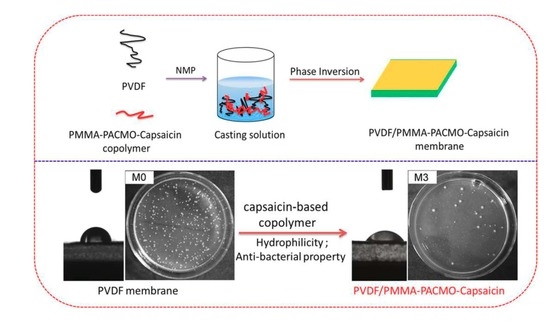
Anti-Fouling and Anti-Bacterial Modification of Poly(vinylidene fluoride) Membrane by Blending with the Capsaicin-Based Copolymer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
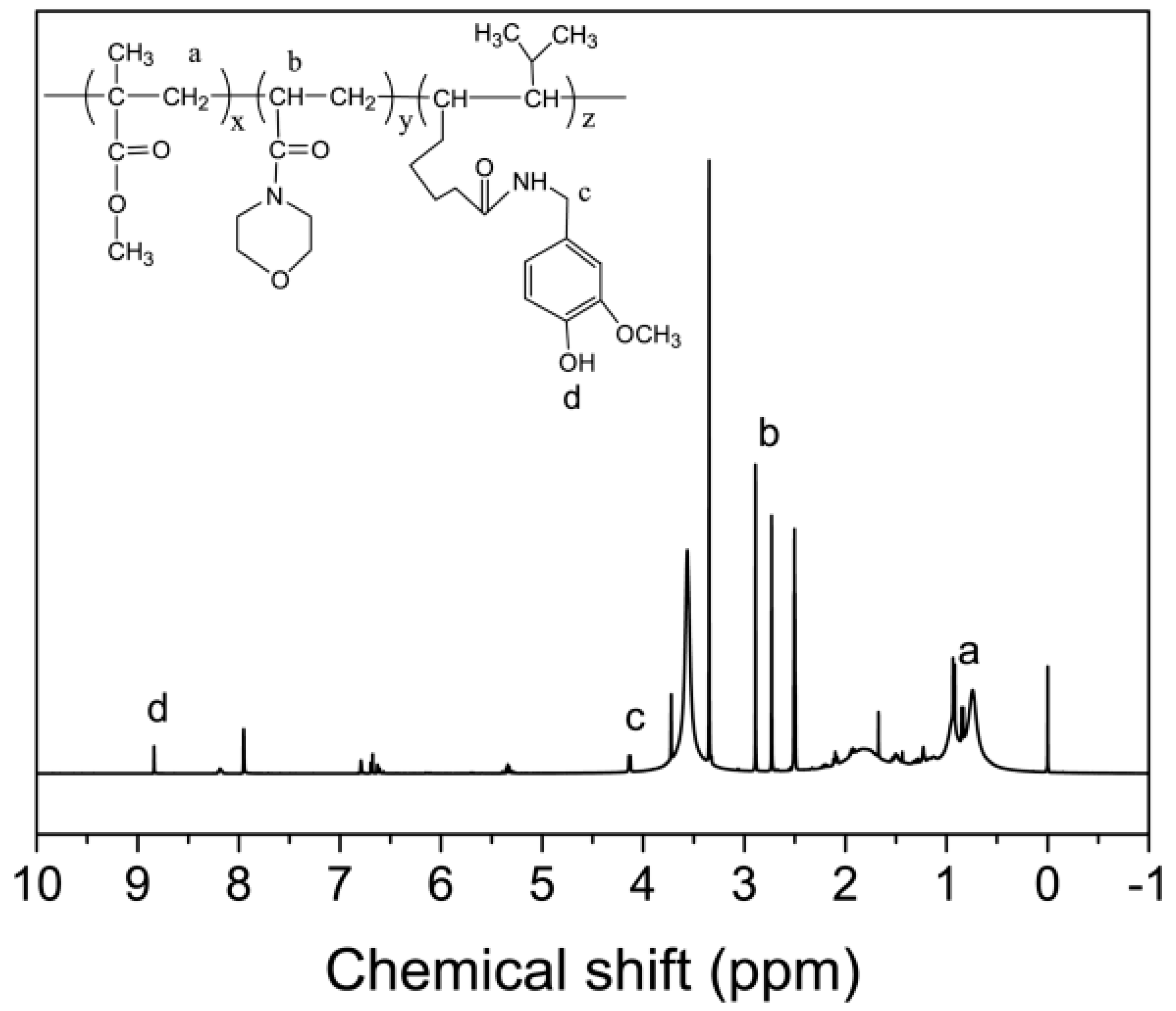
2.2. Synthesis of Capsaicin-Based Copolymer
2.3. Membrane Preparation
2.4. Membrane Characterization
2.5. Filtration Experiment
3. Results
3.1. Characterization of PMMA-PACMO-Capsaicin Copolymer
3.2. Chemical Composition of Membrane Surfaces
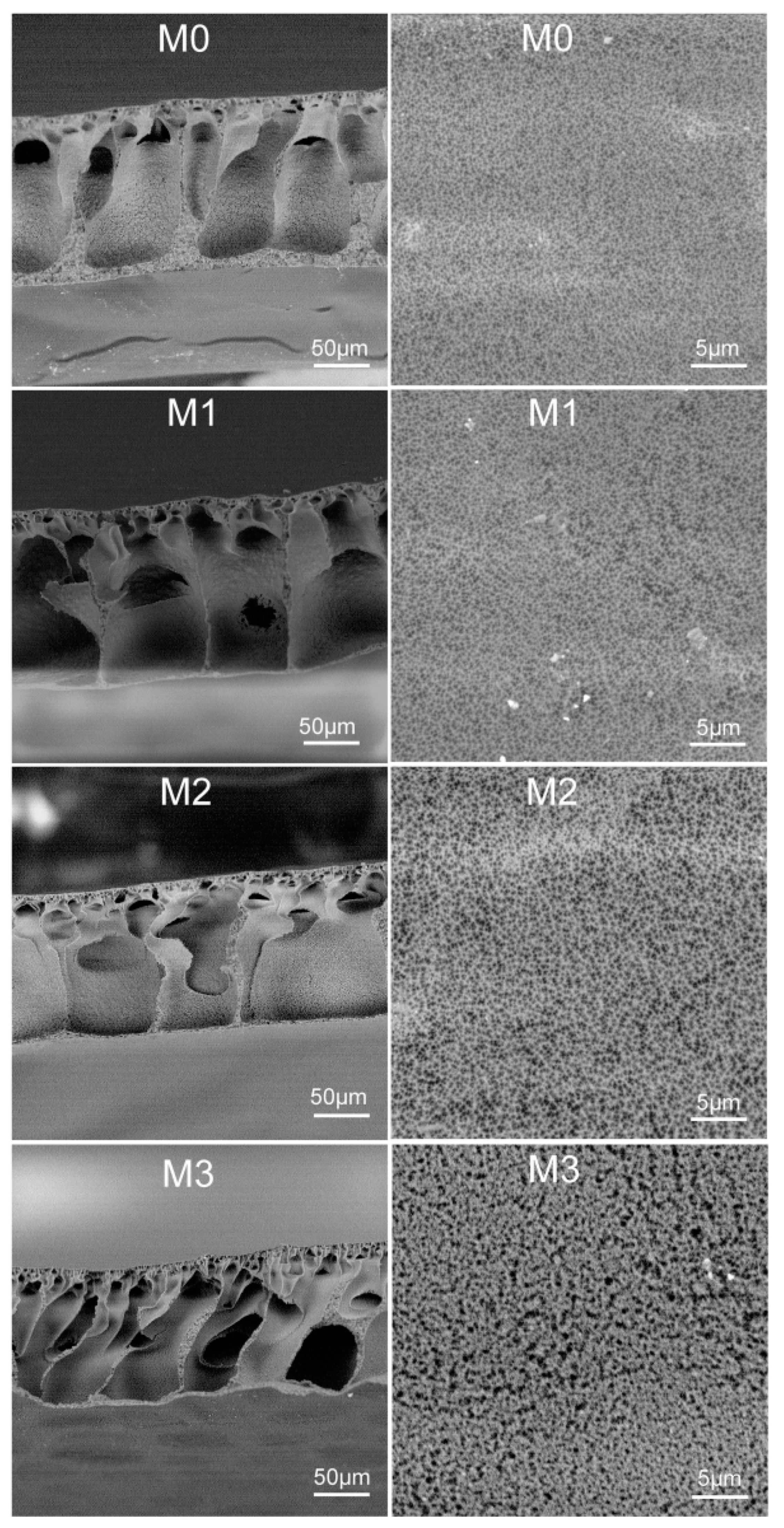
3.3. Membrane Morphologies
3.4. Hydrophilicity of Membranes
3.5. Permeation and Separation Properties of Membranes
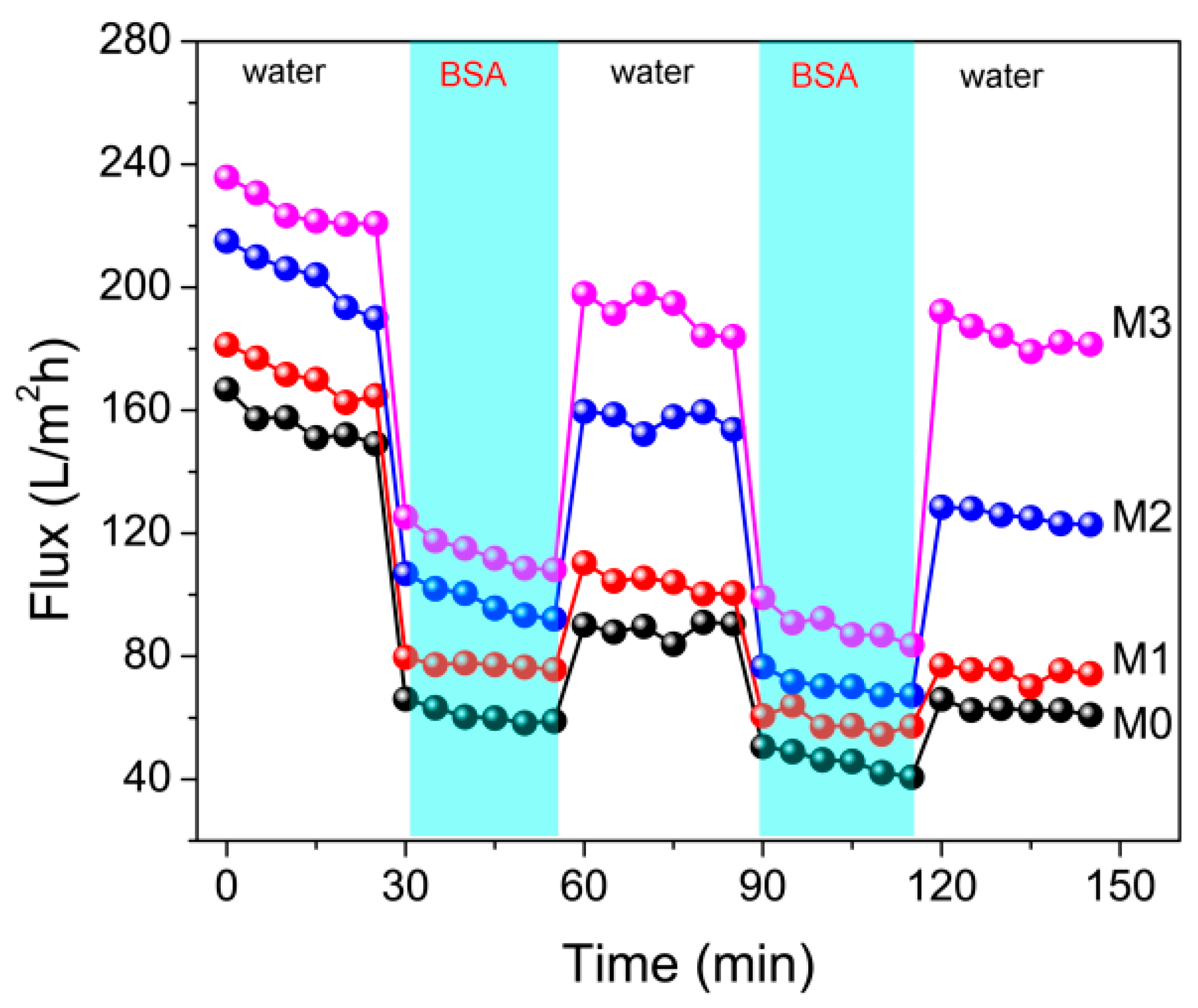
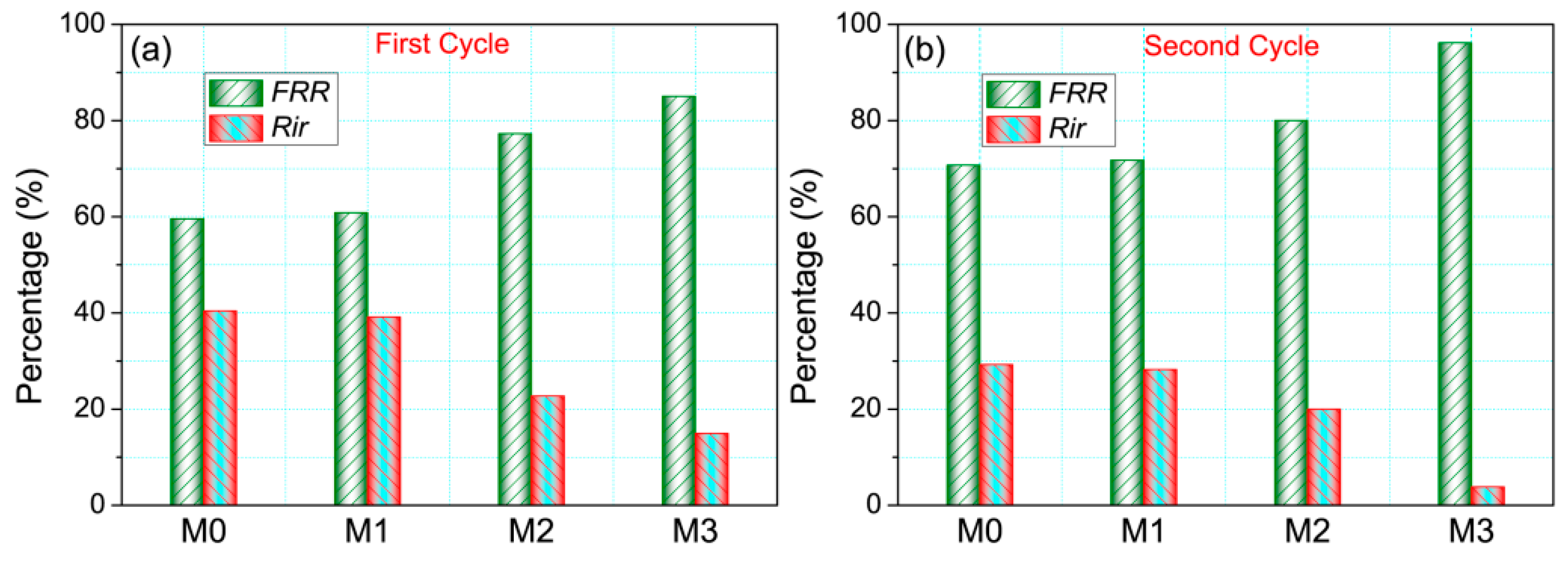
3.6. Anti-Fouling Properties of Membranes
3.7. Anti-Bacterial Properties of Membranes
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chai, M.; Ye, Y.; Chen, V. Separation and concentration of milk proteins with a submerged membrane vibrational system. J. Membr. Sci. 2017, 524, 305–314. [Google Scholar] [CrossRef]
- Javadi, M.; Jafarzadeh, Y.; Yegani, R.; Kazemi, S. PVDF membranes embedded with PVP functionalized nanodiamond for pharmaceutical wastewater treatment. Chem. Eng. Res. Des. 2018, 140, 241–250. [Google Scholar] [CrossRef]
- Younas, H.; Bai, H.W.; Shao, J.H.; Han, Q.C.; Ling, Y.H.; He, Y.L. Super-hydrophilic and fouling resistant PVDF ultrafiltration membranes based on a facile prefabricated surface. J Membr. Sci. 2017, 541, 529–540. [Google Scholar] [CrossRef]
- Li, N.; Zhang, J.; Tian, Y.; Zhao, J.H.; Zhang, J.; Zuo, W. Anti-fouling potential evaluation of PVDF membranes modified with ZnO against polysaccharide. Chem. Eng. J. 2016, 304, 165–174. [Google Scholar] [CrossRef]
- Li, T.T.; Liu, F.; Lin, H.B.; Xiong, Z.; Wang, H.; Zhong, Y.; Xiang, L.C.; Wu, A.G. Fabrication of anti-fouling, anti-bacterial and non-clotting PVDF membranes through one step “outside-in” interface segregation strategy. J. Colloid. Interf. Sci. 2018, 517, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Zhao, Y.P.; Chen, L. The construction of a zwitterionic PVDF membrane surface to improve biofouling resistance. Biofouling 2013, 29, 991–1003. [Google Scholar] [CrossRef] [PubMed]
- Herzberg, M.; Berry, D.; Raskin, L. Impact of microfiltration treatment of secondary wastewater effluent on biofouling of reverse osmosis membranes. Water. Res. 2010, 44, 167–176. [Google Scholar] [CrossRef]
- Hebbar, R.S.; Isloor, A.M.; Ananda, K.; Sohaimi Abdullah, M.; Ismail, A.F. Fabrication of a novel hollow fiber membrane decorated with functionalized Fe2O3 nanoparticles: Towards sustainable water treatment and biofouling control. New J. Chem. 2017, 41, 4197–4211. [Google Scholar] [CrossRef]
- Xu, H.J.; Liu, Y. d-Amino acid mitigated membrane biofouling and promoted biofilm detachment. J. Membr. Sci. 2011, 376, 266–274. [Google Scholar] [CrossRef]
- Zhu, L.J.; Zhu, L.P.; Zhao, Y.F.; Zhu, B.K.; Xu, Y.Y. Anti-fouling and anti-bacterial polyethersulfone membranes quaternized from the additive of poly(2-dimethylamino ethyl methacrylate) grafted SiO2 nanoparticles. J. Mater. Chem. A 2014, 2, 15566–15574. [Google Scholar] [CrossRef]
- Song, W.L.; Li, Z.P.; Li, Y.Z.; You, H.; Qi, P.S.; Liu, F.; Loy, D.A. Facile sol-gel coating process for anti-biofouling modification of poly (vinylidene fluoride) microfiltration membrane based on novel zwitterionic organosilica. J. Membr. Sci. 2018, 550, 266–277. [Google Scholar] [CrossRef]
- Carretier, S.; Chen, L.A.; Venault, A.; Yang, Z.R.; Aimar, P.; Chang, Y. Design of PVDF/PEGMA-b-PS-b-PEGMA membranes by VIPS for improved biofouling mitigation. J. Membr. Sci. 2016, 510, 355–369. [Google Scholar] [CrossRef]
- Li, X.; Hu, X.F.; Cai, T. Construction of hierarchical fouling resistance surfaces onto poly(vinylidene fluoride) membranes for combating membrane biofouling. Langmuir 2017, 33, 4477–4489. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.F.; Li, Y.; Li, Q.L.; Wan, L.S.; Xu, Z.K. Surface hydrophilization of microporous polypropylene membrane by grafting zwitterionic polymer for anti-biofouling. J. Membr. Sci. 2010, 362, 255–264. [Google Scholar] [CrossRef]
- Pan, Y.; Yu, Z.X.; Shi, H.; Chen, Q.; Zeng, G.Y.; Di, H.H.; Ren, X.Q.; He, Y. A novel antifouling and antibacterial surface-functionalized PVDF ultrafiltration membrane via binding Ag/SiO2 nanocomposites. J. Chem. Technol. Biotechnol. 2017, 92, 562–572. [Google Scholar] [CrossRef]
- Wu, Q.; Chen, G.E.; Sun, W.G.; Xu, Z.L.; Kong, Y.F.; Zheng, X.P.; Xu, S.J. Bio-inspired GO-Ag/PVDF/F127 membrane with improved anti-fouling for natural organic matter (NOM) resistance. Chem. Eng. J. 2017, 313, 450–460. [Google Scholar] [CrossRef]
- Huang, L.C.; Zhao, S.; Wang, Z.; Wu, J.H.; Wang, J.X.; Wang, S.C. In situ immobilization of silver nanoparticles for improving permeability, antifouling and anti-bacterial properties of ultrafiltration membrane. J. Membr. Sci. 2016, 499, 269–281. [Google Scholar] [CrossRef]
- Zhao, C.Q.; Lv, J.L.; Xu, X.C.; Zhang, G.Q.; Yang, Y.S.; Yang, F.L. Highly antifouling and antibacterial performance of poly (vinylidene fluoride) ultrafiltration membranes blending with copper oxide and graphene oxide nanofillers for effective wastewater treatment. J. Colloid Interf. Sci. 2017, 505, 341–351. [Google Scholar] [CrossRef]
- Li, R.J.; Wu, Y.T.; Shen, L.G.; Chen, J.R.; Lin, H.J. A novel strategy to develop antifouling and antibacterial conductive Cu/polydopamine/polyvinylidene fluoride membranes for water treatment. J. Colloid Interf. Sci. 2018, 531, 493–501. [Google Scholar] [CrossRef]
- Ronen, A.; Semiat, R.; Dosoretz, C.G. Impact of ZnO embedded feed spacer on biofilm development in membrane systems. Water Res. 2013, 47, 6628–6638. [Google Scholar] [CrossRef]
- Javdaneh, S.; Mehrnia, M.R.; Homayoonfal, M. Engineering design of a biofilm formed on a pH-sensitive ZnO/PSf nanocomposite membrane with antibacterial properties. RSC Adv. 2016, 6, 112269–112281. [Google Scholar] [CrossRef]
- Kakihana, Y.; Cheng, L.; Fang, L.F.; Wang, S.Y.; Jeon, S.; Saeki, D.; Rajabzadeh, S.; Matsuyama, H. Preparation of positively charged PVDF membranes with improved antibacterial activity by blending modification: Effect of change in membrane surface material properties. Colloid Surf. A 2017, 533, 133–139. [Google Scholar] [CrossRef]
- Sui, Y.; Gao, X.; Wang, Z.N.; Gao, C.L. Antifouling and antibacterial improvement of surface-functionalized poly(vinylidene fluoride) membrane prepared via dihydroxyphenylalanine-initiated atom transfer radical graft polymerizations. J. Membr. Sci. 2012, 394–395, 107–119. [Google Scholar] [CrossRef]
- Shen, X.; Zhao, Y.P.; Chen, L. Polycation-grafted poly(vinylidene fluoride) membrane with biofouling resistance. Chem. Eng. Technol. 2015, 38, 859–866. [Google Scholar] [CrossRef]
- Wang, H.; Wang, Z.M.; Yan, X.; Chen, J.; Lang, W.Z.; Guo, Y.J. Novel organic-inorganic hybrid polyvinylidene fluoride ultrafiltration membranes with antifouling and antibacterial properties by embedding N-halamine functionalized silica nanospheres. J. Ind. Eng. Chem. 2017, 52, 295–304. [Google Scholar] [CrossRef]
- Hou, S.H.; Dong, X.; Zhu, J.H.; Zheng, J.F.; Bi, W.H.; Li, S.J.; Zhang, S.B. Preparation and characterization of an antibacterial ultrafiltration membrane with N-chloramine functional groups. J. Colloid Interf. Sci. 2017, 496, 391–400. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.R.; Ma, J.X.; Tang, C.Y.; Wang, Z.W.; Ng, H.Y.; Wu, Z.C. Antibiofouling polyvinylidene fluoride membrane modified by quaternary ammonium compound: Direct contact-killing versus induced indirectcontact-killing. Environ. Sci. Technol. 2016, 50, 5086–5093. [Google Scholar] [CrossRef]
- Zhang, W.; Cheng, W.; Ziemann, E.; Béer, A.; Lu, X.L.; Elimelech, M.; Bernstein, R. Functionalization of ultrafiltration membrane with polyampholyte hydrogel and graphene oxide to achieve dual antifouling and antibacterial properties. J. Membr. Sci. 2018, 565, 293–302. [Google Scholar] [CrossRef]
- Watts, L.J. Anti-Fouling Coating Composition Containing Capsaicin. U.S. Patent US5397385, 14 March 1995. [Google Scholar]
- Wang, J.; Gao, X.L.; Wang, Q.; Sun, H.J.; Wang, X.J.; Gao, C.J. Enhanced biofouling resistance of polyethersulfone membrane surface modified with capsaicin derivative and itaconic acid. Appl. Surf. Sci. 2015, 356, 467–474. [Google Scholar] [CrossRef]
- Xu, J.; Feng, X.; Hou, J.; Wang, X.; Shan, B.T.; Yu, L.M.; Gao, C.J. Preparation and characterization of a novel polysulfone UF membrane using a copolymer with capsaicin-mimic moieties for improved anti-fouling properties. J. Membr. Sci. 2013, 446, 171–180. [Google Scholar] [CrossRef]
- Zhang, L.L.; Xu, J.; Tang, Y.Y.; Hou, J.W.; Yu, L.M.; Gao, C.J. A novel long-lasting antifouling membrane modified with bifunctional capsaicin-mimic moieties via in situ polymerization for efficient water purification. J. Mater. Chem. A 2016, 4, 10352–10362. [Google Scholar] [CrossRef]
- Zhang, L.L.; Shan, C.Y.; Jiang, X.H.; Li, X.; Yu, L.M. High hydrophilic antifouling membrane modified with capsaicin-mimic moieties via microwave assistance (MWA) for efficient water purification. Chem. Eng. J. 2018, 338, 688–699. [Google Scholar] [CrossRef]
- Zhan, X.L.; Zhang, G.F.; Chen, X.; He, R.; Zhang, Q.H.; Chen, F.Q. Improvement of antifouling and antibacterial properties of poly(ether sulfone) UF membrane by blending with a multifunctional comb copolymer. Ind. Eng. Chem. Res. 2015, 54, 11312–11318. [Google Scholar] [CrossRef]
- Gao, X.L.; Wang, H.Z.; Wang, J.; Huang, X.; Gao, C.J. Surface-modified PSf UF membrane by UV-assisted graft polymerization of capsaicin derivative moiety for fouling and bacterial resistance. J. Membr. Sci. 2013, 445, 146–155. [Google Scholar]
- Akyuz, L.; Kaya, M.; Mujtaba, M.; Ilk, S.; Sargin, I.; Salaberria, A.M.; Labidi, J.; Cakmak, Y.S.; Islek, C. Supplementing capsaicin with chitosan-based films enhanced the anti-quorum sensing, antimicrobial, antioxidant, transparency, elasticity and hydrophobicity. Int. J. Biol. Macromol. 2018, 115, 438–446. [Google Scholar] [CrossRef] [PubMed]
- Yao, F.; Fu, G.D.; Zhao, J.P.; Kang, E.T.; Neoh, K.G. Antibacterial effect of surface-functionalized polypropylene hollow fiber membrane from surface-initiated atom transfer radical polymerization. J. Membr. Sci. 2008, 319, 149–157. [Google Scholar] [CrossRef]
- Liu, F.; Xu, Y.Y.; Zhu, B.K.; Zhang, F.; Zhu, L.P. Preparation of hydrophilic and fouling resistant poly(vinylidene fluoride) hollow fiber membranes. J. Membr. Sci. 2009, 345, 331–339. [Google Scholar] [CrossRef]
- Shen, X.; Xie, T.D.; Wang, J.G.; Wang, F. Improved fouling resistance of poly(vinylidene fluoride) membrane modified with poly(acryloyl morpholine)-based amphiphilic copolymer. Colloid Polym. Sci. 2017, 295, 1211–1221. [Google Scholar] [CrossRef]
- Li, F.W.; Lin, Y.L.; Wang, X.; Geng, Y.L.; Wang, D.J. Preparative isolation and purification of capsaicinoids from Capsicum frutescens using high-speed counter-current chromatography. Sep. Purif. Technol. 2009, 64, 304–308. [Google Scholar] [CrossRef]
- Ma, W.Z.; Rajabzadeh, S.; Shaikh, A.R.; Kakihana, Y.; Sun, Y.C.; Matsuyama, H. Effect of type of poly(ethylene glycol) (PEG) based amphiphilic copolymer on antifouling properties of copolymer/poly(vinylidene fluoride) (PVDF) blend membranes. J. Membr. Sci. 2016, 514, 429–439. [Google Scholar] [CrossRef]
- Shen, X.; Liu, J.; Feng, X.; Zhao, Y.P.; Chen, L. Preliminary investigation on hemocompatibility of poly(vinylidene fluoride) membrane grafted with acryloylmorpholine via ATRP. J. Biomed. Mater. Res. Part A 2015, 103, 683–692. [Google Scholar] [CrossRef] [PubMed]
- Hashim, N.A.; Liu, F.; Li, K. A simplified method for preparation of hydrophilic PVDF membranes from an amphiphilic graft copolymer. J. Membr. Sci. 2009, 345, 134–141. [Google Scholar] [CrossRef]
- Khayet, M.; García-Payo, M.C.; Qusay, F.A.; Zubaidy, M.A. Structural and performance studies of poly(vinyl chloride) hollow fiber membranes prepared at different air gap lengths. J. Membr. Sci. 2009, 330, 30–39. [Google Scholar] [CrossRef]
- Chiang, Y.C.; Chang, Y.; Higuchi, A.; Chen, W.Y.; Ruaan, R.C. Sulfobetaine-grafted poly(vinylidene fluoride) ultrafiltration membranes exhibit excellent antifouling property. J. Membr. Sci. 2009, 339, 151–159. [Google Scholar] [CrossRef]





| Membrane Sample | PVDF (wt%) | PMMA-PACMO-Capsaicin (wt%) | PEG (wt%) | NMP (wt%) |
|---|---|---|---|---|
| M0 | 15.0 | 0.0 | 2.5 | 82.5 |
| M1 | 12.5 | 2.5 | 2.5 | 82.5 |
| M2 | 10.0 | 5.0 | 2.5 | 82.5 |
| M3 | 7.5 | 7.5 | 2.5 | 82.5 |
| Sample | Chemical Elements (%) | (N/F)e (%) | (N/F)t (%) | C 1s (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| C1s | F1s | O1s | N1s | CF2 | O–C=O | C=O | CH | C–N/C–OH/C–O–C/CH2 | |||
| M0 | 52.66 | 44.52 | 2.82 | — | — | — | 44.01 | — | — | 4.27 | 51.72 |
| M1 | 55.53 | 35.26 | 7.50 | 1.71 | 4.85 | 0.98 | 29.68 | 4.26 | 1.44 | 13.75 | 50.87 |
| M2 | 57.77 | 30.08 | 9.57 | 2.58 | 8.58 | 2.45 | 25.45 | 7.15 | 2.67 | 18.66 | 46.07 |
| M3 | 60.95 | 22.27 | 13.46 | 3.32 | 14.91 | 4.97 | 16.07 | 8.09 | 3.19 | 29.87 | 42.78 |
| Sample | Ra (nm) | Rq (nm) | Rz (nm) | ε (%) | rm (nm) |
|---|---|---|---|---|---|
| M0 | 14.3 ± 2.1 | 17.3 ± 3.2 | 160.5 ± 6.4 | 68.6 | 100.5 |
| M1 | 16.4 ± 3.6 | 20.1 ± 3.4 | 170.5 ± 14.4 | 69.2 | 146.1 |
| M2 | 15.8 ± 2.3 | 19.1 ± 1.6 | 204.0 ± 11.9 | 72.5 | 164.6 |
| M3 | 19.4 ± 1.7 | 25.4 ± 2.0 | 231.0 ± 18.1 | 75.3 | 179.2 |
| Sample | Pure Water Flux (L/m2h) | BSA Rejection (%) |
|---|---|---|
| M0 | 149.2 | 95.6 |
| M1 | 171.3 | 83.4 |
| M2 | 203.1 | 71.3 |
| M3 | 225.5 | 64.6 |
| Sample | Blank | M0 | M1 | M2 | M3 |
|---|---|---|---|---|---|
| Number of bacteria colonies | 260 | 256 | 89 | 74 | 30 |
| Anti-bacterial efficiency (%) | 0 | 1.5 | 65.8 | 71.5 | 88.5 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, X.; Liu, P.; Xia, S.; Liu, J.; Wang, R.; Zhao, H.; Liu, Q.; Xu, J.; Wang, F. Anti-Fouling and Anti-Bacterial Modification of Poly(vinylidene fluoride) Membrane by Blending with the Capsaicin-Based Copolymer. Polymers 2019, 11, 323. https://doi.org/10.3390/polym11020323
Shen X, Liu P, Xia S, Liu J, Wang R, Zhao H, Liu Q, Xu J, Wang F. Anti-Fouling and Anti-Bacterial Modification of Poly(vinylidene fluoride) Membrane by Blending with the Capsaicin-Based Copolymer. Polymers. 2019; 11(2):323. https://doi.org/10.3390/polym11020323
Chicago/Turabian StyleShen, Xiang, Peng Liu, Shubiao Xia, Jianjun Liu, Rui Wang, Hua Zhao, Qiuju Liu, Jiao Xu, and Fan Wang. 2019. "Anti-Fouling and Anti-Bacterial Modification of Poly(vinylidene fluoride) Membrane by Blending with the Capsaicin-Based Copolymer" Polymers 11, no. 2: 323. https://doi.org/10.3390/polym11020323