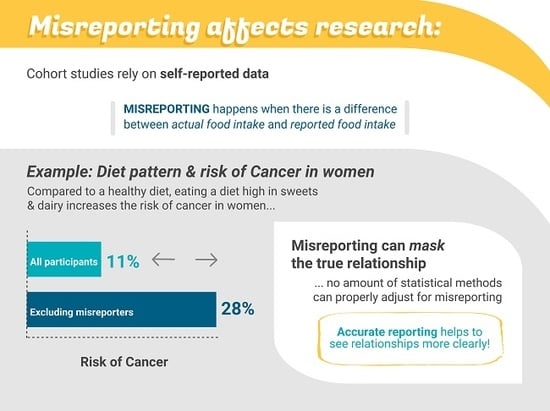
Strategies to Address Misestimation of Energy Intake Based on Self-Report Dietary Consumption in Examining Associations Between Dietary Patterns and Cancer Risk
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Source
2.2. Dietary Intake Assessment
2.3. Physical Activity Assessment
2.4. Energy Intake Estimation
2.5. Cancer Incidence and Sub-Groups
2.6. Statistical Analysis
3. Results
3.1. Participant Baseline Sociodemographic Characteristics
3.2. Dietary Patterns in Relation to Methods for Accounting for Misestimation of Energy Intake
3.3. Association between Dietary Patterns and Cancer Risk
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Ethics of Human Participation
References
- Murray, C.J.L.; Lopez, A.D. Measuring the global burden of disease. N. Engl. J. Med. 2013, 369, 448–457. [Google Scholar] [CrossRef] [PubMed]
- World Cancer Research Fund/American Institute for Cancer Research. Diet, Nutrition, Physical Activity and Cancer: A Global Perspective. Continuous Update Project Expert Report; World Cancer Research Fund: London, UK; American Institute for Cancer Research: Washington, DC, USA, 2018. [Google Scholar]
- Reedy, J.; Subar, A.F.; George, S.M.; Krebs-Smith, S.M. Extending Methods in Dietary Patterns Research. Nutrients 2018, 10, 571. [Google Scholar] [CrossRef] [PubMed]
- Schulze, M.B.; Hoffmann, K. Methodological approaches to study dietary patterns in relation to risk of coronary heart disease and stroke. Br. J. Nutr. 2006, 95, 860–869. [Google Scholar] [CrossRef] [PubMed]
- Grosso, G.; Bella, F.; Godos, J.; Sciacca, S.; Del Rio, D.; Ray, S.; Galvano, F.; Giovannucci, E.L. Possible role of diet in cancer: Systematic review and multiple meta-analyses of dietary patterns, lifestyle factors, and cancer risk. Nutr. Rev. 2017, 75, 405–419. [Google Scholar] [CrossRef]
- Illner, A.-K.; Freisling, H.; Boeing, H.; Huybrechts, I.; Crispim, S.P.; Slimani, N. Review and evaluation of innovative technologies for measuring diet in nutritional epidemiology. Int. J. Epidemiol. 2012, 41, 1187–1203. [Google Scholar] [CrossRef] [Green Version]
- Subar, A.F.; Freedman, L.S.; Tooze, J.A.; Kirkpatrick, S.I.; Boushey, C.; Neuhouser, M.L.; Thompson, F.E.; Potischman, N.; Guenther, P.M.; Tarasuk, V.; et al. Addressing Current Criticism Regarding the Value of Self-Report Dietary Data. J. Nutr. 2015, 145, 2639–2645. [Google Scholar] [CrossRef] [Green Version]
- Freedman, L.S.; Schatzkin, A.; Midthune, D.; Kipnis, V. Dealing with dietary measurement error in nutritional cohort studies. J. Natl. Cancer Inst. 2011, 103, 1086–1092. [Google Scholar] [CrossRef]
- Mayne, S.T.; Playdon, M.C.; Rock, C.L. Diet, nutrition, and cancer: Past, present and future. Nat. Rev. Clin. Oncol. 2016, 13, 504–515. [Google Scholar] [CrossRef]
- Watanabe, D.; Nanri, H.; Sagayama, H.; Yoshida, T.; Itoi, A.; Yamaguchi, M.; Yokoyama, K.; Watanabe, Y.; Goto, C.; Ebine, N.; et al. Estimation of Energy Intake by a Food Frequency Questionnaire: Calibration and Validation with the Doubly Labeled Water Method in Japanese Older People. Nutrients 2019, 11, 1546. [Google Scholar] [CrossRef]
- Banna, J.C.; McCrory, M.A.; Fialkowski, M.K.; Boushey, C. Examining Plausibility of Self-Reported Energy Intake Data: Considerations for Method Selection. Front. Nutr. 2017, 4, 45. [Google Scholar] [CrossRef] [Green Version]
- Park, J.; Kazuko, I.-T.; Kim, E.; Kim, J.; Yoon, J. Estimating free-living human energy expenditure: Practical aspects of the doubly labeled water method and its applications. Nutr. Res. Pract. 2014, 8, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Freedman, L.S.; Commins, J.M.; Moler, J.E.; Arab, L.; Baer, D.J.; Kipnis, V.; Midthune, D.; Moshfegh, A.J.; Neuhouser, M.L.; Prentice, R.L.; et al. Pooled results from 5 validation studies of dietary self-report instruments using recovery biomarkers for energy and protein intake. Am. J. Epidemiol. 2014, 180, 172–188. [Google Scholar] [CrossRef] [PubMed]
- Freedman, L.S.; Commins, J.M.; Moler, J.E.; Willett, W.; Tinker, L.F.; Subar, A.F.; Spiegelman, D.; Rhodes, D.; Potischman, N.; Neuhouser, M.L.; et al. Pooled results from 5 validation studies of dietary self-report instruments using recovery biomarkers for potassium and sodium intake. Am. J. Epidemiol. 2015, 181, 473–487. [Google Scholar] [CrossRef] [PubMed]
- Black, A. Critical evaluation of energy intake using the Goldberg cut-off for energy intake:basal metabolic rate. A practical guide to its calculation, use and limitations. Int. J. Obes. 2000, 24, 1119–1130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCrory, M.A.; Hajduk, C.L.; Roberts, S.B. Procedures for screening out inaccurate reports of dietary energy intake. Public Health Nutr. 2002, 5, 873–882. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.T.-K.; Roberts, S.B.; Howarth, N.C.; Mccrory, M.A. Diet and Physical Activity Effect of Screening Out Implausible Energy Intake Reports on Relationships between Diet and BMI. Obes. Res. 2005, 13, 1205–1217. [Google Scholar] [CrossRef] [PubMed]
- Tooze, J.; Krebs-Smith, S.; Troiano, R.; Subar, A. The accuracy of the Goldberg method for classifying misreporters of energy intake on a food frequency questionnaire and 24-h recalls: Comparison with doubly labeled water. Eur. J. Clin. Nutr. 2012, 66, 569–576. [Google Scholar] [CrossRef]
- Tooze, J.A.; Freedman, L.S.; Carroll, R.J.; Midthune, D.; Kipnis, V. The impact of stratification by implausible energy reporting status on estimates of diet-health relationships. Biom. J. 2016, 58, 1538–1551. [Google Scholar] [CrossRef] [Green Version]
- McNaughton, S.A.; Mishra, G.D.; Brunner, E.J. Food patterns associated with blood lipids are predictive of coronary heart disease: The Whitehall II study. Br. J. Nutr. 2009, 102, 619–624. [Google Scholar] [CrossRef]
- McNaughton, S.A.; Mishra, G.D.; Brunner, E.J. Dietary patterns, insulin resistance, and incidence of type 2 diabetes in the Whitehall II Study. Diabetes Care 2008, 31, 1343–1348. [Google Scholar] [CrossRef]
- Brunner, E.J.; Mosdøl, A.; Witte, D.R.; Martikainen, P.; Stafford, M.; Shipley, M.J.; Marmot, M.G. Dietary patterns and 15-y risks of major coronary events, diabetes, and mortality. Am. J. Clin. Nutr. 2008, 87, 1414–1421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bryant, H.; Robson, P.J.; Ullman, R.; Friedenreich, C.; Dawe, U. Population-based cohort development in Alberta, Canada: A feasibility study. Chronic Dis. Can. 2006, 27, 51–59. [Google Scholar] [PubMed]
- Robson, P.J.; Solbak, N.M.; Haig, T.R.; Whelan, H.K.; Vena, J.E.; Akawung, A.K.; Rosner, W.K.; Brenner, D.R.; Cook, L.S.; Csizmadi, I.; et al. Design, methods and demographics from phase I of Alberta’s Tomorrow Project cohort: A prospective cohort profile. CMAJ Open 2016, 4, E515–E527. [Google Scholar] [CrossRef] [PubMed]
- Ye, M.; Robson, P.J.; Eurich, D.T.; Vena, J.E.; Xu, J.-Y.; Johnson, J.A. Cohort Profile: Alberta’s Tomorrow Project. Int. J. Epidemiol. 2017, 46, 1097–1098l. [Google Scholar] [CrossRef] [PubMed]
- Dobner, J.; Kaser, S. Body mass index and the risk of infection—From underweight to obesity. Clin. Microbiol. Infect. 2018, 24, 24–28. [Google Scholar] [CrossRef]
- National Institutes of Health Diet History Questionnaire; NIH: Bethesda, MD, USA, 2007.
- Csizmadi, I.; Kahle, L.; Ullman, R.; Dawe, U.; Zimmerman, T.P.; Friedenreich, C.M.; Bryant, H.; Subar, A.F. Adaptation and evaluation of the National Cancer Institute’s Diet History Questionnaire and nutrient database for Canadian populations. Public Health Nutr. 2007, 10, 88–96. [Google Scholar] [CrossRef]
- Csizmadi, I.; Boucher, B.A.; Lo Siou, G.; Massarelli, I.; Rondeau, I.; Garriguet, D.; Koushik, A.; Elenko, J.; Subar, A.F. Using national dietary intake data to evaluate and adapt the US Diet History Questionnaire: The stepwise tailoring of an FFQ for Canadian use. Public Health Nutr. 2016, 19, 3247–3255. [Google Scholar] [CrossRef]
- Lo Siou, G.; Yasui, Y.; Csizmadi, I.; McGregor, S.E.; Robson, P.J. Exploring statistical approaches to diminish subjectivity of cluster analysis to derive dietary patterns: The Tomorrow Project. Am. J. Epidemiol. 2011, 173, 956–967. [Google Scholar] [CrossRef]
- Friedenreich, C.M.; Courneya, K.S.; Neilson, H.K.; Matthews, C.E.; Willis, G.; Irwin, M.; Troiano, R.; Ballard-Barbash, R. Reliability and validity of the Past Year Total Physical Activity Questionnaire. Am. J. Epidemiol. 2006, 163, 959–970. [Google Scholar] [CrossRef]
- Goldberg, G.R.; Black, A.E.; Jebb, S.A.; Cole, T.J.; Murgatroyd, P.R.; Coward, W.A.; Prentice, A.M. Critical evaluation of energy intake data using fundamental principles of energy physiology: 1. Derivation of cut-off limits to identify under-recording. Eur. J. Clin. Nutr. 1991, 45, 569–581. [Google Scholar]
- Mifflin, M.D.; St Jeor, S.T.; Hill, L.A.; Scott, B.J.; Daugherty, S.A.; Koh, Y.O. A new predictive equation for resting energy expenditure in healthy individuals. Am. J. Clin. Nutr. 1990, 51, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Csizmadi, I.; Lo Siou, G.; Friedenreich, C.M.; Owen, N.; Robson, P.J. Hours spent and energy expended in physical activity domains: Results from the Tomorrow Project cohort in Alberta, Canada. Int. J. Behav. Nutr. Phys. Act. 2011, 8, 110. [Google Scholar] [CrossRef] [PubMed]
- World Cancer Research Fund/American Institute for Cancer Research. Continuous Update Project: Diet, Nutrition, Physical Activity and the Prevention of Cancer. Summary of Strong Evidence; World Cancer Research Fund: London, UK; American Institute for Cancer Research: Washington, DC, USA, 2018. [Google Scholar]
- Hamilton, S.R.; Aaltonen, L. World Health Organization Classification of Tumours. Pathology and Genetics of Tumours of the Digestive System; IARC Press: Lyon, France, 2000. [Google Scholar]
- Forgy, E. Cluster analysis of Multivariate Data: Efficiency Versus Interpretability of Classifications. Biometrics 1965, 21, 768–769. [Google Scholar]
- Hu, L.-Y.; Huang, M.-W.; Ke, S.-W.; Tsai, C.-F. The distance function effect on k-nearest neighbor classification for medical datasets. Springerplus 2016, 5, 1304. [Google Scholar] [CrossRef] [PubMed]
- Sauvageot, N.; Schritz, A.; Leite, S.; Alkerwi, A.; Stranges, S.; Zannad, F.; Streel, S.; Hoge, A.; Donneau, A.-F.; Albert, A.; et al. Stability-based validation of dietary patterns obtained by cluster analysis. Nutr. J. 2017, 16, 4. [Google Scholar] [CrossRef] [PubMed]
- Cooper, M.C.; Milligan, G.W. A study of standardization of variables in cluster analysis. J. Classif. 1988, 5, 181–204. [Google Scholar]
- Prentice, R.L.; Kalbfleisch, J.D.; Peterson, A.V.; Flournoy, N.; Farewell, V.T.; Breslow, N.E. The analysis of failure times in the presence of competing risks. Biometrics 1978, 34, 541–554. [Google Scholar] [CrossRef]
- Greenland, S.; Senn, S.J.; Rothman, K.J.; Carlin, J.B.; Poole, C.; Goodman, S.N.; Altman, D.G. Statistical tests, P values, confidence intervals, and power: A guide to misinterpretations. Eur. J. Epidemiol. 2016, 31, 337–350. [Google Scholar] [CrossRef]
- Health Canada. Canadian guidelines for body weight classification in adults. Available online: http://www.hc-sc.gc.ca/fn-an/nutrition/weights-poids/guide-ld-adult/index-eng.php (accessed on 28 November 2016).
- Markussen, M.S.; Veierød, M.B.; Ursin, G.; Andersen, L.F. The effect of under-reporting of energy intake on dietary patterns and on the associations between dietary patterns and self-reported chronic disease in women aged 50-69 years. Br. J. Nutr. 2016, 116, 547–558. [Google Scholar] [CrossRef]
- Mattisson, I.; Wirfält, E.; Aronsson, C.A.; Wallström, P.; Sonestedt, E.; Gullberg, B.; Berglund, G. Misreporting of energy: Prevalence, characteristics of misreporters and influence on observed risk estimates in the Malmö Diet and Cancer cohort. Br. J. Nutr. 2005, 94, 832–842. [Google Scholar] [CrossRef]
- Prentice, R.L.; Shaw, P.A.; Bingham, S.A.; Beresford, S.A.A.; Caan, B.; Neuhouser, M.L.; Patterson, R.E.; Stefanick, M.L.; Satterfield, S.; Thomson, C.A.; et al. Biomarker-calibrated energy and protein consumption and increased cancer risk among postmenopausal women. Am. J. Epidemiol. 2009, 169, 977–989. [Google Scholar] [CrossRef] [PubMed]
- Kye, S.; Kwon, S.O.; Lee, S.Y.; Lee, J.; Kim, B.H.; Suh, H.J.; Moon, H.K. Under-reporting of energy intake from 24-hour dietary recalls in the korean national health and nutrition examination survey. Osong Public Heal. Res. Perspect. 2014, 5, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Heerstrass, D.W.; Ocké, M.C.; Bueno-de-Mesquita, H.B.; Peeters, P.H.; Seidell, J.C. Underreporting of energy, protein and potassium intake in relation to body mass index. Int. J. Epidemiol. 1998, 27, 186–193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Freedman, L.S.; Midthune, D.; Carroll, R.J.; Tasevska, N.; Schatzkin, A.; Mares, J.; Tinker, L.; Potischman, N.; Kipnis, V. Using regression calibration equations that combine self-reported intake and biomarker measures to obtain unbiased estimates and more powerful tests of dietary associations. Am. J. Epidemiol. 2011, 174, 1238–1245. [Google Scholar] [CrossRef]
- Shim, J.-S.; Oh, K.; Kim, H.C. Dietary assessment methods in epidemiologic studies. Epidemiol. Health 2014, 36, e2014009. [Google Scholar] [CrossRef]
- Carroll, R.J.; Midthune, D.; Subar, A.F.; Shumakovich, M.; Freedman, L.S.; Thompson, F.E.; Kipnis, V. Taking advantage of the strengths of 2 different dietary assessment instruments to improve intake estimates for nutritional epidemiology. Am. J. Epidemiol. 2012, 175, 340–347. [Google Scholar] [CrossRef]
- Hebert, J.R.; Hurley, T.G.; Peterson, K.E.; Resnicow, K.; Thompson, F.E.; Yaroch, A.L.; Ehlers, M.; Midthune, D.; Williams, G.C.; Greene, G.W.; et al. Social Desirability Trait Influences on Self-Reported Dietary Measures among Diverse Participants in a Multicenter Multiple Risk Factor Trial. J. Nutr. 2008, 138, 226S–234S. [Google Scholar] [CrossRef] [Green Version]
- Cook, A.; Pryer, J.; Shetty, P. The problem of accuracy in dietary surveys. Analysis of the over 65 UK National Diet and Nutrition Survey. J. Epidemiol. Community Health 2000, 54, 611–616. [Google Scholar] [CrossRef]
- Hearty, A.P.; Gibney, M.J. Comparison of cluster and principal component analysis techniques to derive dietary patterns in Irish adults. Br. J. Nutr. 2009, 101, 598–608. [Google Scholar] [CrossRef]
- Bamia, C.; Orfanos, P.; Ferrari, P.; Overvad, K.; Hundborg, H.H.; Tjønneland, A.; Olsen, A.; Kesse, E.; Boutron-Ruault, M.-C.; Clavel-Chapelon, F.; et al. Dietary patterns among older Europeans: The EPIC-Elderly study. Br. J. Nutr. 2005, 94, 100–113. [Google Scholar] [CrossRef]
- Wirfält, E.; Mattisson, I.; Gullberg, B.; Berglund, G. Food patterns defined by cluster analysis and their utility as dietary exposure variables: A report from the Malmö Diet and Cancer Study. Public Health Nutr. 2000, 3, 159–173. [Google Scholar] [CrossRef] [PubMed]
- Reedy, J.; Wirfält, E.; Flood, A.; Mitrou, P.N.; Krebs-Smith, S.M.; Kipnis, V.; Midthune, D.; Leitzmann, M.; Hollenbeck, A.; Schatzkin, A.; et al. Comparing 3 dietary pattern methods—Cluster analysis, factor analysis, and index analysis—With colorectal cancer risk: The NIH-AARP Diet and Health Study. Am. J. Epidemiol. 2010, 171, 479–487. [Google Scholar] [CrossRef] [PubMed]
- Newby, P.K.; Muller, D.; Tucker, K.L. Associations of empirically derived eating patterns with plasma lipid biomarkers: A comparison of factor and cluster analysis methods. Am. J. Clin. Nutr. 2004, 80, 759–767. [Google Scholar] [CrossRef] [PubMed]
- Costacou, T.; Bamia, C.; Ferrari, P.; Riboli, E.; Trichopoulos, D.; Trichopoulou, A. Tracing the Mediterranean diet through principal components and cluster analyses in the Greek population. Eur. J. Clin. Nutr. 2003, 57, 1378–1385. [Google Scholar] [CrossRef] [PubMed]
- Newby, P.K.; Muller, D.; Hallfrisch, J.; Qiao, N.; Andres, R.; Tucker, K.L. Dietary patterns and changes in body mass index and waist circumference in adults. Am. J. Clin. Nutr. 2003, 77, 1417–1425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schulze, M.B.; Hoffmann, K.; Kroke, A.; Boeing, H. Dietary patterns and their association with food and nutrient intake in the European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam study. Br. J. Nutr. 2001, 85, 363–373. [Google Scholar] [CrossRef]
- Wirfält, E.; Midthune, D.; Reedy, J.; Mitrou, P.; Flood, A.; Subar, A.F.; Leitzmann, M.; Mouw, T.; Hollenbeck, A.R.; Schatzkin, A.; et al. Associations between food patterns defined by cluster analysis and colorectal cancer incidence in the NIH-AARP diet and health study. Eur. J. Clin. Nutr. 2009, 63, 707–717. [Google Scholar] [CrossRef]
- Berg, C.M.; Lappas, G.; Strandhagen, E.; Wolk, A.; Torén, K.; Rosengren, A.; Aires, N.; Thelle, D.S.; Lissner, L. Food patterns and cardiovascular disease risk factors: The Swedish INTERGENE research program. Am. J. Clin. Nutr. 2008, 88, 289–297. [Google Scholar] [CrossRef] [Green Version]
- Martikainen, P.; Brunner, E.; Marmot, M. Socioeconomic differences in dietary patterns among middle-aged men and women. Soc. Sci. Med. 2003, 56, 1397–1410. [Google Scholar] [CrossRef]
- Milligan, G.W. An examination of the effect of six types of error perturbation on fifteen clustering algorithms. Psychometrika 1980, 45, 325–342. [Google Scholar] [CrossRef]
- Gan, G.; Wu, J. Subspace clustering for high dimensional categorical data. ACM SIGKDD Explor. Newsl. 2004, 6, 87. [Google Scholar] [CrossRef]
- Smith, A.D.A.C.; Emmett, P.M.; Newby, P.K.; Northstone, K. Dietary patterns obtained through principal components analysis: The effect of input variable quantification. Br. J. Nutr. 2013, 109, 1881–1891. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, A.; Koju, R.P.; Beresford, S.A.A.; Gary Chan, K.C.; Karmacharya, B.M.; Fitzpatrick, A.L. Food patterns measured by principal component analysis and obesity in the Nepalese adult. Heart Asia 2016, 8, 46–53. [Google Scholar] [CrossRef]
- Mullie, P.; Clarys, P. Relation between dietary pattern analysis (principal component analysis) and body mass index: A 5-year follow-up study in a Belgian military population. J. R. Army Med. Corps 2016, 162, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Thorpe, M.G.; Milte, C.M.; Crawford, D.; McNaughton, S.A. A comparison of the dietary patterns derived by principal component analysis and cluster analysis in older Australians. Int. J. Behav. Nutr. Phys. Act. 2016, 13, 30. [Google Scholar] [CrossRef] [PubMed]
- Freitas-Vilela, A.A.; Smith, A.D.A.C.; Kac, G.; Pearson, R.M.; Heron, J.; Emond, A.; Hibbeln, J.R.; Castro, M.B.T.; Emmett, P.M. Dietary patterns by cluster analysis in pregnant women: Relationship with nutrient intakes and dietary patterns in 7-year-old offspring. Matern. Child Nutr. 2017, 13. [Google Scholar] [CrossRef]
- Wilkins, R.; Berthelot, J.M.; Ng, E. Trends in Mortality by Neighbourhood Income in Urban Canada from 1971 to 1996; Health Reports; Toronto Public Library: Toronto, ON, Canada, 2002; pp. 45–72. [Google Scholar]
- Parkin, D.; Muir, C.; Whelan, S. Cancer Incidence in Five Continents; WHO: Geneva, Switzerland, 2012; pp. 128–153. [Google Scholar]
- Livingstone, M.B.E.; Black, A.E. Markers of the validity of reported energy intake. J. Nutr. 2003, 133, 895S–920S. [Google Scholar] [CrossRef]
- Pérez-Rodrigo, C.; Gil, Á.; González-Gross, M.; Ortega, R.M.; Serra-Majem, L.; Varela-Moreiras, G.; Aranceta-Bartrina, J. Clustering of dietary patterns, lifestyles, and overweight among Spanish children and adolescents in the ANIBES study. Nutrients 2015, 8, 11. [Google Scholar] [CrossRef]
- Whelan, H.K.; Xu, J.-Y.; Vaseghi, S.; Lo Siou, G.; McGregor, S.E.; Robson, P.J. Alberta’s Tomorrow Project: Adherence to cancer prevention recommendations pertaining to diet, physical activity and body size. Public Health Nutr. 2017, 20, 1143–1153. [Google Scholar] [CrossRef]

| Cancer location | ICD Code | Morphology Code c |
|---|---|---|
| Dietary-Cancers a | ||
| Mouth | C1-C6, C9 | |
| Pharynx | C10, C11, C13 | |
| Larynx | C32 | |
| Esophagus-squamous cell carcinoma | C15 | Include only 8051, 8070, 8074, 8083 |
| Lung | C34 | |
| Stomach | C16 | |
| Liver | C22 | |
| Colon | C18, C26.0 | |
| Rectosigmoid and rectum | C19, C20 | |
| Breast | C50 | |
| Endometrium | C54.1 | |
| Kidney | C64 | |
| Digestive-Cancers b | ||
| Esophagus | C15 | |
| Stomach | C16 | |
| Small Intestine | C17 | |
| Colon | C18, C26.0 | |
| Rectosigmoid and rectum | C19, C20 | |
| Anus, anal canal and anorectum | C21 | |
| Liver and intrahepatic bile ducts | C22 | |
| Gall bladder and extrahepatic bile ducts | C23-24 | |
| Exocrine pancreas | C25 | Include only 8500, 8480, 8490, 8560, 8020, 8035,8154, 8441, 8470,8453, 8550, 8551, 8154, 8971, 8452 |
| Reporting Status | Dietary Pattern | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Healthy | Sweets & Dairy | Meats & Pizza | |||||||
| Total | Plausible Reporters | Misreporters | Total | Plausible Reporters | Misreporters | Total | Plausible Reporters | Misreporters | |
| Men | |||||||||
| n = 2690 | n = 1205 | n = 1485 | n = 3233 | n = 1758 | n = 1475 | n = 3924 | n = 2165 | n = 1759 | |
| Age at enrollment, median (IQR) | 52.0 (15.3) | 51.5 (15.9) | 52.3 (14.8) | 52.4 (15.6) | 52.3 (16.1) | 52.6 (15.0) | 48.3 (12.4) | 48.2 (12.9) | 48.3 (12.1) |
| Body mass indexb, % | |||||||||
| <25.0 | 26.7 | 33.6 | 21.0 | 25.3 | 30.3 | 19.5 | 18.8 | 22.9 | 13.8 |
| 25.0–29.9 | 49.6 | 49.3 | 49.8 | 50.1 | 49.5 | 50.8 | 48.2 | 49.6 | 46.6 |
| ≥30.0 | 23.8 | 17.1 | 29.2 | 24.6 | 20.3 | 29.8 | 33.0 | 27.5 | 39.7 |
| Leisure-time physical activity(MET hrs/week) median (IQR) | 27.5 (36.6) | 26.5 (34.1) | 28.6 (38.4) | 17.9 (28.2) | 18.0 (26.9) | 17.8 (19.7) | 18.0 (28.8) | 17.8 (28.8) | 18.2 (29.0) |
| Marital status, % | |||||||||
| Married/with partner | 82.8 | 83.8 | 81.9 | 84.0 | 85.1 | 82.7 | 82.7 | 82.4 | 83.1 |
| Single | 7.1 | 7.1 | 7.1 | 5.9 | 6.4 | 5.3 | 6.4 | 6.5 | 6.2 |
| Divorced/separated/widowed | 10.1 | 9.1 | 11.0 | 10.1 | 8.5 | 11.9 | 10.9 | 11.1 | 10.7 |
| Education, % | |||||||||
| Post-secondary complete | 66.0 | 69.5 | 63.2 | 54.7 | 57.5 | 51.4 | 51.9 | 54.0 | 49.2 |
| Some post-secondary | 17.9 | 16.2 | 19.3 | 17.9 | 16.1 | 19.9 | 19.0 | 17.4 | 20.9 |
| High school complete | 8.9 | 7.6 | 10.0 | 14.9 | 14.6 | 15.3 | 18.4 | 16.8 | 20.2 |
| High school not complete | 7.2 | 6.8 | 7.5 | 12.6 | 11.8 | 13.4 | 10.8 | 11.7 | 9.7 |
| Annual household income, % | |||||||||
| <$50,000 | 20.9 | 21.2 | 20.5 | 29.5 | 29.9 | 29.0 | 21.3 | 22.7 | 19.6 |
| $50,000–$99,999 | 42.0 | 41.0 | 42.8 | 44.4 | 44.8 | 43.9 | 45.7 | 44.7 | 47.0 |
| ≥$100,000 | 36.0 | 36.2 | 35.8 | 24.5 | 23.7 | 25.4 | 31.6 | 31.1 | 32.3 |
| Smoking status, % | |||||||||
| Never smoked | 51.2 | 52.1 | 50.5 | 41.6 | 41.0 | 42.3 | 36.1 | 35.6 | 36.7 |
| Former smoker | 41.6 | 40.9 | 42.2 | 40.9 | 40.4 | 41.5 | 37.9 | 36.3 | 39.8 |
| Current smoker | 7.1 | 7.0 | 7.3 | 17.5 | 18.5 | 16.2 | 25.9 | 28.0 | 23.3 |
| Family history of cancer, % | |||||||||
| No | 50.2 | 50.0 | 50.0 | 47.9 | 40.0 | 47.8 | 51.1 | 51.3 | 50.9 |
| Yes | 49.9 | 50.0 | 50.0 | 52.1 | 60.0 | 52.2 | 48.9 | 48.7 | 49.1 |
| Personal history of chronic disease a, % | |||||||||
| None | 48.8 | 50.9 | 47.1 | 52.5 | 52.3 | 52.8 | 54.5 | 56.7 | 51.7 |
| One | 29.5 | 28.4 | 30.4 | 28.6 | 28.9 | 28.3 | 28.7 | 27.7 | 29.9 |
| Two or more | 21.6 | 20.8 | 22.4 | 18.8 | 18.8 | 18.9 | 16.8 | 15.6 | 18.3 |
| Women | |||||||||
| n = 4808 | n = 2239 | n = 2469 | n = 4790 | n = 2667 | n = 2123 | n = 6643 | n = 3621 | n = 3022 | |
| Age at enrollment, median (IQR) | 51.9 (14.0) | 52.6 (14.2) | 51.3 (13.8) | 51.9 (16.0) | 52.4 (16.7) | 51.6 (15.2) | 47.6 (13.4) | 47.8 (13.4) | 47.5 (13.3) |
| Body mass index b, % | |||||||||
| <25.0 | 43.4 | 51.1 | 36.2 | 42.7 | 49.9 | 33.6 | 35.7 | 41.1 | 29.3 |
| 25.0–29.9 | 34.6 | 32.7 | 36.4 | 33.2 | 32.0 | 34.8 | 33.2 | 33.0 | 33.5 |
| ≥30.0 | 22.0 | 16.3 | 27.4 | 24.1 | 18.2 | 31.5 | 31.0 | 25.9 | 37.2 |
| Leisure-time physical activity (MET hrs/week) median (IQR) | 23.1 (30.0) | 22.1 (29.4) | 23.8 (30.3) | 16.3 (23.7) | 16.0 (22.9) | 16.9 (24.8) | 13.7 (22.2) | 13.5 (22.0) | 14.1 (22.3) |
| Marital status, % | |||||||||
| Married/with partner | 73.2 | 74.4 | 72.1 | 74.2 | 77.2 | 70.5 | 78.9 | 81.3 | 75.9 |
| Single | 6.4 | 6.1 | 6.8 | 5.2 | 4.7 | 5.7 | 4.8 | 4.5 | 5.1 |
| Divorced/separated/widowed | 20.3 | 19.5 | 21.1 | 20.6 | 18.0 | 23.8 | 16.4 | 14.2 | 19.0 |
| Education, % | |||||||||
| Post-secondary complete | 53.7 | 55.5 | 51.9 | 49.2 | 51.3 | 46.5 | 43.8 | 44.5 | 42.9 |
| Some post-secondary | 21.4 | 20.2 | 22.4 | 21.0 | 20.7 | 21.2 | 22.9 | 22.4 | 23.4 |
| High school complete | 17.7 | 17.3 | 18.1 | 20.0 | 19.0 | 21.3 | 23.7 | 23.5 | 23.9 |
| High school not complete | 7.2 | 6.9 | 7.5 | 9.8 | 8.9 | 19.3 | 9.7 | 9.5 | 9.9 |
| Annual household income, % | |||||||||
| <$50,000 | 31.8 | 31.8 | 31.7 | 39.0 | 37.6 | 40.9 | 34.5 | 33.4 | 35.9 |
| $50,000–$99,999 | 38.3 | 36.9 | 39.7 | 37.9 | 38.9 | 36.7 | 40.2 | 40.0 | 40.4 |
| ≥$100,000 | 26.9 | 27.9 | 26.0 | 20.0 | 20.6 | 19.3 | 22.6 | 23.7 | 21.2 |
| Smoking status, % | |||||||||
| Never smoked | 49.7 | 50.4 | 49.1 | 51.4 | 53.7 | 48.6 | 40.8 | 41.5 | 39.9 |
| Former smoker | 40.6 | 40.4 | 40.8 | 34.7 | 33.0 | 36.8 | 34.5 | 33.5 | 35.7 |
| Current smoker | 9.6 | 9.2 | 10.0 | 13.8 | 13.3 | 14.4 | 24.7 | 25.0 | 24.3 |
| Family history of cancer, % | |||||||||
| No | 45.3 | 47.0 | 43.7 | 45.4 | 45.0 | 45.9 | 47.1 | 47.5 | 46.8 |
| Yes | 54.7 | 52.9 | 56.3 | 54.6 | 55.0 | 54.0 | 52.9 | 52.6 | 53.2 |
| Personal history of chronic disease a | |||||||||
| None | 57.2 | 58.1 | 56.4 | 57.2 | 59.4 | 54.6 | 60.1 | 61.6 | 58.3 |
| One | 28.2 | 28.1 | 28.3 | 28.8 | 27.8 | 29.9 | 27.0 | 25.9 | 28.2 |
| Two or more | 14.5 | 13.7 | 15.3 | 14.0 | 12.8 | 15.5 | 12.9 | 12.5 | 13.5 |
| Menopausal status, % | |||||||||
| Pre-menopause | 58.9 | 59.5 | 58.4 | 59.4 | 59.1 | 59.9 | 51.8 | 50.6 | 53.3 |
| Post-menopause | 40.7 | 40.1 | 41.4 | 40.0 | 40.5 | 39.3 | 47.8 | 49.1 | 46.2 |
| Hormone replacement therapy use, % | |||||||||
| Never used | 84.8 | 83.3 | 86.2 | 82.8 | 82.9 | 82.8 | 86.2 | 86.9 | 85.4 |
| Ever used | 15.0 | 16.5 | 13.6 | 16.8 | 16.8 | 16.9 | 13.5 | 12.8 | 14.4 |
| Men | |||||||
| Healthy Pattern | |||||||
| Inclusion a (n = 2690) | ExBefore b (n = 1780) | ExAfter c (n = 1205) | InclusionNN d (n = 3468) | ||||
| Food groups | Mean e (SD) | Food groups | Mean e (SD) | Food groups | Mean e (SD) | Food groups | Mean e (SD) |
| Fruit | 9.9 (5.4) | Fruit | 7.8 (5.0) | Fruit | 9.3 (5.2) | Fruit | 8.1 (5.4) |
| Breakfast cereal | 4.6 (4.1) | Low-fat dairy | 6.0 (6.7) | Fruit juice | 4.6 (5.7) | Low-fat dairy | 5.9 (6.8) |
| Fruit juice | 4.5 (5.4) | Fruit juice | 4.5 (5.6) | Breakfast cereal | 4.2 (3.5) | Fruit juice | 4.4 (5.4) |
| Rice | 3.6 (6.0) | Breakfast cereal | 4.2 (3.4) | Rice | 4.0 (6.4) | Breakfast cereal | 4.4 (3.8) |
| Nuts | 3.1 (5.0) | Rice | 3.3 (5.7) | Nuts | 3.7 (5.5) | Rice | 3.1 (5.5) |
| Poultry no skin | 3.0 (3.5) | Nuts | 3.2 (4.9) | Poultry no skin | 3.2 (3.7) | Nuts | 2.7 (4.6) |
| Regular fat dairy | 2.7 (3.2) | Poultry no skin | 2.9 (3.4) | Regular fat dairy | 2.6 (2.9) | Poultry no skin | 2.7 (3.3) |
| Cooked vegetables | 1.9 (1.7) | Regular fat dairy | 2.1 (2.6) | Cooked vegetables | 2.0 (1.8) | Regular fat dairy | 2.2 (2.9) |
| Soup | 1.8 (2.1) | Soup | 1.7 (1.9) | Soup | 1.8 (2.1) | Soup | 1.7 (2.0) |
| Fish | 1.6 (1.6) | Cooked vegetables | 1.7 (1.6) | Fish | 1.6 (1.6) | Cooked vegetables | 1.6 (1.5) |
| Wine | 1.5 (3.3) | Fish | 1.4 (1.5) | Wine | 1.5 (3.5) | Fish | 1.4 (1.4) |
| Legumes | 1.2 (1.6) | Wine | 1.4 (3.4) | Meal replacement | 1.5 (5.3) | Wine | 1.4 (3.3) |
| Meats/Pizza Pattern | |||||||
| Inclusion a (n =3924) | ExBefore b (n = 2127) | ExAfter c (n = 2165) | InclusionNN d (n = 3760) | ||||
| Food groups | Mean e (SD) | Food groups | Mean e (SD) | Food groups | Mean e (SD) | Food groups | Mean e (SD) |
| Meat | 11.6 (5.4) | Meat | 10.6 (5.4) | Meat | 11.6 (5.4) | Meat | 10.3 (5.4) |
| Pasta/pizza | 6.8 (4.7) | Pasta/pizza | 6.8 (4.8) | Pasta/pizza | 6.9 (4.9) | Pasta/pizza | 6.7 (4.6) |
| Beer | 5.6 (11.0) | Beer | 5.2 (10.8) | Beer | 5.8 (11.1) | Beer | 5.0 (11.0) |
| Regular soda | 4.3 (6.4) | Regular soda | 5.0 (7.2) | Regular soda | 4.5 (6.7) | Regular soda | 4.7 (6.9) |
| Chips | 3.6 (3.6) | Chips | 3.9 (3.7) | Chips | 3.6 (3.5) | Chips | 3.8 (3.8) |
| Other breads | 3.5 (3.7) | Processed meat | 3.4 (2.6) | Other bread | 3.5 (3.8) | Processed meat | 3.3 (2.6) |
| Processed meat | 3.5 (2.6) | Regular fat cheese | 2.6 (2.8) | Processed meat | 3.5 (2.6) | Regular fat cheese | 2.4 (2.7) |
| Regular fat cheese | 2.4 (2.8) | French fries | 2.2 (2.0) | Regular fat cheese | 2.5 (2.8) | French fries | 2.1 (2.1) |
| French fries | 2.3 (2.2) | Confectionary | 2.2 (3.0) | French fries | 2.3 (2.1) | Confectionary | 2.1 (2.9) |
| Eggs | 2.2 (2.1) | Liquor | 1.9 (5.3) | Eggs | 2.0 (1.8) | Liquor | 1.9 (5.1) |
| Liquor | 1.9 (5.0) | Regular fat salad dressing | 1.5 (1.9) | Liquor | 1.9 (5.1) | Regular fat salad dressing | 1.5 (1.9) |
| Regular fat salad dressing | 1.5 (2.0) | Mexican | 1.2 (1.6) | Regular fat salad dressing | 1.5 (1.9) | Mexican | 1.3 (1.6) |
| Sweets/Dairy Pattern | |||||||
| Inclusion a (n = 3233) | ExBefore b (n = 1221) | ExAfter c (n = 1758) | InclusionNN d (n =2619) | ||||
| Food groups | Mean e (SD) | Food groups | Mean e (SD) | Food groups | Mean e (SD) | Food groups | Mean e (SD) |
| Low fat dairy | 7.3 (7.5) | Jam | 5.0 (4.7) | Low fat dairy | 7.2 (7.3) | Jam | 4.5 (4.6) |
| Wholemeal bread | 5.0 (4.9) | Wholemeal bread | 4.8 (4.6) | Cake | 5.1 (4.6) | Wholemeal bread | 4.5 (4.8) |
| Jam | 4.8 (4.5) | Cake | 3.9 (4.1) | Wholemeal bread | 4.9 (4.5) | Cake | 3.5 (3.7) |
| Cake | 4.7 (4.3) | Other bread | 3.5 (4.2) | Jam | 4.8 (4.5) | Other bread | 3.4 (4.1) |
| Cooked potatoes | 3.1 (2.6) | Cooked potatoes | 3.2 (2.3) | Cooked potatoes | 2.9 (2.3) | Cooked potatoes | 3.2 (2.6) |
| Dessert | 2.2 (2.3) | Margarine | 2.5 (2.4) | Confectionary | 2.3 (3.4) | Margarine | 2.1 (2.3) |
| Confectionary | 2.2 (3.2) | Eggs | 2.2 (2.0) | Dessert | 2.2 (2.3) | Eggs | 2.3 (2.3) |
| Margarine | 1.8 (2.1) | Dessert | 1.9 (1.9) | Ice cream | 1.9 (2.6) | Dessert | 1.8 (1.9) |
| Ice cream | 1.8 (2.6) | Coffee | 1.8 (0.8) | Margarine | 1.9 (2.1) | Coffee | 2.1 (1.2) |
| Coffee | 1.3 (1.2) | Ice cream | 1.6 (2.4) | Coffee | 1.0 (0.9) | Ice cream | 1.5 (2.3) |
| Mayonnaise | 0.7 (1.1) | High fat dairy | 1.6 (3.9) | Mayonnaise | 0.7 (1.1) | High fat dairy | 1.4 (3.7) |
| Women | |||||||
| Healthy Pattern | |||||||
| Inclusion a (n = 4808) | ExBefore b (n = 2919) | ExAfter c (n = 2239) | InclusionNN d (n = 5633) | ||||
| Food groups | Mean e (SD) | Food groups | Mean e (SD) | Food groups | Mean e (SD) | Food groups | Mean e (SD) |
| Fruit | 13.3 (6.3) | Fruit | 11.6 (6.0) | Fruit | 12.9 (6.0) | Fruit | 11.6 (6.5) |
| Regular fat dairy | 5.1 (4.6) | Regular fat dairy | 4.4 (3.9) | Regular fat dairy | 4.9 (4.1) | Regular fat dairy | 4.4 (4.3) |
| Poultry no skin | 4.6 (4.6) | Poultry no skin | 4.3 (4.2) | Poultry no skin | 4.6 (4.4) | Poultry no skin | 4.3 (4.4) |
| Nuts | 3.5 (5.5) | Nuts | 4.2 (6.1) | Nuts | 4.4 (6.3) | Nuts | 3.4 (5.3) |
| Rice | 3.0 (3.7) | Wholemeal bread | 3.2 (3.2) | Rice | 3.2 (3.9) | Wholemeal bread | 3.2 (3.3) |
| Cooked vegetables | 2.6 (2.3) | Rice | 3.1 (3.8) | Cooked vegetables | 2.6 (2.4) | Rice | 3.0 (3.9) |
| Fish | 1.9 (2.2) | Cooked vegetables | 2.4 (2.3) | Fish | 1.9 (2.1) | Cooked vegetables | 2.4 (2.2) |
| Soup | 1.9 (2.2) | Soup | 1.9 (2.1) | Soup | 1.9 (2.0) | Soup | 2.0 (2.3) |
| Wine | 1.7 (3.4) | Fish | 1.9 (2.0) | Wine | 1.7 (3.6) | Fish | 1.9 (2.1) |
| Legumes | 1.5 (1.6) | Wine | 1.8 (3.7) | Legumes | 1.5 (1.6) | Wine | 1.7 (3.6) |
| Raw vegetables | 1.5 (1.1) | Legumes | 1.5 (1.5) | Legumes | 1.5 (1.6) | ||
| Cabbage | 1.3 (1.6) | Raw vegetables | 1.4 (0.9) | Raw vegetables | 1.4 (1.1) | ||
| Meats/Pizza Pattern | |||||||
| Inclusion a (n = 6643) | ExBefore b (n = 3835) | ExAfter c (n = 3621) | InclusionNN d (n = 7049) | ||||
| Food groups | Mean e (SD) | Food groups | Mean e (SD) | Food groups | Mean e (SD) | Food groups | Mean e (SD) |
| Meat | 9.2 (4.8) | Meat | 8.6 (4.7) | Meat | 9.2 (4.7) | Meat | 8.4 (4.8) |
| Pasta/pizza | 6.5 (4.4) | Pasta/pizza | 6.2 (4.2) | Pasta/pizza | 6.4 (4.3) | Pasta/pizza | 6.2 (4.3) |
| Chips | 3.8 (4.0) | Chips | 3.8 (4.0) | Chips | 3.9 (4.1) | Chips | 3.7 (4.0) |
| Regular soda | 3.5 (6.6) | Regular soda | 3.5 (6.7) | Regular soda | 3.6 (6.7) | Regular soda | 3.4 (6.6) |
| Other bread | 3.4 (3.6) | Cake | 3.3 (3.4) | Other bread | 3.3 (3.4) | Cake | 3.1 (3.2) |
| Cooked potatoes | 2.8 (2.2) | Other bread | 3.1 (3.2) | Cooked potatoes | 2.7 (2.0) | Other bread | 3.1 (3.4) |
| Regular fat cheese | 2.7 (3.3) | Jam | 2.8 (2.9) | Regular fat cheese | 2.7 (3.2) | Jam | 2.7 (3.0) |
| Processed meat | 2.5 (1.9) | Regular fat cheese | 2.7 (3.2) | Confectionary | 2.6 (3.9) | Regular fat cheese | 2.6 (3.2) |
| Confectionary | 2.5 (3.7) | Cooked potatoes | 2.7 (2.0) | Processed meat | 2.5 (1.9) | Cooked potatoes | 2.8 (2.2) |
| Eggs | 2.2 (2.4) | Confectionary | 2.7 (4.0) | Eggs | 2.1 (2.1) | Confectionary | 2.5 (3.8) |
| Regular fat salad dressing | 2.1 (2.7) | Processed meat | 2.4 (1.8) | Regular fat salad dressing | 2.1 (2.6) | Processed meat | 2.4 (1.9) |
| Dessert | 1.7 (1.9) | Eggs | 2.1 (2.1) | Dessert | 1.8 (1.9) | Eggs | 2.1 (2.3) |
| Sweets/Dairy Pattern | |||||||
| Inclusion a (n = 4790) | ExBefore b (n = 1873) | ExAfter c (n = 2667) | InclusionNN c (n = 3559) | ||||
| Food groups | Mean e (SD) | Food groups | Mean e (SD) | Food groups | Mean e (SD) | Food groups | Mean e (SD) |
| Low-fat dairy | 10.3 (8.1) | Low-fat dairy | 14.3 (6.5) | Low-fat dairy | 10.3 (7.6) | Low-fat dairy | 13.3 (7.7) |
| Breakfast cereal | 5.1 (4.2) | Breakfast cereal | 5.0 (3.7) | Breakfast cereal | 4.6 (3.5) | Breakfast cereal | 5.2 (4.3) |
| Wholemeal bread | 4.5 (4.3) | Fruit juice | 3.8 (4.7) | Wholemeal bread | 4.5 (4.0) | Fruit juice | 3.7 (4.7) |
| Fruit juice | 4.2 (5.6) | Fruit juice | 4.3 (5.5) | ||||
| Cake | 3.4 (3.4) | Cake | 3.7 (3.7) | ||||
| Jam | 2.9 (2.9) | Jam | 3.0 (2.8) | ||||
| Ice cream | 1.1 (1.9) | Ice cream | 1.2 (2.0) | ||||
| Men | |||||
| Accounting for Misreporters | Dietary Pattern | n | Cancer Cases a | % of Cases Misreport | Cancer Risk–HR (95%) b |
| Inclusion | Healthy | 2690 | 257 | 57.2 | 1.00 |
| Sweets/Dairy | 3233 | 384 | 46.6 | 1.13 (0.96–1.33) | |
| Meats/Pizza | 3924 | 341 | 45.6 | 1.10 (0.93–1.30) | |
| InclusionNN | Healthy | 3468 | 349 | 47.0 | 1.00 |
| Sweets/Dairy | 2619 | 336 | 47.5 | 1.11 (0.95–1.30) | |
| Meats/Pizza | 3760 | 297 | 52.4 | 0.95 (0.81–1.11) | |
| ExBefore | Healthy | 1780 | 185 | 1.00 | |
| Sweets/Dairy | 1221 | 160 | 1.08 (0.87–1.35) | ||
| Meats/Pizza | 2127 | 156 | -- | 0.85 (0.68–1.06) | |
| ExAfter | Healthy | 1205 | 110 | 1.00 | |
| Sweets/Dairy | 1758 | 209 | 1.17 (0.93–1.48) | ||
| Meats/Pizza | 2165 | 182 | 1.08 (0.84–1.39) | ||
| Women | |||||
| Accounting for Misreporters | Dietary Pattern | n | Cancer Cases a | % of Cases Misreport | Cancer Risk–HR (95%) c |
| Inclusion | Healthy | 4808 | 347 | 54.2 | 1.00 |
| Sweets/Dairy | 4790 | 419 | 48.7 | 1.11 (0.96–1.28) | |
| Meats/Pizza | 6643 | 528 | 43.9 | 1.14 (0.99–1.32) | |
| InclusionNN | Healthy | 5633 | 426 | 51.9 | 1.00 |
| Sweets/Dairy | 3559 | 287 | 49.0 | 1.10 (0.94–1.28) | |
| Meats/Pizza | 7049 | 581 | 42.9 | 1.14 (1.00–1.30) | |
| ExBefore | Healthy | 2919 | 205 | 1.00 | |
| Sweets/Dairy | 1873 | 164 | 1.28 (1.04–1.58) | ||
| Meats/Pizza | 3835 | 296 | 1.12 (0.93–1.35) | ||
| ExAfter | Healthy | 2239 | 159 | 1.00 | |
| Sweets/Dairy | 2667 | 235 | 1.17 (0.96–1.44) | ||
| Meats/Pizza | 3621 | 271 | 1.12 (0.91–1.38) | ||
| Men | |||||
| Accounting for Misreporters | Dietary Pattern | n | Cancer Cases a | % of Cases Misreport | Cancer Risk-HR(95%) b |
| Inclusion | Healthy | 2690 | 52 | 63.5 | 1.00 |
| Sweets/Dairy | 3233 | 107 | 50.5 | 1.34 (0.96–1.89) | |
| Meats/Pizza | 3924 | 105 | 38.1 | 1.42 (1.00–2.02) | |
| InclusionNN | Healthy | 3468 | 73 | 53.4 | 1.00 |
| Sweets/Dairy | 2619 | 110 | 48.2 | 1.45 (1.07–1.97) | |
| Meats/Pizza | 3760 | 81 | 43.2 | 1.13 (0.82–1.57) | |
| ExBefore | Healthy | 1780 | 34 | 1.00 | |
| Sweets/Dairy | 1221 | 57 | 1.74 (1.12–2.72) | ||
| Meats/Pizza | 2127 | 46 | 1.23 (0.77–1.95) | ||
| ExAfter | Healthy | 1205 | 19 | 1.00 | |
| Sweets/Dairy | 1758 | 53 | 1.50 (0.88–2.56) | ||
| Meats/Pizza | 2165 | 65 | 1.92 (1.12–3.29) | ||
| Women | |||||
| Accounting for Misreporters | Dietary Pattern | n | Cancer Cases a | % of Cases Misreport | Cancer Risk-HR (95%) c |
| Inclusion | Healthy | 4808 | 241 | 52.7 | 1.00 |
| Sweets/Dairy | 4790 | 284 | 43.7 | 1.05 (0.88–1.25) | |
| Meats/Pizza | 6643 | 380 | 50.0 | 1.13 (0.96–1.34) | |
| InclusionNN | Healthy | 5633 | 303 | 52.1 | 1.00 |
| Sweets/Dairy | 3559 | 191 | 43.5 | 1.00 (0.84–1.21) | |
| Meats/Pizza | 7049 | 411 | 48.7 | 1.10 (0.94–1.28) | |
| ExBefore | Healthy | 2919 | 145 | 1.00 | |
| Sweets/Dairy | 1873 | 108 | 1.17 (0.91–1.50) | ||
| Meats/Pizza | 3835 | 211 | 1.07 (0.85–1.34) | ||
| ExAfter | Healthy | 2239 | 114 | 1.00 | |
| Sweets/Dairy | 2667 | 160 | 1.09 (0.86–1.40) | ||
| Meats/Pizza | 3621 | 190 | 1.02 (0.79–1.30) | ||
| Men | |||||
| Accounting for Misreporters | Dietary Pattern | n | Cancer Cases a | % of Cases Misreport | Cancer Risk-HR (95%) b |
| Inclusion | Healthy | 2690 | 38 | 57.9 | 1.00 |
| Sweets/Dairy | 3233 | 76 | 51.3 | 1.43 (0.96–2.13) | |
| Meats/Pizza | 3924 | 77 | 40.3 | 1.45 (0.96–2.17) | |
| InclusionNN | Healthy | 3468 | 58 | 44.8 | 1.00 |
| Sweets/Dairy | 2619 | 69 | 56.5 | 1.23 (0.86–1.76) | |
| Meats/Pizza | 3760 | 64 | 42.2 | 1.10 (0.77–1.60) | |
| ExBefore | Healthy | 1780 | 32 | 1.00 | |
| Sweets/Dairy | 1221 | 30 | 1.08 (0.64–1.82) | ||
| Meats/Pizza | 2127 | 37 | 1.01 (0.61–1.67) | ||
| ExAfter | Healthy | 1205 | 16 | 1.00 | |
| Sweets/Dairy | 1758 | 37 | 1.37 (0.76–2.49) | ||
| Meats/Pizza | 2165 | 46 | 1.62 (0.89–2.95) | ||
| Women | |||||
| Accounting for Misreporters | Dietary Pattern | n | Cancer Cases a | % of Cases Misreport | Cancer Risk-HR (95%) c |
| Inclusion | Healthy | 4808 | 51 | 52.9 | 1.00 |
| Sweets/Dairy | 4790 | 69 | 34.8 | 1.17 (0.81–1.69) | |
| Meats/Pizza | 6643 | 81 | 50.6 | 1.22 (0.84–1.77) | |
| InclusionNN | Healthy | 5633 | 60 | 51.7 | 1.00 |
| Sweets/Dairy | 3559 | 46 | 34.8 | 1.25 (0.84–1.84) | |
| Meats/Pizza | 7049 | 98 | 49.0 | 1.43 (1.02–2.01) | |
| ExBefore | Healthy | 2919 | 29 | 1.00 | |
| Sweets/Dairy | 1873 | 30 | 1.73 (1.03–2.89) | ||
| Meats/Pizza | 3835 | 50 | 1.43 (0.88–2.33) | ||
| ExAfter | Healthy | 2239 | 24 | 1.00 | |
| Sweets/Dairy | 2667 | 45 | 1.42 (0.86–2.35) | ||
| Meats/Pizza | 3621 | 40 | 1.13 (0.66–1.93) | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Solbak, N.M.; Al Rajabi, A.; Akawung, A.K.; Lo Siou, G.; Kirkpatrick, S.I.; Robson, P.J. Strategies to Address Misestimation of Energy Intake Based on Self-Report Dietary Consumption in Examining Associations Between Dietary Patterns and Cancer Risk. Nutrients 2019, 11, 2614. https://doi.org/10.3390/nu11112614
Solbak NM, Al Rajabi A, Akawung AK, Lo Siou G, Kirkpatrick SI, Robson PJ. Strategies to Address Misestimation of Energy Intake Based on Self-Report Dietary Consumption in Examining Associations Between Dietary Patterns and Cancer Risk. Nutrients. 2019; 11(11):2614. https://doi.org/10.3390/nu11112614
Chicago/Turabian StyleSolbak, Nathan M., Ala Al Rajabi, Alianu K. Akawung, Geraldine Lo Siou, Sharon I. Kirkpatrick, and Paula J. Robson. 2019. "Strategies to Address Misestimation of Energy Intake Based on Self-Report Dietary Consumption in Examining Associations Between Dietary Patterns and Cancer Risk" Nutrients 11, no. 11: 2614. https://doi.org/10.3390/nu11112614