewicz W, Malysa A, Smardz J, Wieckiewicz M. Modifications of Polymeric Membranes Used in Guided Tissue and Bone Regeneration. Polymers. 2019; 11(5):782. https://doi.org/10.3390/polym11050782
Chicago/Turabian StyleFlorjanski, Wojciech, Sylwia Orzeszek, Anna Olchowy, Natalia Grychowska, Wlodzimierz Wieckiewicz, Andrzej Malysa, Joanna Smardz, and Mieszko Wieckiewicz. 2019. "Modifications of Polymeric Membranes Used in Guided Tissue and Bone Regeneration" Polymers 11, no. 5: 782. https://doi.org/10.3390/polym11050782