Associations between B Vitamins and Parkinson’s Disease
Abstract
:1. Introduction
2. Methods
2.1. Literature Search
2.2. Inclusion Criteria
2.3. Statistical Analysis
3. Results

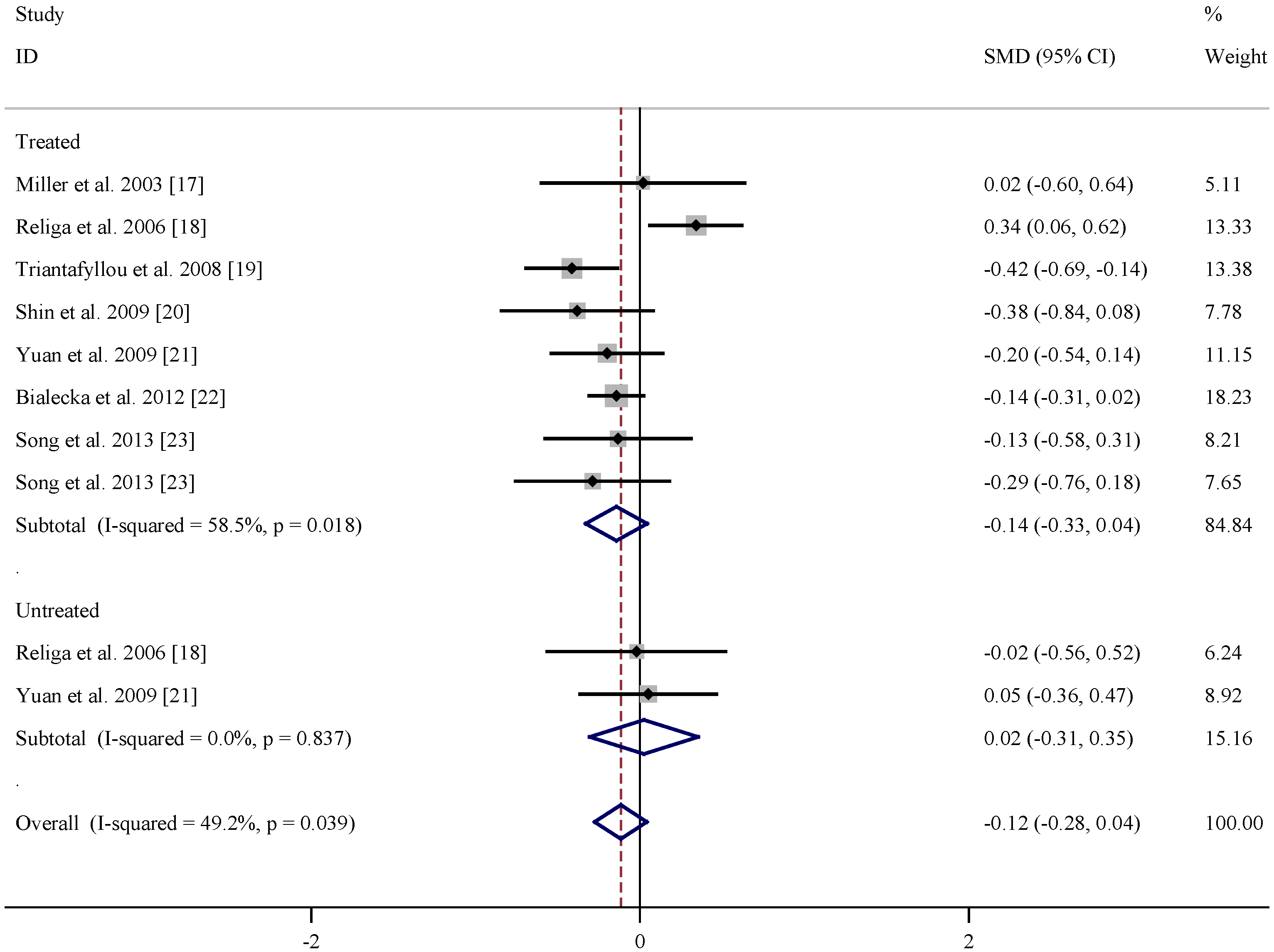
| References | Subgroup | Mean age (years) | n | Folate levels (nmol/L) (Mean ± SD) | |||
|---|---|---|---|---|---|---|---|
| PD | Control | PD | Control | PD | Control | ||
| Miller et al. 2003 [17] | Treated | 64 ± 9 | 60 ± 10 | 20 | 20 | 12.8 ± 11.6 | 12.6 ± 10.8 |
| Religa et al. 2006 [18] | Treated | 70.5 ± 7.57 | 71.2 ± 6.0 | 99 | 100 | 20.87 ± 9.58 | 17.13 ± 12.21 |
| Triantafyllou et al. 2008 [19] | Treated | 70.1 ± 8.0 | 69.6 ± 8.1 | 111 | 93 | 9.92 ± 5.17 | 12.35 ± 6.57 |
| Shin et al. 2009 [20] | Treated | 63.5 ± 7.8 | 65.4 ± 7.8 | 33 | 41 | 19.26 ± 9.06 | 23.11 ± 10.87 |
| Yuan et al. 2009 [21] | Treated | 71.83 ± 10.34 | 69.95 ± 8.46 | 48 | 110 | 18.28 ± 10.8 | 20.37 ± 10.33 |
| Białecka et al. 2012 [22] | Treated | 64.4 ± 10.1 | 64.8 ± 9.6 | 320 | 254 | 20.16 ± 9.52 | 21.52 ± 9.28 |
| Song et al. 2013 [23] | Treated | 66.45 ± 6.60 | 66.23 ± 11.83 | 33 | 48 | 34.46 ± 25.69 | 38.54 ± 33.57 |
| Song et al. 2013 [23] | Treated | 70.50 ± 6.75 | 66.23 ± 11.83 | 28 | 48 | 29.47 ± 27.21 | 38.54 ± 33.57 |
| Religa et al. 2006 [18] | Untreated | 66.0 ± 7.11 | 71.2 ± 6.0 | 15 | 100 | 16.88 ± 6.39 | 17.13 ± 12.21 |
| Yuan et al. 2009 [21] | Untreated | 70.57 ± 9.09 | 69.95 ± 8.46 | 28 | 110 | 20.87 ± 8.11 | 20.37 ± 10.33 |
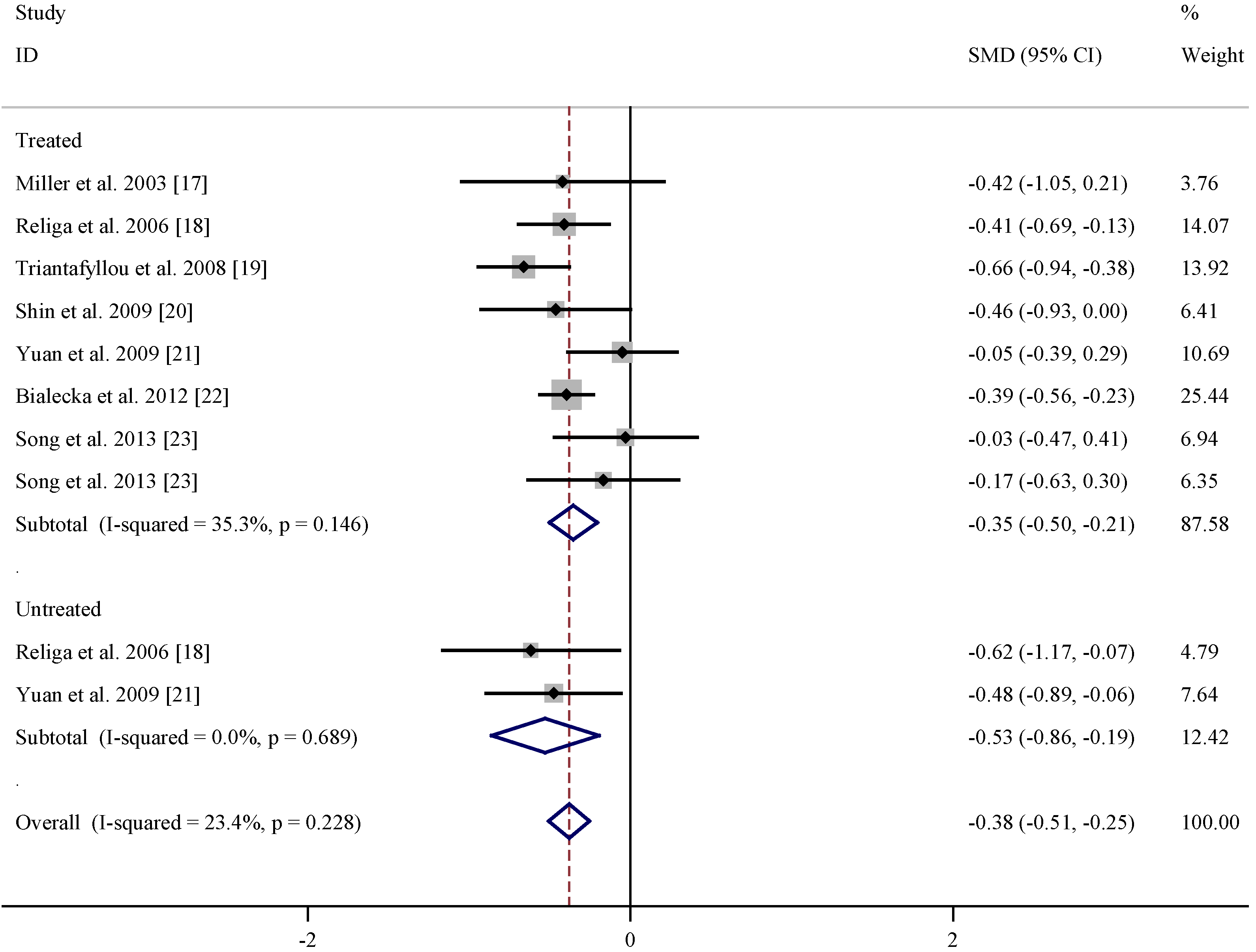
| References | Subgroup | Mean age (years) | n | Vitamin B12 levels (pmol/L) (Mean ± SD) | |||
|---|---|---|---|---|---|---|---|
| PD | Control | PD | Control | PD | Control | ||
| Miller et al. 2003 [17] | Treated | 64 ± 9 | 60 ± 10 | 20 | 20 | 375 ± 167 | 464 ± 249 |
| Religa et al. 2006 [18] | Treated | 70.5 ± 7.57 | 71.2 ± 6.0 | 99 | 100 | 241.04 ± 129.04 | 17.13 ± 12.21 |
| Triantafyllou et al. 2008 [19] | Treated | 70.1 ± 8.0 | 69.6 ± 8.1 | 111 | 93 | 216.03 ± 90.5 | 283.54 ± 114.43 |
| Shin et al. 2009 [20] | Treated | 63.5 ± 7.8 | 65.4 ± 7.8 | 33 | 41 | 528.71 ± 299.70 | 651.93 ± 236.25 |
| Yuan et al. 2009 [21] | Treated | 71.83 ± 10.34 | 69.95 ± 8.46 | 48 | 110 | 354.76 ± 191.32 | 362.46 ± 135.82 |
| Białecka et al. 2012 [22] | Treated | 64.4 ± 10.1 | 64.8 ± 9.6 | 320 | 254 | 244.95 ± 104.77 | 295.12 ± 150.51 |
| Song et al. 2013 [23] | Treated | 66.45 ± 6.60 | 66.23 ± 11.83 | 33 | 48 | 473.61 ± 220.53 | 480.53 ± 239.45 |
| Song et al. 2013 [23] | Treated | 70.50 ± 6.75 | 66.23 ± 11.83 | 28 | 48 | 428.84 ± 265.56 | 470.61 ± 239.45 |
| Religa et al. 2006 [18] | Untreated | 66.0 ± 7.11 | 71.2 ± 6.0 | 15 | 100 | 201.42 ± 63.82 | 305.08 ± 178.03 |
| Yuan et al. 2009 [21] | Untreated | 70.57 ± 9.09 | 69.95 ± 8.46 | 28 | 110 | 299.95 ± 112.20 | 362.46 ± 135.82 |
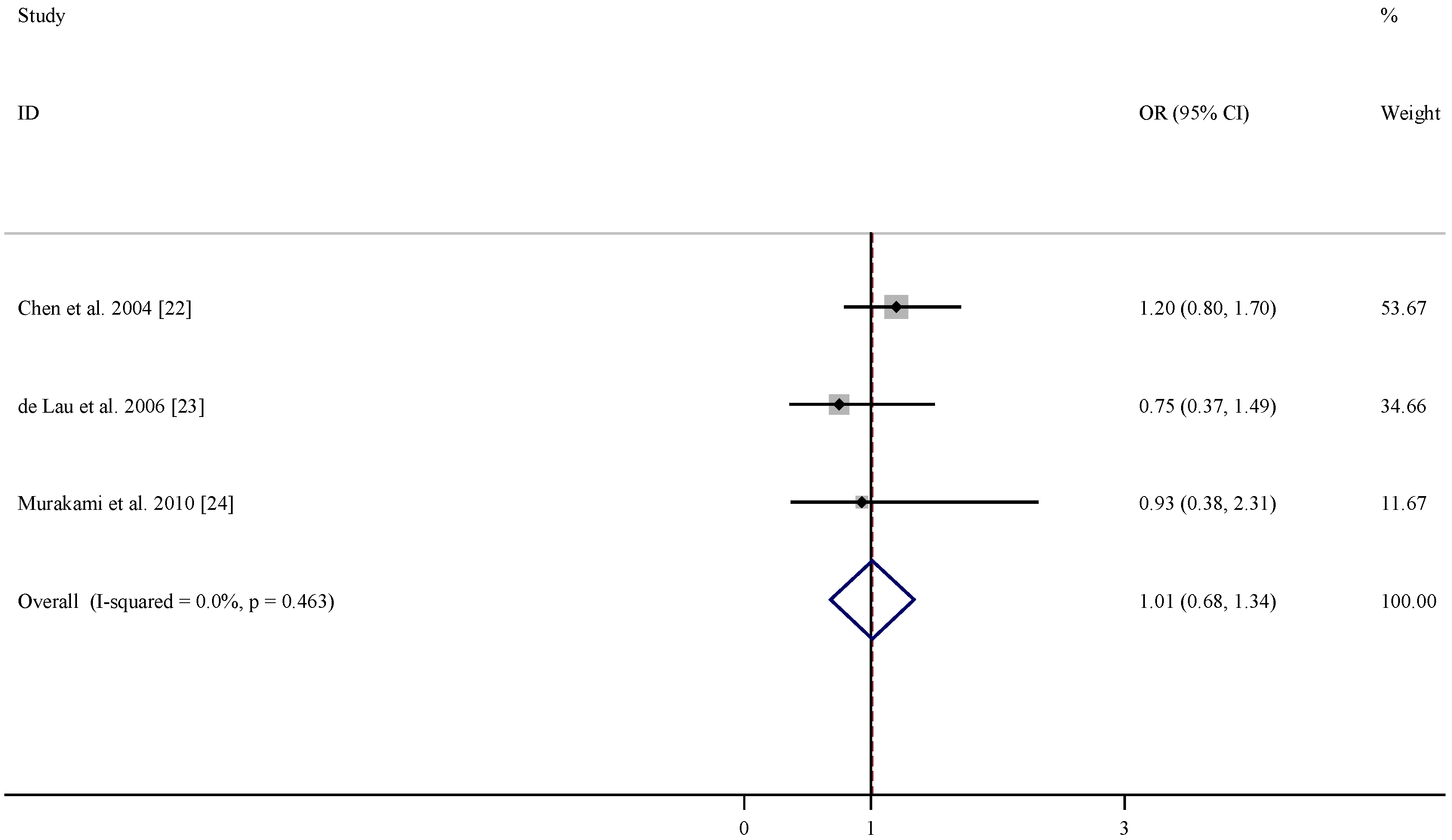
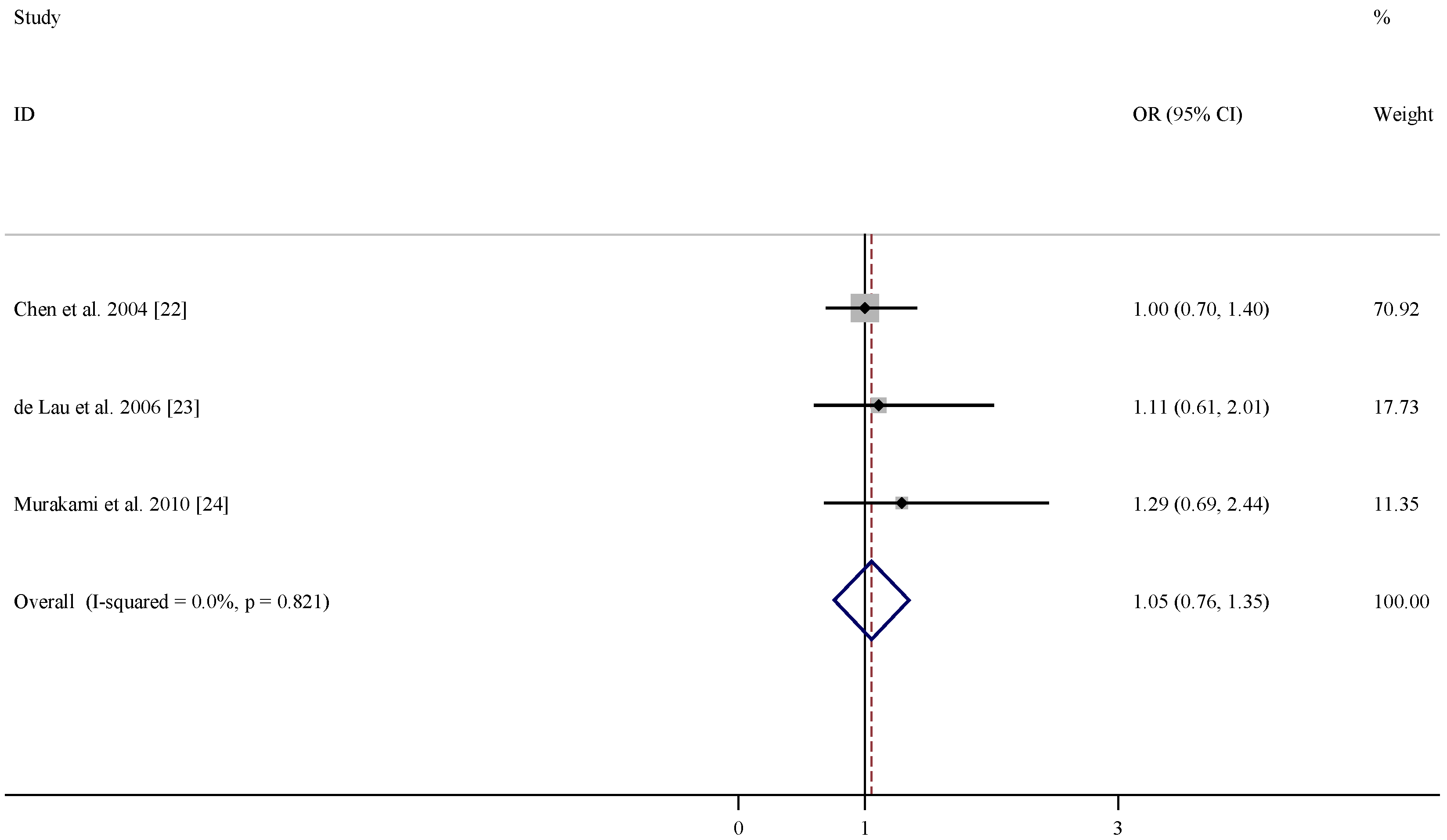
| References | Relative risk (95% CI) for folate | Relative risk (95% CI) for vitamin B6 | Relative risk (95% CI) for vitamin B12 | Adjustment |
|---|---|---|---|---|
| Chen et al. 2004 [22] | 1.2 (0.8, 1.7) | 1.0 (0.7, 1.4) | 1.0 (0.7, 1.4) | age, smoking, total energy intake, alcohol consumption, caffeine intake, and lactose intake |
| De Lau et al. 2006 [23] | 0.75 (0.37, 1.49) | 0.46 (0.22, 0.96) | 1.11 (0.61, 2.01) | age, sex, and total energy intake |
| Murakami et al. 2010 [24] | 0.93 (0.38, 2.31) | 0.48 (0.23, 0.99) | 1.29 (0.69, 2.44) | age, sex, region, smoking, education, BMI, and dietary factors, including cholesterol, dietary glycaemic index, vitamin E , vitamin C, β-carotene, alcohol, caffeine and Fe, and intake of other B vitamins |

4. Discussion
Acknowledgments
Conflicts of Interest
References
- Lees, A.J.; Hardy, J.; Revesz, T. Parkinson’s disease. Lancet 2009, 373, 2055–2066. [Google Scholar] [CrossRef]
- Connolly, B.S.; Lang, A.E. Pharmacological treatment of Parkinson disease: A review. JAMA 2014, 311, 1670–1683. [Google Scholar] [CrossRef] [PubMed]
- De Lau, L.M.; Breteler, M.M. Epidemiology of Parkinson’s disease. Lancet Neurol. 2006, 5, 525–535. [Google Scholar] [CrossRef]
- Jenner, P. Oxidative stress in Parkinson’s disease. Ann. Neurol. 2003, 53, S26–S36. [Google Scholar] [CrossRef] [PubMed]
- Dias, V.; Junn, E.; Mouradian, M.M. The role of oxidative stress in Parkinson’s disease. J. Parkinsons. Dis. 2013, 3, 461–491. [Google Scholar] [PubMed]
- Seshadri, S.; Beiser, A.; Selhub, J.; Jacques, P.F.; Rosenberg, I.H.; D’Agostino, R.B.; Wilson, P.W.; Wolf, P.A. Plasma homocysteine as a risk factor for dementia and Alzheimer’s disease. N. Engl. J. Med. 2002, 346, 476–483. [Google Scholar] [CrossRef] [PubMed]
- Prins, N.D.; den Heijer, T.; Hofman, A.; Koudstaal, P.J.; Jolles, J.; Clarke, R.; Breteler, M.M. Homocysteine and cognitive function in the elderly: The Rotterdam Scan Study. Neurology 2002, 59, 1375–1380. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, W.; Roebroek, R.; Blom, H.; van Oppenraaij, D.; Muller, T. Hyperhomocysteinaemia in Parkinson’s disease. J. Neurol. 1998, 245, 811–812. [Google Scholar] [CrossRef] [PubMed]
- Postuma, R.B.; Lang, A. Homocysteine and levodopa. Should parkinson disease patients receive preventative therapy? Neurology 2004, 63, 886–891. [Google Scholar] [CrossRef] [PubMed]
- Müller, T. Role of homocysteine in the treatment of Parkinson’s disease. Expert Rev. Neurother. 2008, 8, 957–967. [Google Scholar] [CrossRef] [PubMed]
- Papazisis, K.; Kyriazis, G.; Kiosseoglou, G.; Kazis, A. Endothelial function markers in parkinsonian patients with hyperhomocysteinemia. J. Clin. Neurosci. 2005, 12, 669–672. [Google Scholar]
- Todorović, Z.; Dzoljić, E.; Novaković, I.; Mirković, D.; Stojanović, R.; Nesić, Z.; Krajinović, M.; Prostran, M.; Kostić, V. Homocysteine serum levels and MTHFR C677T genotype in patients with Parkinson’s disease, with and without levodopa therapy. J. Neurol. Sci. 2006, 248, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Oroz, M.C.; Lage, P.M.; Sanchez-Mut, J.; Lamet, I.; Pagonabarraga, J.; Toledo, J.B.; García-Garcia, D.; Clavero, P.; Samaranch, L.; Irurzun, C.; et al. Homocysteine and cognitive impairment in Parkinson’s disease: A biochemical, neuroimaging, and genetic study. Mov. Disord. 2009, 24, 1437–1444. [Google Scholar] [CrossRef] [PubMed]
- Duan, W.; Ladenheim, B.; Cutler, R.G.; Kruman, I.I.; Cadet, J.L.; Mattson, M.P. Dietary folate deficiency anded elevated homocysteine levels endanger dopaminergic neurons in models of Parkinson’s disease. J. Neurochem. 2002, 80, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Kruman, I.I.; Culmsee, C.; Chan, S.L.; Kruman, Y.; Guo, Z.; Penix, L.; Mattson, M.P. Homocysteine elicits a DNA damage response in neurons that promotes apoptosis and hypersensitivity to excitotoxicity. J. Neurosci. 2000, 20, 6920–6926. [Google Scholar] [PubMed]
- Lipton, S.A.; Kim, W.K.; Choi, Y.B.; Kumar, S.; D’Emilia, D.M.; Rayudu, P.V.; Arnelle, D.R.; Stamler, J.S. Neurotoxicity associated with dual actions of homocysteine at the N-methyl-d-aspartate receptor. Proc. Natl. Acad. Sci. USA 1997, 94, 5923–5928. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.W.; Selhub, J.; Nadeau, M.R.; Thomas, C.A.; Feldman, R.G.; Wolf, P.A. Effect of l-dopa on plasma homocysteine in PD patients: Relationship to B-vitamin status. Neurology 2003, 60, 1125–1129. [Google Scholar] [CrossRef] [PubMed]
- Religa, D.; Czyzewski, K.; Styczynska, M.; Peplonska, B.; Lokk, J.; Chodakowska-Zebrowska, M.; Stepien, K.; Winblad, B.; Barcikowska, M. Hyperhomocysteinemia and methylenetetrahydrofolate reductase polymorphism in patients with Parkinson’s disease. Neurosci. Lett. 2006, 404, 56–60. [Google Scholar] [CrossRef] [PubMed]
- Triantafyllou, N.I.; Nikolaou, C.; Boufidou, F.; Angelopoulos, E.; Rentzos, M.; Kararizou, E.; Evangelopoulos, M.E.; Vassilopoulos, D. Folate and vitamin B12 levels in levodopa-treated Parkinson’s disease patients: Their relationship to clinical manifestations, mood and cognition. Parkinsonism Relat. Disord. 2008, 14, 321–325. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.W.; Sohn, Y.H. Hyperhomocysteinemia in patients with Parkinson’s disease and relationship to vitamin B level. J. Mov. Disord. 2009, 2, 33–36. [Google Scholar] [CrossRef] [PubMed]
- Yuan, R.Y.; Sheu, J.J.; Yu, J.M.; Hu, C.J.; Tseng, I.J.; Ho, C.S.; Yeh, C.Y.; Hung, Y.L.; Chiang, T.R. Methylenetetrahydrofolate reductase polymorphisms and plasma homocysteine in levodopa-treated and non-treated Parkinson’s disease patients. J. Neurol. Sci. 2009, 287, 64–68. [Google Scholar] [CrossRef] [PubMed]
- Białecka, M.; Kurzawski, M.; Roszmann, A.; Robowski, P.; Sitek, E.J.; Honczarenko, K.; Gorzkowska, A.; Budrewicz, S.; Mak, M.; Jarosz, M.; et al. Association of COMT, MTHFR, and SLC19A1(RFC-1) polymorphisms with homocysteine blood levels and cognitive impairment in Parkinson’s disease. Pharmacogenet Genomics 2012, 22, 716–724. [Google Scholar] [CrossRef] [PubMed]
- Song, I.U.; Kim, J.S.; Park, I.S.; Kim, Y.D.; Cho, H.J.; Chung, S.W.; Lee, K.S. Clinical significance of homocysteine (hcy) on dementia in Parkinson’s disease (PD). Arch. Gerontol. Geriatr. 2013, 57, 288–291. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhang, S.M.; Schwarzschild, M.A.; Hernán, M.A.; Logroscino, G.; Willett, W.C.; Ascherio, A. Folate intake and risk of Parkinson’s disease. Am. J. Epidemiol. 2004, 160, 368–375. [Google Scholar] [CrossRef] [PubMed]
- De Lau, L.M.; Koudstaal, P.J.; Witteman, J.C.; Hofman, A.; Breteler, M.M. Dietary folate, vitamin B12, and vitamin B6 and the risk of Parkinson disease. Neurology 2006, 67, 315–318. [Google Scholar] [CrossRef] [PubMed]
- Murakami, K.; Miyake, Y.; Sasaki, S.; Tanaka, K.; Fukushima, W.; Kiyohara, C.; Tsuboi, Y.; Yamada, T.; Oeda, T.; Miki, T.; et al. Dietary intake of folate, vitamin B6, vitamin B12 and riboflavin and risk of Parkinson’s disease: A case-control study in Japan. Br. J. Nutr. 2010, 104, 757–764. [Google Scholar] [CrossRef] [PubMed]
- Ehrenshaft, M.; Bilski, P.; Li, M.Y.; Chignell, C.F.; Daub, M. A highly conserved sequence is a novel gene involved in de novo vitamin B6 biosynthesis. Proc. Natl. Acad. Sci. USA 1999, 96, 9374–9378. [Google Scholar] [CrossRef] [PubMed]
- Stocker, P.; Lesgards, J.F.; Vidal, N.; Chalier, F.; Prost, M. ESR study of a biological assay on whole blood: Antioxidant efficiency of various vitamins. Biochim. Biophys. Acta 2003, 1621, 1–8. [Google Scholar] [CrossRef]
- Cabrini, L.; Bergami, R.; Fiorentini, D.; Marchetti, M.; Landi, L.; Tolomelli, B. Vitamin B6 deficiency affects antioxidant defences in rat liver and heart. Biochem. Mol. Biol. Int. 1998, 46, 689–697. [Google Scholar] [CrossRef] [PubMed]
- Mahfouz, M.M.; Kummerow, F.A. Vitamin C or vitamin B6 supplementation prevent the oxidative stress and decrease of prostacyclin generation in homocysteinemic rats. Int. J. Biochem. Cell Biol. 2004, 36, 1919–1932. [Google Scholar] [CrossRef] [PubMed]
- Endo, N.; Nishiyama, K.; Otsuka, A.; Kanouchi, H.; Taga, M.; Oka, T. Antioxidant activity of vitamin B6 delays homocysteine-induced atherosclerosis in rats. Br. J. Nutr. 2006, 95, 1088–1093. [Google Scholar] [CrossRef] [PubMed]
- Ullegaddi, R.; Powers, H.J.; Gariballa, S.E. B-group vitamin supplementation mitigates oxidative damage after acute ischemic stroke. Clin. Sci. 2004, 107, 477–484. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, L. Associations between B Vitamins and Parkinson’s Disease. Nutrients 2015, 7, 7197-7208. https://doi.org/10.3390/nu7095333
Shen L. Associations between B Vitamins and Parkinson’s Disease. Nutrients. 2015; 7(9):7197-7208. https://doi.org/10.3390/nu7095333
Chicago/Turabian StyleShen, Liang. 2015. "Associations between B Vitamins and Parkinson’s Disease" Nutrients 7, no. 9: 7197-7208. https://doi.org/10.3390/nu7095333




