Sub-Chronic Consumption of Dark Chocolate Enhances Cognitive Function and Releases Nerve Growth Factors: A Parallel-Group Randomized Trial
Abstract
:1. Introduction
2. Materials and Methods
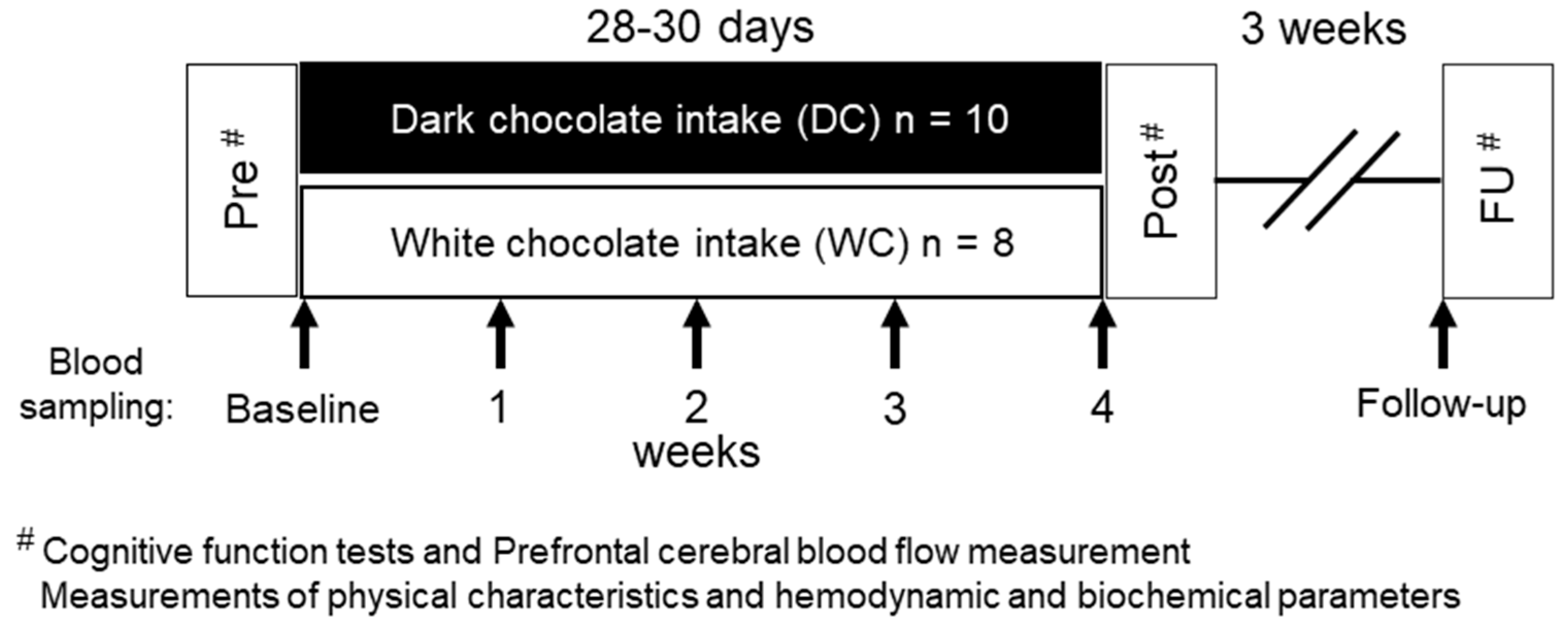
2.1. Subjects
2.2. Experimental Protocol
2.3. Chocolates and Chocolate-Intake Interventions
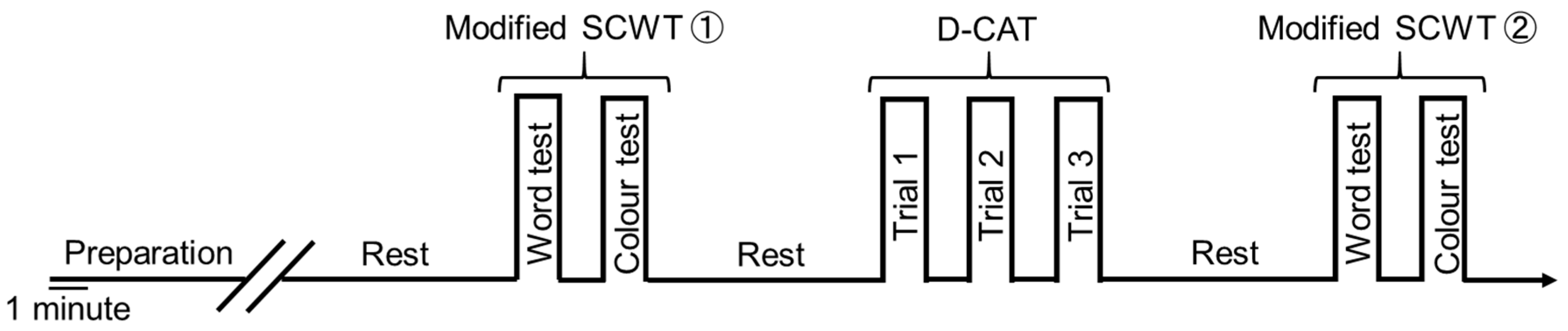
2.4. Experimental Conditions for Cognitive Function Tests and PFCBF Measurement
2.5. Modified Stroop Color Word Test (Modified SCWT)
2.6. Digital Cancellation Test (D-CAT)
2.7. Prefrontal Cerebral Blood Flow (PFCBF)
2.8. Blood Samples
2.9. Biochemical Analyses
2.10. Measurements of Theobromine and Caffeine Content in Plasma
2.11. Enzyme-Linked Immunosorbent Assay (ELISA)
2.12. Outcome Measures
2.13. Statistics
3. Results
3.1. Baseline Scores
3.2. Adherence to Daily Chocolate Consumption and Intake of Caffeinated Beverages
3.3. Physical Characteristics and Appearances, Hemodynamic Parameters, and Biochemical Analysis
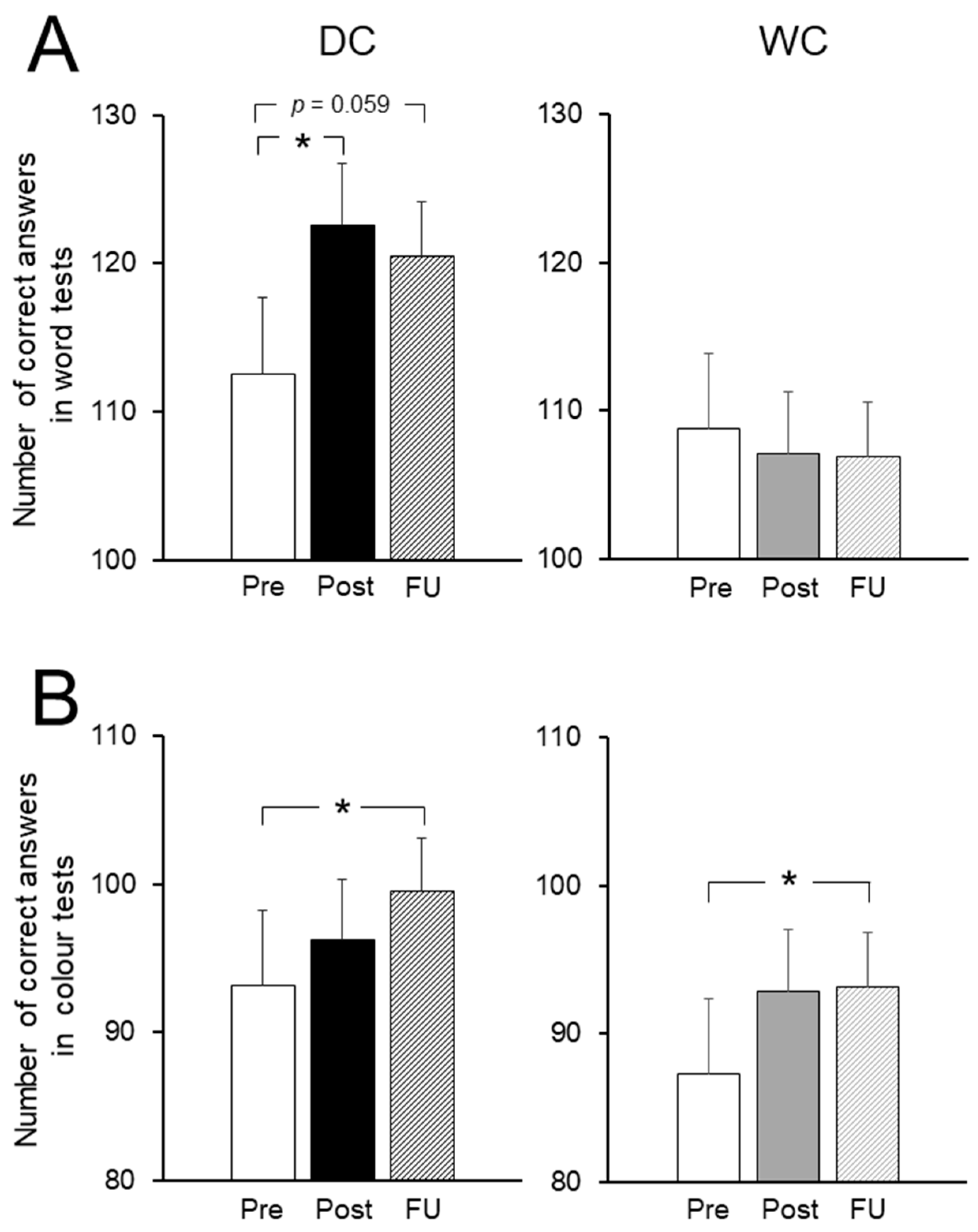
3.4. Cognitive Function Tests
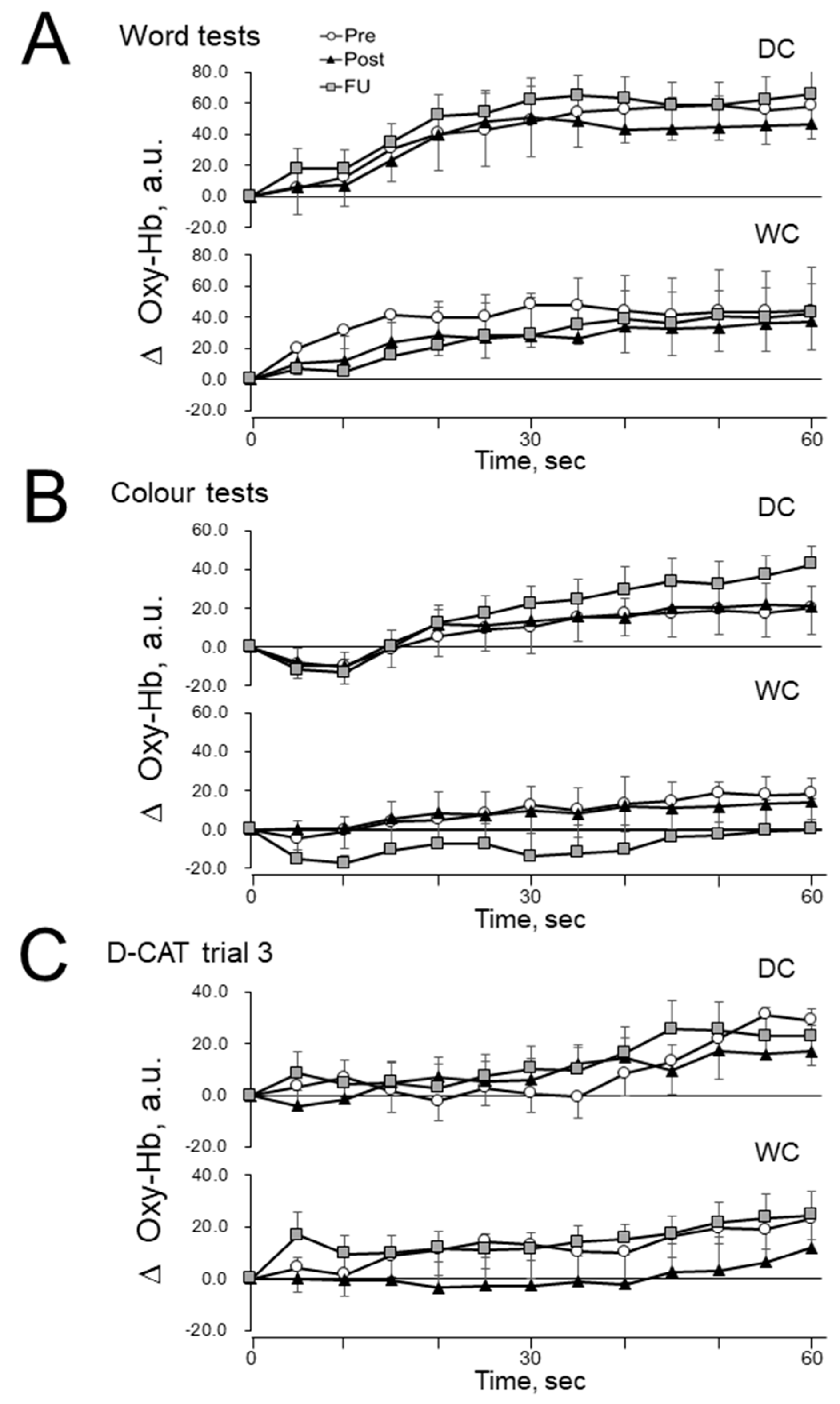
3.5. Prefrontal Cerebral Blood Flow (PFCBF)
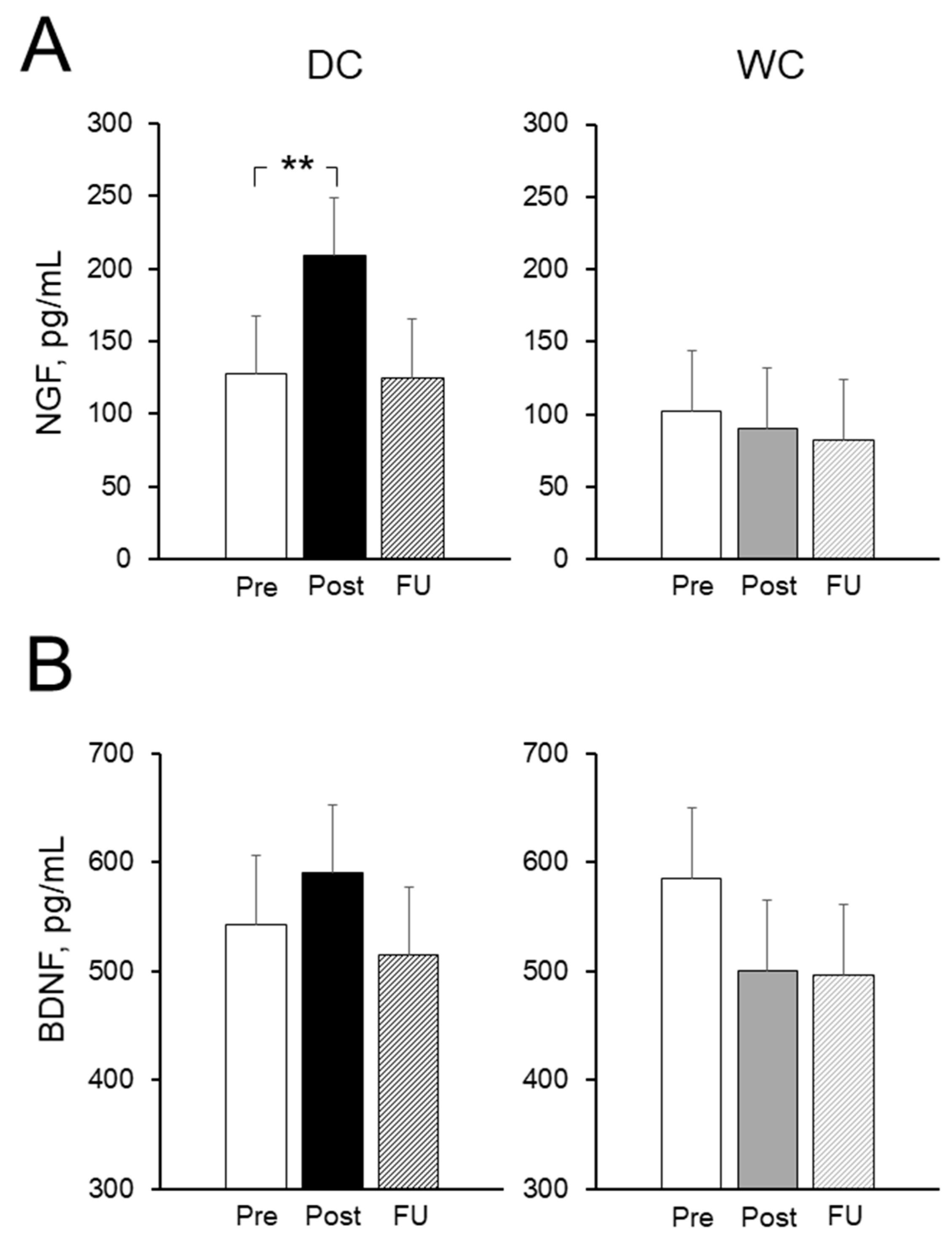
3.6. Plasma Nerve Growth Factor (NGF) and Brain-Derived Neurotrophic Factor (BDNF) Levels
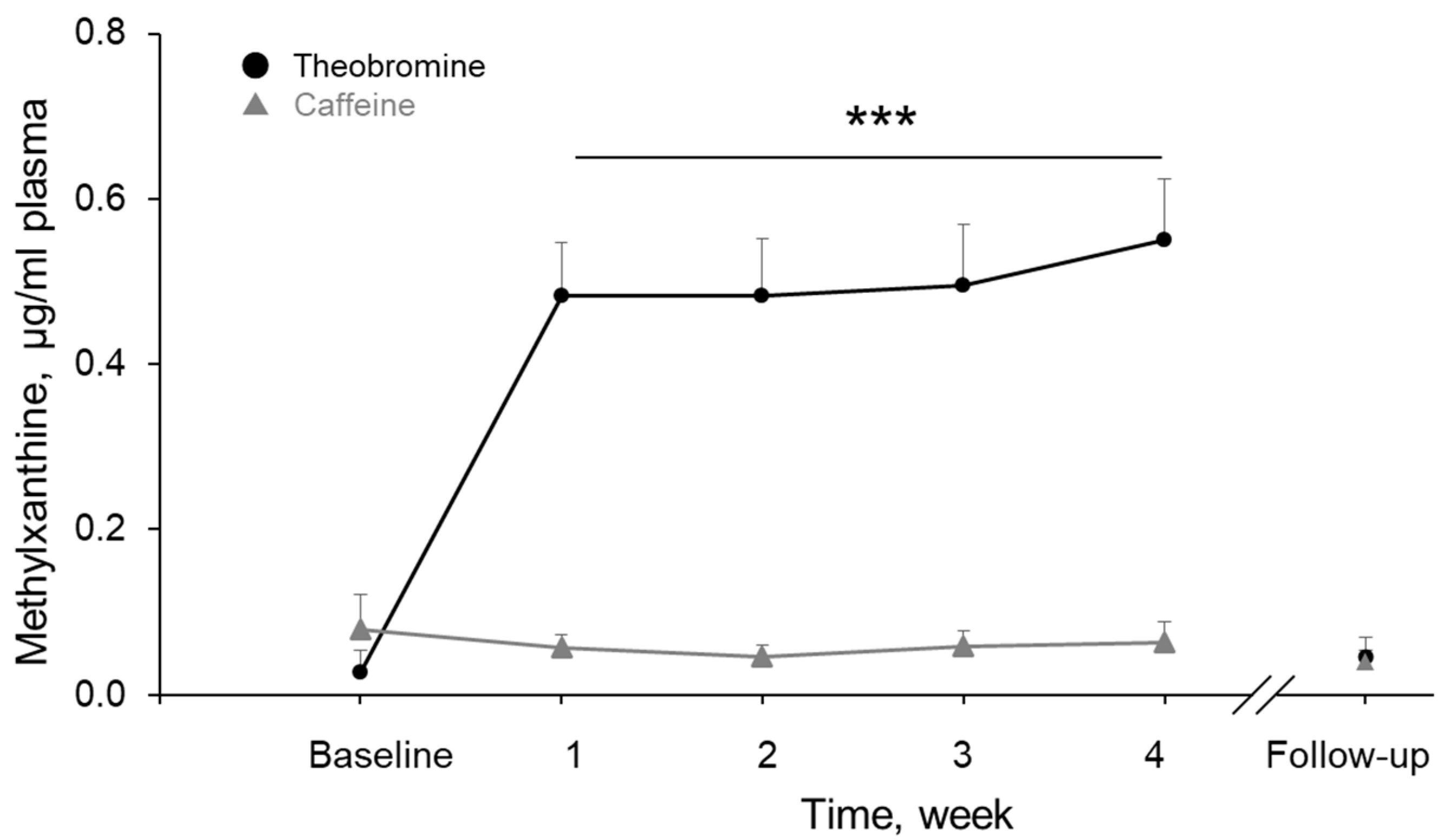
3.7. Theobromine and Caffeine Concentrations in Plasma
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Messerli, F.H. Chocolate consumption, cognitive function, and nobel laureates. N. Engl. J. Med. 2012, 367, 1562–1564. [Google Scholar] [CrossRef] [PubMed]
- Grassi, D.; Desideri, G.; Ferri, C. Protective effects of dark chocolate on endothelial function and diabetes. Curr. Opin. Clin. Nutr. Metab. Care 2013, 16, 662–668. [Google Scholar] [CrossRef] [PubMed]
- Scapagnini, G.; Davinelli, S.; di Renzo, L.; de Lorenzo, A.; Olarte, H.H.; Micali, G.; Cicero, A.F.; Gonzalez, S. Cocoa bioactive compounds: Significance and potential for the maintenance of skin health. Nutrients 2014, 6, 3202–3213. [Google Scholar] [CrossRef] [PubMed]
- Davinelli, S.; Corbi, G.; Zarrelli, A.; Arisi, M.; Calzavara-Pinton, P.; Grassi, D.; de Vivo, I.; Scapagnini, G. Short-term supplementation with flavanol-rich cocoa improves lipid profile, antioxidant status and positively influences the AA/EPA ratio in healthy subjects. J. Nutr. Biochem. 2018, 61, 33–39. [Google Scholar] [CrossRef]
- Brickman, A.M.; Khan, U.A.; Provenzano, F.A.; Yeung, L.; Suzuki, W.; Schroeter, H.; Wall, M.; Sloan, R.P.; Small, S.A. Enhancing dentate gyrus function with dietary flavanols improves cognition in older adults. Nat. Neurosci. 2014, 17, 1798–1803. [Google Scholar] [CrossRef]
- Jalil, A.M.; Ismail, A. Polyphenols in cocoa and cocoa products: Is there a link between antioxidant properties and health? Molecules 2008, 13, 2190–2219. [Google Scholar] [CrossRef]
- Crichton, G.E.; Elias, M.F.; Alkerwi, A. Chocolate intake is associated with better cognitive function: The Maine-Syracuse Longitudinal Study. Appetite 2016, 100, 126–132. [Google Scholar] [CrossRef]
- Taubert, D.; Roesen, R.; Lehmann, C.; Jung, N.; Schomig, E. Effects of low habitual cocoa intake on blood pressure and bioactive nitric oxide—A randomized controlled trial. Jama 2007, 298, 49–60. [Google Scholar] [CrossRef]
- Buijsse, B.; Weikert, C.; Drogan, D.; Bergmann, M.; Boeing, H. Chocolate consumption in relation to blood pressure and risk of cardiovascular disease in German adults. Eur. Heart J. 2010, 31, 1616–1623. [Google Scholar] [CrossRef]
- Nehlig, A. The neuroprotective effects of cocoa flavanol and its influence on cognitive performance. Br. J. Clin. Pharmacol. 2013, 75, 716–727. [Google Scholar] [CrossRef]
- Katz, D.L.; Doughty, K.; Ali, A. Cocoa and chocolate in human health and disease. Antioxid. Redox Signal. 2011, 15, 2779–2811. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Pinilla, E.; Onatibia-Astibia, A.; Franco, R. The relevance of theobromine for the beneficial effects of cocoa consumption. Front. Pharmacol. 2015, 6, 30. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, N.; Miwa, S.; Hitomi, Y.; Nakamura, H.; Tsuchiya, H.; Yachie, A. Theobromine, the primary methylxanthine found in Theobroma cacao, prevents malignant glioblastoma proliferation by negatively regulating phosphodiesterase-4, extracellular signal-regulated kinase, Akt/mammalian target of rapamycin kinase, and nuclear factor-kappa B. Nutr. Cancer Int. J. 2014, 66, 419–423. [Google Scholar] [CrossRef]
- Yoneda, M.; Sugimoto, N.; Katakura, M.; Matsuzaki, K.; Tanigami, H.; Yachie, A.; Ohno-Shosaku, T.; Shido, O. Theobromine up-regulates cerebral brain-derived neurotrophic factor and facilitates motor learning in mice. J. Nutr. Biochem. 2017, 39, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, N.; Katakura, M.; Matsuzaki, K.; Sumiyoshi, E.; Yachie, A.; Shido, O. Chronic administration of theobromine inhibits mTOR signal in rats. Basic Clin. Pharmacol. Toxicol. 2019, 124, 575–581. [Google Scholar] [CrossRef] [PubMed]
- Kandel, E.R. The molecular biology of memory: cAMP, PKA, CRE, CREB-1, CREB-2, and CPEB. Mol. Brain 2012, 5, 14. [Google Scholar] [CrossRef]
- Lee, D. Global and local missions of cAMP signaling in neural plasticity, learning, and memory. Front. Pharmacol. 2015, 6, 161. [Google Scholar] [CrossRef]
- Li, Y.F.; Cheng, Y.F.; Huang, Y.; Conti, M.; Wilson, S.P.; O’Donnell, J.M.; Zhang, H.T. Phosphodiesterase-4D knock-out and RNA interference-mediated knock-down enhance memory and increase hippocampal neurogenesis via increased cAMP signaling. J. Neurosci. 2011, 31, 172–183. [Google Scholar] [CrossRef]
- Islam, R.; Matsuzaki, K.; Sumiyoshi, E.; Hossain, M.E.; Hashimoto, M.; Katakura, M.; Sugimoto, N.; Shido, O. Theobromine improves working memory by activating the CaMKII/CREB/BDNF pathway in rats. Nutrients 2019, 11, 888. [Google Scholar] [CrossRef]
- Afoakwa, E.O. Chapter 2: World cocoa production, processing and chocolate consumption pattern. In Chocolate Science and Technology, 2nd ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2016; pp. 17–48. [Google Scholar]
- Fox, M.; Meyer-Gerspach, A.C.; Wendebourg, M.J.; Gruber, M.; Heinrich, H.; Sauter, M.; Woelnerhanssen, B.; Koeberle, D.; Juengling, F. Effect of cocoa on the brain and gut in healthy subjects: A randomised controlled trial. Br. J. Nutr. 2019, 121, 654–661. [Google Scholar] [CrossRef]
- Pruijm, M.; Hofmann, L.; Charollais-Thoenig, J.; Forni, V.; Maillard, M.; Coristine, A.; Stuber, M.; Burnier, M.; Vogt, B. Effect of dark chocolate on renal tissue oxygenation as measured by BOLD-MRI in healthy volunteers. Clin. Nephrol. 2013, 80, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Grassi, D.; Lippi, C.; Necozione, S.; Desideri, G.; Ferri, C. Short-term administration of dark chocolate is followed by a significant increase in insulin sensitivity and a decrease in blood pressure in healthy persons. Am. J. Clin. Nutr. 2005, 81, 611–614. [Google Scholar] [CrossRef]
- Gottumukkala, R.V.; Nadimpalli, N.; Sukala, K.; Subbaraju, G.V. Determination of Catechin and Epicatechin content in chocolates by high-performance liquid chromatography. Int. Sch. Res. Not. 2014, 2014, 628196. [Google Scholar] [CrossRef] [PubMed]
- Pereira, T.; Bergqvist, J.; Vieira, C.; Sveälv, B.G.; Castanheira, J.; Conde, J. Randomized study of the effects of cocoa-rich chocolate on the ventricle-arterial coupling and vascular function of young, healthy adults. Nutrition 2019, 63–64, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Alanon, M.E.; Castle, S.M.; Siswanto, P.J.; Cifuentes-Gomez, T.; Spencer, J.P.E. Assessment of flavanol stereoisomers and caffeine and theobromine content in commercial chocolates. Food Chem. 2016, 208, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Stroop, J.R. Studies of interference in serial verbal reactions. J. Exp. Psychol. 1935, 18, 643–662. [Google Scholar] [CrossRef]
- Hatta, T.; Yoshizaki, K.; Ito, Y.; Mase, M.; Kabasawa, H. Reliability and validity of the digit cancellation test, a brief screen of attention. Psychologia 2012, 55, 246–256. [Google Scholar] [CrossRef]
- Miyamoto, M.; Matsuzaki, K.; Katakura, M.; Hara, T.; Tanabe, Y.; Shido, O. Oral intake of encapsulated dried ginger root powder hardly affects human thermoregulatory function, but appears to facilitate fat utilization. Int. J. Biometeorol. 2015, 59, 1461–1474. [Google Scholar] [CrossRef]
- Massee, L.A.; Ried, K.; Pase, M.; Travica, N.; Yoganathan, J.; Scholey, A.; Macpherson, H.; Kennedy, G.; Sali, A.; Pipingas, A. The acute and sub-chronic effects of cocoa flavanols on mood, cognitive and cardiovascular health in young healthy adults: A randomized, controlled trial. Front. Pharmacol. 2015, 6, 93. [Google Scholar] [CrossRef]
- Smit, H.J.; Gaffan, E.A.; Rogers, P.J. Methylxanthines are the psycho-pharmacologically active constituents of chocolate. Psychopharmacology 2004, 176, 412–419. [Google Scholar] [CrossRef]
- Faridi, Z.; Njike, V.Y.; Dutta, S.; Ali, A.; Katz, D.L. Acute dark chocolate and cocoa ingestion and endothelial function: A randomized controlled crossover trial. Am. J. Clin. Nutr. 2008, 88, 58–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mumford, G.K.; Benowitz, N.L.; Evans, S.M.; Kaminski, B.J.; Preston, K.L.; Sannerud, C.A.; Silverman, K.; Griffiths, R.R. Absorption rate of methylxanthines following capsules, cola and chocolate. Eur. J. Clin. Pharmacol. 1996, 51, 319–325. [Google Scholar] [CrossRef] [PubMed]
- El Mohsen, M.M.A.; Kuhnle, G.; Rechner, A.R.; Schroeter, H.; Rose, S.; Jenner, P.; Rice-Evans, C.A. Uptake and metabolism of epicatechin and its access to the brain after oral ingestion. Free Radic. Biol. Med. 2002, 33, 1693–1702. [Google Scholar] [CrossRef]
- Steiner, J.P.; Nath, A. Neurotrophin strategies for neuroprotection: Are they sufficient? J. Neuroimmune Pharmacol. 2014, 9, 182–194. [Google Scholar] [CrossRef]
- Keefe, K.M.; Sheikh, I.S.; Smith, G.M. Targeting neurotrophins to specific populations of neurons: NGF, BDNF, and NT-3 and their relevance for treatment of spinal cord injury. Int. J. Mol. Sci. 2017, 18, 548. [Google Scholar] [CrossRef]
- Kowianski, P.; Lietzau, G.; Czuba, E.; Waskow, M.; Steliga, A.; Morys, J. BDNF: A key factor with multipotent impact on brain signaling and synaptic plasticity. Cell. Mol. Neurobiol. 2018, 38, 579–593. [Google Scholar] [CrossRef]
- Lim, S.; Moon, M.; Oh, H.; Kim, H.G.; Kim, S.Y.; Oh, M.S. Ginger improves cognitive function via NGF-induced ERK/CREB activation in the hippocampus of the mouse. J. Nutr. Biochem. 2014, 25, 1058–1065. [Google Scholar] [CrossRef]
- Lu, B.; Nagappan, G.; Lu, Y. BDNF and synaptic plasticity, cognitive function, and dysfunction. In Neurotrophic Factors; Springer: Berlin/Heidelberg, Germany, 2014; pp. 223–250. [Google Scholar]
- Francis, S.T.; Head, K.; Morris, P.G.; Macdonald, I.A. The effect of flavanol-rich cocoa on the fMRI response to a cognitive task in healthy young people. J. Cardiovasc. Pharmacol. 2006, 47, S215–S220. [Google Scholar] [CrossRef]
- Sorond, F.A.; Lipsitz, L.A.; Hollenberg, N.K.; Fisher, N.D. Cerebral blood flow response to flavanol-rich cocoa in healthy elderly humans. Neuropsychiatr. Dis. Treat. 2008, 4, 433–440. [Google Scholar]
- Monahan, K.D.; Feehan, R.P.; Kunselman, A.R.; Preston, A.G.; Miller, D.L.; Lott, M.E.J. Dose-dependent increases in flow-mediated dilation following acute cocoa ingestion in healthy older adults. J. Appl. Physiol. 2011, 111, 1568–1574. [Google Scholar] [CrossRef] [Green Version]
- Njike, V.Y.; Faridi, Z.; Shuval, K.; Dutta, S.; Kay, C.D.; West, S.G.; Kris-Etherton, P.M.; Katz, D.L. Effects of sugar-sweetened and sugar-free cocoa on endothelial function in overweight adults. Int. J. Cardiol. 2011, 149, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Pomportes, L.; Brisswalter, J.; Casini, L.; Hays, A.; Davranche, K. Cognitive performance enhancement induced by caffeine, carbohydrate and guarana mouth rinsing during submaximal exercise. Nutrients 2017, 9, 589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loprinzi, P.D.; Lovorn, A. Exercise and cognitive function. J. Clin. Med. 2019, 8, 1707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| DC (24.0 g/Day) | WC (24.5 g/Day) | |
|---|---|---|
| Energy, kcal | 135 | 145 |
| Protein, g | 2.0 | 2.0 |
| Fat, g | 10.0 | 9.5 |
| Carbohydrates, g | 9.0 | 12.5 |
| Cacao polyphenol, mg | 540.0 | ND |
| Epicatechin, mg | 34.8 | ND |
| Caffeine, mg | 26.8 | ND |
| Theobromine, mg | 197.5 | ND |
| DC (n = 10) | |||||||||
| Trial 1 | Trial 2 | Trial 3 | |||||||
| Pre | Post | FU | Pre | Post | FU | Pre | Post | FU | |
| TP, count | 380 ± 18 | 359 ± 18 | 372 ± 18 | 288 ± 16 | 275 ± 14 | 286 ± 13 | 218 ± 15 | 233 ± 13 * | 230 ± 12 * |
| Omission ratio, % | 5.1 ± 1.4 | 1.6 ± 1.0 * | 3.1 ± 1.5 | 8.1 ± 1.9 | 4.0 ± 1.6 * | 6.2 ± 2.1 | 7.3 ± 1.9 | 5.8 ± 1.7 | 4.9 ± 1.5 |
| WC (n = 8) | |||||||||
| Trial 1 | Trial 2 | Trial 3 | |||||||
| Pre | Post | FU | Pre | Post | FU | Pre | Post | FU | |
| TP, count | 329 ± 10 | 349 ± 10 | 335 ± 21 | 264 ± 12 | 272 ± 14 | 264 ± 20 | 211 ± 14 | 221 ± 15 | 217 ± 20 |
| Omission ratio, % | 1.5 ± 1.2 | 3.1 ± 1.1 | 1.8 ± 1.1 | 4.0 ± 1.1 | 1.9 ± 0.5 | 2.2 ± 0.8 | 5.5 ± 2.1 | 5.1 ± 1.3 | 4.4 ± 1.7 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sumiyoshi, E.; Matsuzaki, K.; Sugimoto, N.; Tanabe, Y.; Hara, T.; Katakura, M.; Miyamoto, M.; Mishima, S.; Shido, O. Sub-Chronic Consumption of Dark Chocolate Enhances Cognitive Function and Releases Nerve Growth Factors: A Parallel-Group Randomized Trial. Nutrients 2019, 11, 2800. https://doi.org/10.3390/nu11112800
Sumiyoshi E, Matsuzaki K, Sugimoto N, Tanabe Y, Hara T, Katakura M, Miyamoto M, Mishima S, Shido O. Sub-Chronic Consumption of Dark Chocolate Enhances Cognitive Function and Releases Nerve Growth Factors: A Parallel-Group Randomized Trial. Nutrients. 2019; 11(11):2800. https://doi.org/10.3390/nu11112800
Chicago/Turabian StyleSumiyoshi, Eri, Kentaro Matsuzaki, Naotoshi Sugimoto, Yoko Tanabe, Toshiko Hara, Masanori Katakura, Mayumi Miyamoto, Seiji Mishima, and Osamu Shido. 2019. "Sub-Chronic Consumption of Dark Chocolate Enhances Cognitive Function and Releases Nerve Growth Factors: A Parallel-Group Randomized Trial" Nutrients 11, no. 11: 2800. https://doi.org/10.3390/nu11112800





