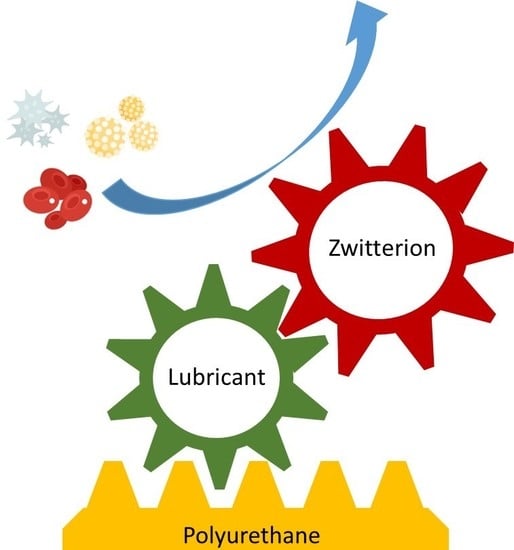
The Grafting of Multifunctional Antithrombogenic Chemical Networks on Polyurethane Intravascular Catheters
Abstract
:1. Introduction
2. Experimental
2.1. Materials
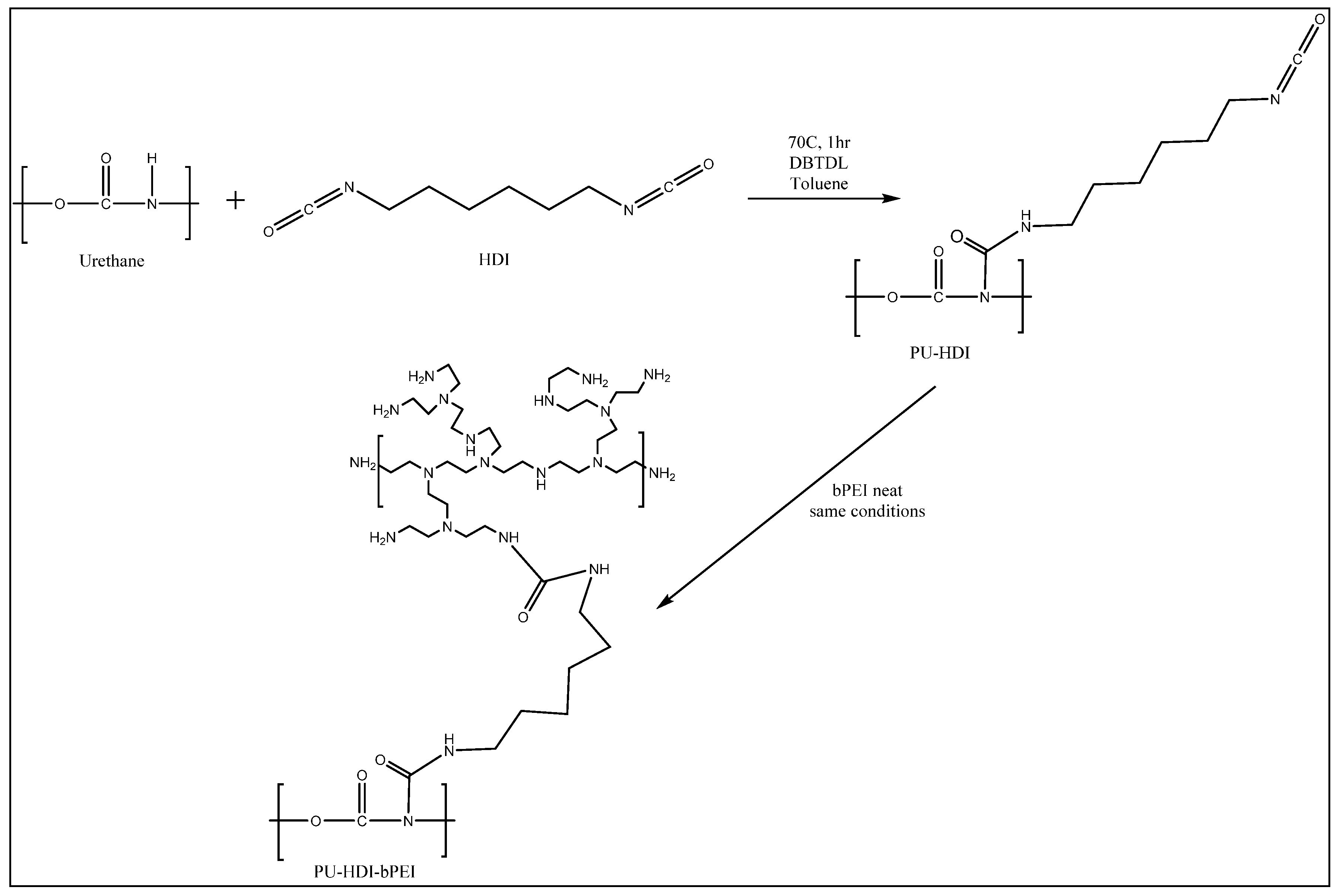
2.2. Synthesis of the Lubricious, Antimicrobial, and Antithrombogenic Grafting Complex
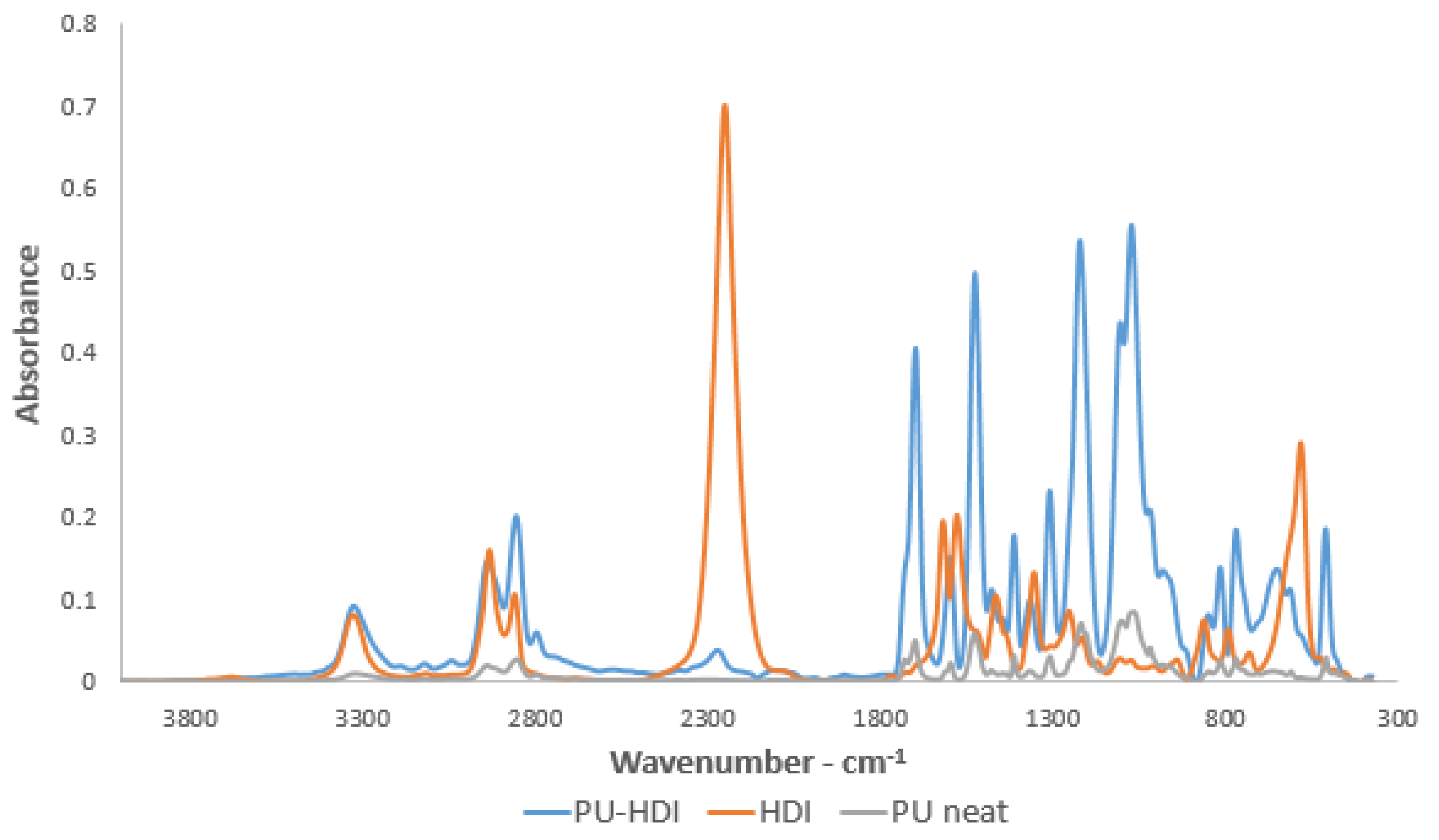
2.3. IR Spectroscopy
2.4. UV–VIS Spectroscopy
2.5. Fluorescent Probe
2.6. Elemental Analysis
2.7. Preparation of PU Surfaces`
2.8. Grafting on PU Surfaces
2.9. X-ray Photoelectron Spectroscopy
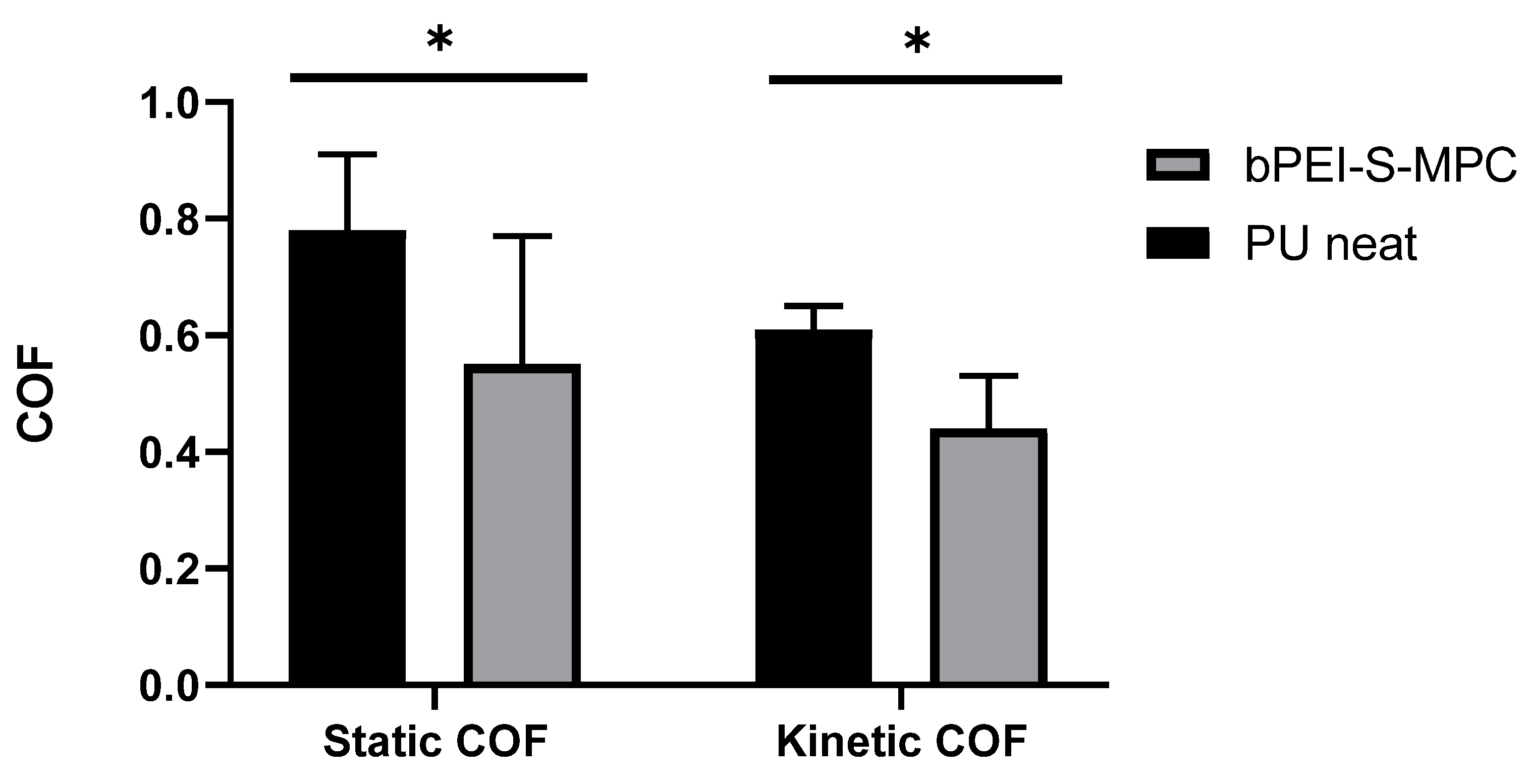
2.10. Coefficient of Friction Measurements
2.11. Ex-Vivo Evaluations
2.12. In-Vivo Thrombogenicity Evaluation
3. Results and Discussion
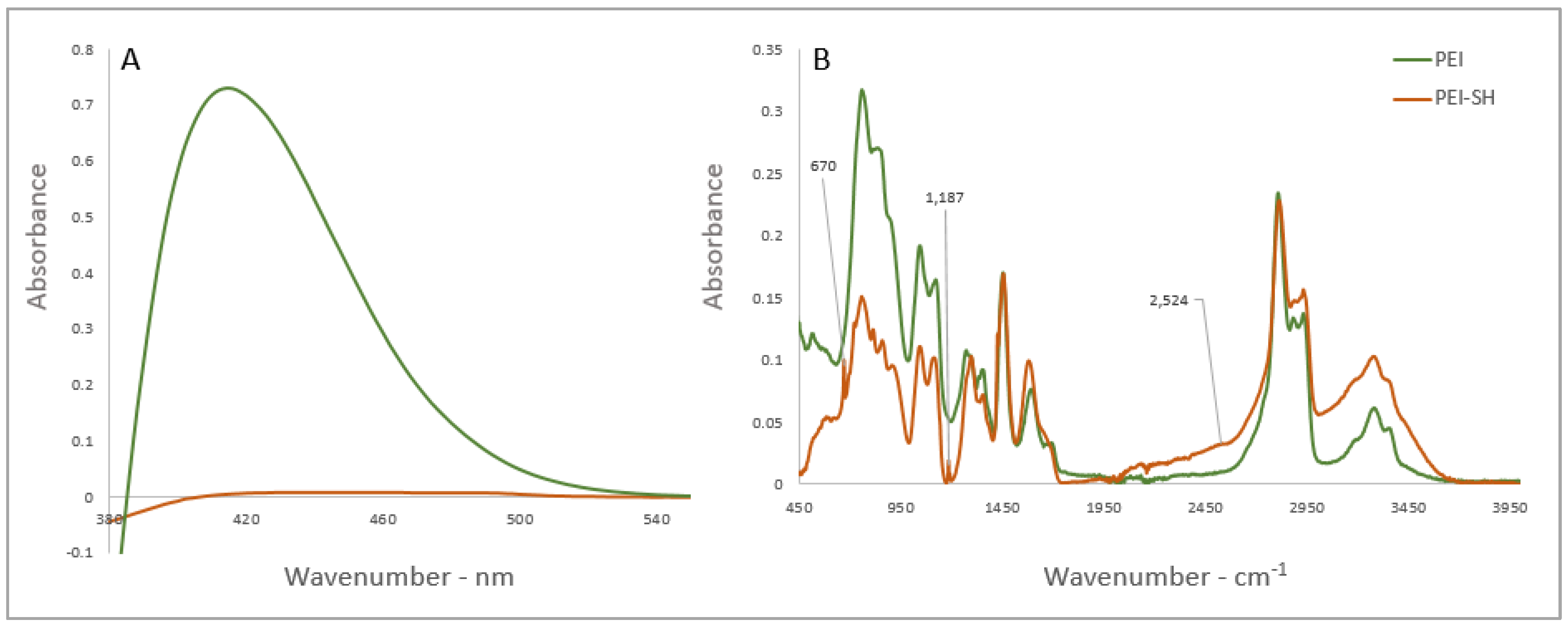
3.1. Synthesis of bPEI-SH
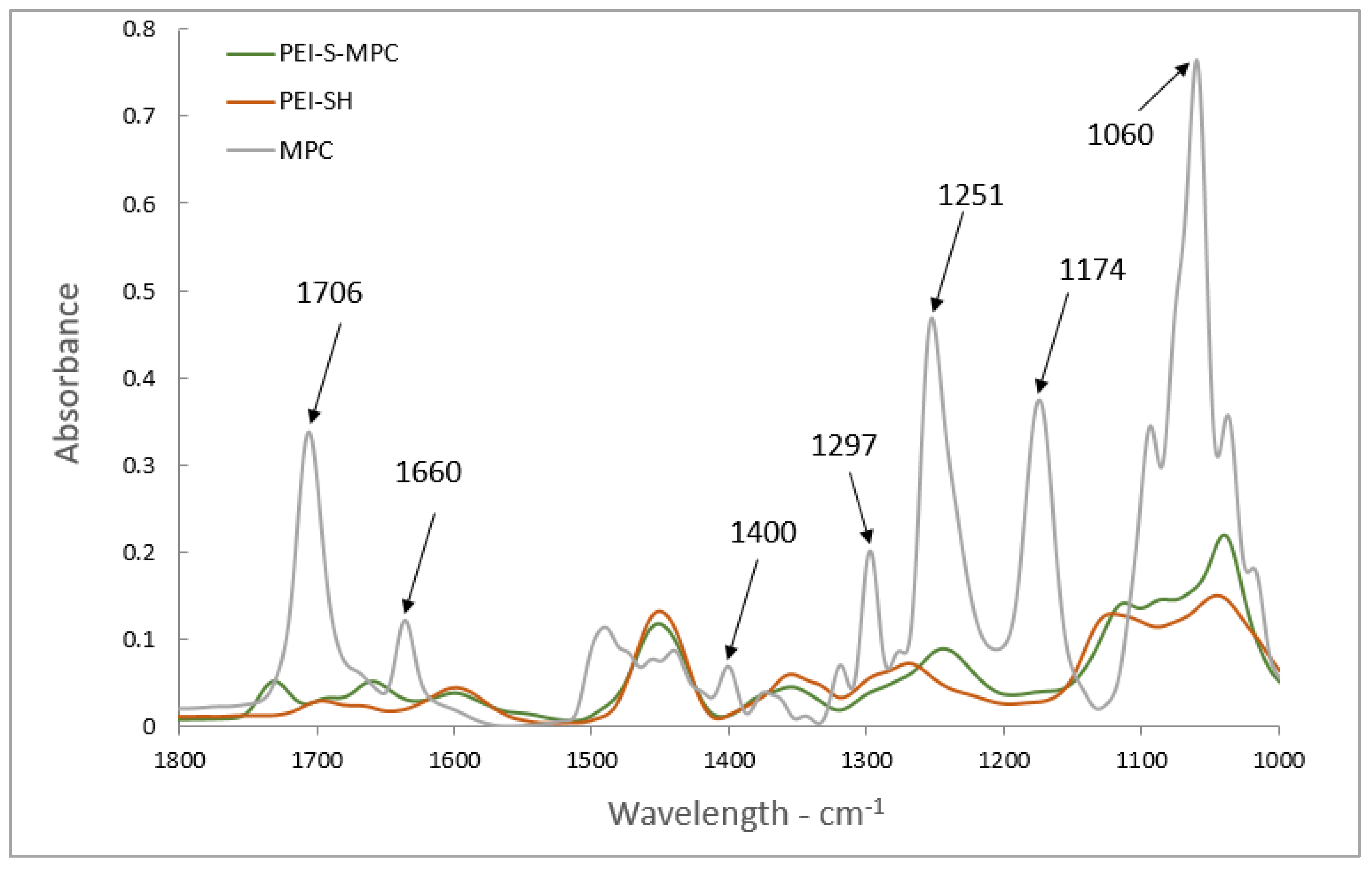
3.2. Thiol-Ene Click for Conjugation of MPC
3.3. Functionalization of PU Surfaces Using Toluenesulfonyl Isocyanate
3.4. Isocyanate Functionalization
3.5. The Preparation of PU-HDI-bPEI-S-MPC
3.6. Mechanical Properties
3.7. Coefficient of Friction
3.8. Antimicrobial Activity
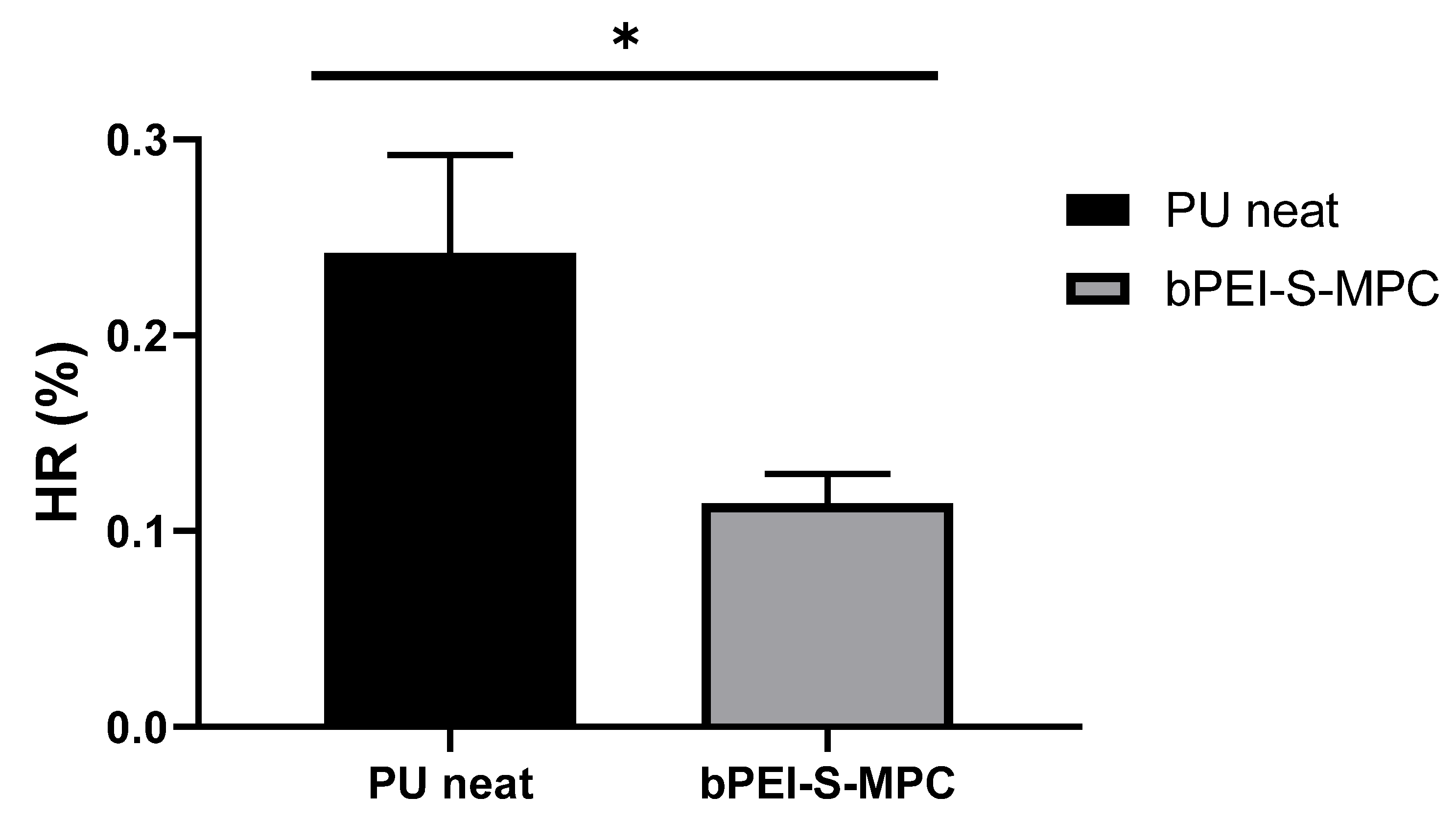
3.9. Hemolysis Assay
3.10. In-Vivo Thrombogenicity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| bPEI | Branched polyethyleneimine |
| bPEI-SH | Thiol terminated branched polyethyleneimine (thiolated bPEI) |
| bPEI-S-MPC | MPC terminated branched polyethyleneimine, mediated by ethylene sulfide. Also called the grafting complex |
| PU-HDI | Hexamethylene diamine (HDI) grafted on PU surface |
| PU-HDI-bPEI | bPEI terminated PU surface, mediated by HDI |
| PU-HDI-bPEI-SH | bPEI-SH terminated PU surface, mediated by HDI |
| PU-HDI-bPEI-S-MPC | bPEI-S-MPC terminated PU surface, mediated by HDI. Also referred as the end product |
References
- Alomari, A.I.; Falk, A. The Natural History of Tunneled Hemodialysis Catheters Removed or Exchanged : A Single-Institution Experience. SIR 2004, 9, 227–235. [Google Scholar] [CrossRef]
- Van Rooden, C.J.; Schippers, E.F.; Barge, R.M.Y.; Rosendaal, F.R.; Guiot, H.F.L. Infectious Complications of Central Venous Catheters Increase the Risk of Catheter-Related Thrombosis in Hematology Patients: A Prospective Study. J. Clin. Oncol. 2005, 23, 2655–2660. [Google Scholar] [CrossRef]
- Mehall, J.R.; Saltzman, D.A.; Jackson, R.J.; Smith, S.D. Fibrin Sheath Enhances Central Venous Catheter Infection. Crit Care Med 2002, 30, 908–912. [Google Scholar] [CrossRef]
- Gorbet, M.B.; Sefton, M.V. Biomaterial-Associated Thrombosis: Roles of Coagulation Factors, Complement, Platelets and Leukocytes. Biomater. Silver Jubil. Compend. 2006, 25, 219–241. [Google Scholar] [CrossRef]
- Takashima, K.; Shimomura, R.; Kitou, T.; Terada, H. Contact and Friction between Catheter and Blood Vessel. Elsevier Sci. 2007, 40, 319–328. [Google Scholar] [CrossRef]
- Hsu, L.-C.; Viejo, M.; Hu, C.B.F.P. Lubricious Coatings for Medical Devices. U.S. Patent 634 22 January, 2002.
- Haslam John, H. Method of Coating Surface with Transparent Film and Product Resulting Therefrom. U.S. Patent 2768909A, 30 October 1956. [Google Scholar]
- Yang, D.; Wang, L.; Stanslaski, J.; Tang, L. Hydrophilic lubricity coating for medical devices comprising a hydrophobic top coat. U.S. Patent 176849B1, 23 January 2001. [Google Scholar]
- Mehta, R.I.; Mehta, R.I. Hydrophilic Polymer Embolism: An Update for Physicians. HHS Public Access 2017, 130, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Munisso, M.C.; Mahara, A.; Kambe, Y.; Fukazawa, K.; Ishihara, K.; Yamaoka, T. A Surface Graft Polymerization Process on Chemically Stable Medical EPTFE for Suppressing Platelet Adhesion and Activation†. Biomater. Sci. 2018. [Google Scholar] [CrossRef] [PubMed]
- Khalifehzadeh, R.; Ratner, B.D. Trifluoromethyl-Functionalized Poly(Lactic Acid): A Fluoropolyester Designed for Blood Contact Applications†. Biomater. Sci. 2019. [Google Scholar] [CrossRef] [PubMed]
- Kaèmierska, K.A.; Kuc, K.; Ciach, T. Polyvinylpyrrolidone-polyurethane interpolymer hydrogel coating as a local drug delivery system. Acta Pol. Pharm. Drug Res. 2008, 65, 763–766. [Google Scholar]
- Kenward, M.; Slater, G.W. Combinatorial Design of Passive Drug Delivery Platforms. Int. J. Pharm. 2007, 339, 91–102. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. Intravascular Medical Devices—FDA Safety Communication—Lubricious Coating Separation; U.S. Food and Drug Administration: White Oak, MD, USA, 2015.
- Chen, S.; Zheng, J.; Li, L.; Jiang, S. Strong Resistance of Phosphorylcholine Self-Assembled Monolayers to Protein Adsorption : Insights into Nonfouling Properties of Zwitterionic Materials. Am. Chem. Soc. 2005, 127, 14473–14478. [Google Scholar] [CrossRef] [PubMed]
- Membrane Asymmetry and Blood Coagulation. Nature 1977, 268, 358–360. [CrossRef] [PubMed]
- Tan, M.; Feng, Y.; Wang, H.; Zhang, L.; Khan, M.; Guo, J. Immobilized Bioactive Agents onto Polyurethane Surface with Heparin and Phosphorylcholine Group. Macromol. Res. 2013, 21, 541–549. [Google Scholar] [CrossRef]
- Chen, S.H.; Chang, Y.; Ishihara, K. Reduced Blood Cell Adhesion on Polypropylene Substrates through a Simple Surface Zwitterionization. Langmuir 2017, 33, 611–621. [Google Scholar] [CrossRef]
- Ishihara, K. Blood-Compatible Surfaces with Phosphorylcholine-Based Polymers for Cardiovascular Medical Devices. Langmuir 2019, 35, 1778–1787. [Google Scholar] [CrossRef]
- Lewis, A.L. Phosphorylcholine-Based Polymers and Their Use in the Prevention of Biofouling. Elsevier Sci. 2000, 18, 261–275. [Google Scholar] [CrossRef]
- Long, S.F.; Clarke, S.; Davies, M.C.; Lewis, A.L.; Hanlon, G.W.; Lloyd, A.W. Controlled Biological Response on Blends of a Phosphorylcholine-Based Copolymer with Poly (Butyl Methacrylate). Biomaterials 2003, 24, 4115–4121. [Google Scholar] [CrossRef]
- Adipurnama, I.; Yang, M.-C.; Ciach, T.; Butruk-Raszeja, B. Surface Modification and Endothelialization of Polyurethane for Vascular Tissue Engineering Applications: A Review. Biomater. Sci. 2017, 5, 22–37. [Google Scholar] [CrossRef]
- De Mel, A.; Cousins, B.G.; Seifalian, A.M. Surface Modification of Biomaterials: A Quest for Blood Compatibility. Int. J. Biomater. 2012, 2012. [Google Scholar] [CrossRef]
- Liu, X.; Yuan, L.; Li, D.; Tang, Z.; Wang, Y.; Chen, G.; Chen, H.; Brash, J.L. Blood Compatible Materials: State of the Art. J. Mater. Chem. B 2014, 2, 5718–5738. [Google Scholar] [CrossRef] [PubMed]
- Bayer MaterialScience, A.G. The Chemistry of Polyurethane Coatings. Digit. Collect. 2005, 31. [Google Scholar]
- Yuan, J.; Zhu, J.; Zhu, C.H.; Shen, J.; Lin, S.C. Platelet Adhesion on a Polyurethane Surface Grafted with a Zwitterionic Monomer of Sulfobetaine via a Jeffamine Spacer. Polym. Int. 2004, 53, 1722–1728. [Google Scholar] [CrossRef]
- Jones, G.D.; Asperger, R.G. Ethylene Sulfide Graft on Polyamine as a Nonfouling Anticorrosion Agent. J. Macromol. Sci. Part A Chem. 1979, 13, 835–852. [Google Scholar] [CrossRef]
- Wang, Q.; Tan, L.; Yang, K. Cytocompatibility and Hemolysis of AZ31B Magnesium Alloy with Si-Containing Coating. J. Mater. Sci. Technol. 2015, 31, 845–851. [Google Scholar] [CrossRef]
- Sousa, K.S.; Silva, E.C.; Airoldi, C. Ethylenesulfide as a Useful Agent for Incorporation into the Biopolymer Chitosan in a Solvent-Free Reaction for Use in Cation Removal. Carbohydr. Res. 2009, 344, 1716–1723. [Google Scholar] [CrossRef]
- Silva, E.C.; Lima, L.C.B.; Silva, F.C.; Sousa, K.S.; Fonseca, M.G.; Santana, S.A.A. Immobilization of Ethylene Sulfide in Aminated Cellulose for Removal of the Divalent Cations. Carbohydr. Polym. 2013, 92, 1203–1210. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.-L.; Li, C.-J.; Gray, D.G. Functionalization of cellulose nanocrystal films via ‘thiol–ene’ click reaction. RSC Adv. 2014, 4, 6965–6969. [Google Scholar] [CrossRef]
- Coates, J. Interpretation of Infrared Spectra, A Practical Approach. In Encyclopedia of Analytical Chemistry R.A.; Meyers, R.A., Ed.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2000; pp. 1–23. [Google Scholar]
- Oelichmann, J. Surface and Depth-Profile Analysis Using FTIR Spectroscopy. Fresenius’ Zeitschrift für Anal. Chemie 1989, 333, 353–359. [Google Scholar] [CrossRef]
- Japanese Standards Association. Antimicrobial Products Test for Antimicrobial Activity and Efficacy, Japanese Industrial Standard JIS Z 2801. Ref. number JIS Z 2801 2000 (E), First English Ed. Publ. 2001 2000, 2000, 1–14. [Google Scholar]
- Thasneem, Y.M.; Sajeesh, S.; Sharma, C.P. Effect of Thiol Functionalization on the Hemo-Compatibility of PLGA Nanoparticles. J. Biomed. Mater. Res. Part A 2011, 99, 607–617. [Google Scholar] [CrossRef] [PubMed]
- Katyevansnhsnet, K.E.; Amenhotep, Z.; Dawson, D.; Waters, H.; Ardern, J. Hematology. In Immunoassay Handbook—Theory and Applications of Ligand Binding, ELISA and Related Techniques; Wild, D.G., Ed.; Elsevier Science: London, UK, 2013; Volume 1, pp. 795–815. [Google Scholar]



| Atomic% | bPEI | bPEI-SH | bPEI-S-MPC |
|---|---|---|---|
| C | 51.2 | 52.3 | 48.9 |
| H | 11.1 | 8.3 | 10.3 |
| N | 32.7 | 29.6 | 21.9 |
| S | 0 | 2.6 | 2.1 |
| O | 2.3 | 3.2 | 10.3 |
| P | 0 | 0 | 1.62 |
| Atomic content (%) | Atomic Ratio | ||||
|---|---|---|---|---|---|
| C | N | O | O/N | N/C | |
| PU neat | 71.49 | 2.05 | 50.17 | 24.47 | 0.03 |
| PU-HDI | 69.61 | 12.07 | 15.64 | 1.3 | 0.17 |
| PU-HDI-bPEI | 71.29 | 8.69 | 18.28 | 2.1 | 0.12 |
| CFU | |
|---|---|
| PU | ˃200 |
| PU-HDI | ˃200 |
| PU-HDI-bPEI | ~50 |
| PU-HDI-bPEI-SH | 0 |
| PU-HDI-bPEI-MPC | 0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roth, Y.; Y. Lewitus, D. The Grafting of Multifunctional Antithrombogenic Chemical Networks on Polyurethane Intravascular Catheters. Polymers 2020, 12, 1131. https://doi.org/10.3390/polym12051131
Roth Y, Y. Lewitus D. The Grafting of Multifunctional Antithrombogenic Chemical Networks on Polyurethane Intravascular Catheters. Polymers. 2020; 12(5):1131. https://doi.org/10.3390/polym12051131
Chicago/Turabian StyleRoth, Yael, and Dan Y. Lewitus. 2020. "The Grafting of Multifunctional Antithrombogenic Chemical Networks on Polyurethane Intravascular Catheters" Polymers 12, no. 5: 1131. https://doi.org/10.3390/polym12051131