Abstract
Equol is a metabolite of isoflavone daidzein and has an affinity to estrogen receptors. Although equol is produced by intestinal bacteria, the association between the status of equol production and the gut microbiota has not been fully investigated. The aim of this study was to compare the intestinal bacteria responsible for equol production in gut microbiota between equol producer and non-producer subjects regarding the intake of daidzein. A total of 1044 adult subjects who participated in a health survey in Hirosaki city were examined. The concentration of equol in urine was measured by high-performance liquid chromatography. The relative abundances of 8 bacterial species responsible for equol production in the gut microbiota was assessed using 16S rRNA amplification. There were 458 subjects identified as equol producers. The proportion of equol production status and the intake of daidzein increased with age. Daily intake of daidzein was larger in equol-producer. The intestinal bacteria, which convert daidzein to equol were present in both equol producers and non-producers. However, the relative abundance and the prevalence of Asaccharobacter celatus and Slackia isoflavoniconvertens were significantly higher in equol producers than those in equol non-producers. The intestinal bacteria that convert daidzein to equol are present in not only the equol producers but also in the non-producers. The daidzein intake is associated with the equol production status through an increase of A. celatus and S. isoflavoniconvertens in the gut microbiota.
1. Introduction
Equol, a metabolite of the soy isoflavone daidzein produced by the gut bacteria, has been reported to have a great affinity to estrogen receptors [1]. Equol has been associated with decreased risks of breast, prostate, and colon cancers; cardiovascular diseases; osteoporosis; and hormone-dependent diseases [2,3,4,5,6]. However, an ability to produce equol varies among the individuals, because only people who possess the intestinal bacteria capable of producing equol are regarded as equol producers. The equol production status has been associated with the consumption of isoflavone daidzein. Previous studies have been demonstrated that only 20–30% of the people in Western countries are equol producers [7,8,9], while the prevalence of equol producers is 40–60% among people in Asian countries where the isoflavone daidzein is regularly consumed [3,10,11].
To date, more than 10 species of gut microbes have been recognized to be responsible for equol production. Several studies have investigated the relationship between the intake of isoflavone daidzein and the equol-production status [12,13,14,15]. However, these studies were regardless of the gut microbiota, which plays a significant role in the equol production. Furthermore, few studies have been conducted to examine the influence of daily intake of daidzein on the equol-production status considering the gut microbiota.
We compared the prevalence of 8 bacterial species in the gut microbiota that convert daidzein to equol between the equol producers and non-producers to investigate the association between the equol-production status and the equol-producing microbiota. We also assessed the daily intake of daidzein to evaluate whether the daily intake of daidzein is different between the equol-producers and non-producers.
2. Material and Methods
2.1. Study Subjects
There were 1118 adult participants in the Iwaki Health Promotion Projects held in June 2015, in Iwaki District of Hirosaki City located in north Japan (Figure 1). Of these, we excluded 11 subjects whose urine sample were not collected; 11 subjects whose urine concentrations of daidzein were under the detectable level; and 52 subjects whose stool samples were not collected. Finally, 1044 subjects (411 men and 633 women) were included.
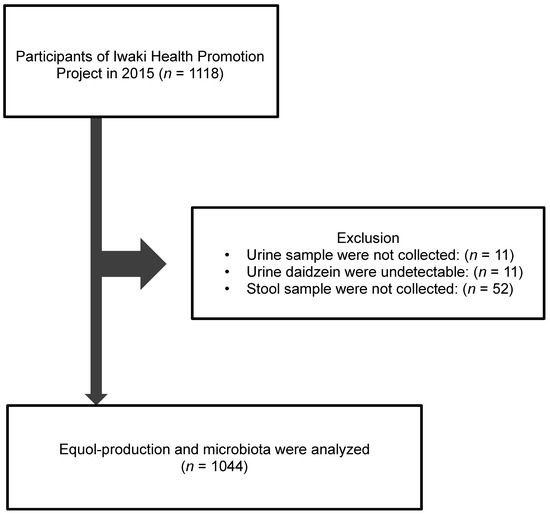
Figure 1.
Study flow of subjects. A total 1044 subjects were enrolled from 1118 adults who participated in Iwaki Health Promotion Projects in 2015.
2.2. Urine Concentration of Equol and Daidzein
The concentration of equol and daidzein in urine were measured by using a modified high-performance liquid chromatography method (HPLC) [16]. The equol production status was defined by a urinary log 10 - transformed equol/daidzein ratio of −1.75 or more as described previously [17].
2.3. Intake of Daidzein
The intake of daidzein was calculated based on a brief self-administered diet history questionnaire (BDHQ), a convenient diet assessment questionnaire developed in Japan [18,19,20]. The BDHQ included questions concerning the intake frequency of 58 food and beverage items commonly consumed in Japan. Those foods included three traditional Japanese soybean products: natto, tofu, and fried tofu.
2.4. Next Generation Sequence Analysis of Gut Microbiota
Fecal samples were collected in commercial containers (TechnoSuruga Laboratory Co., Ltd., Shizuoka, Japan) and were suspended in a guanidine thiocyanate solution (100 mM Tris-HCl (pH 9.0), 40 mM Tris-EDTA (pH 8.0), 4M Guanidine Thiocyanate). These samples were kept at −80 °C until DNA extraction. According to previous studies, a series of representative bacterial species in the human gut microbiota were analyzed using the primers for the V3 - V4 region of 16S rDNA of the prokaryotes [21,22]. The sequencing was conducted using an Illumina MiSeq system (Illumina, San Diego, CA, USA). The methods for quality filtering of the sequence was as follows: the only reads that had quality value scores of scores of ≥20 for more than 99% of the sequence were extracted for the analysis. Detection and identification of the bacteria from the sequences were performed using Metagenome@KIN software (World Fusion Co., Tokyo, Japan) and the TechnoSuruga Lab Microbial Identification database DB-BA 10.0 (TechnoSuruga Laboratory) at 97% sequence similarity.
We compared the relative abundance of the bacterial species in the gut microbiota between the equol producers and non-producers. The relative abundance is the percentage composition of the reads of a bacterium to the total reads of the gut microbiota. To estimate the contribution of each bacterium, the weighted average difference rank was calculated. The following 8 bacterial species that convert daidzein to equol were surveyed; Adlercreutzia equolifaciens [23], Asaccharobacter celatus [24], Bacteroides ovatus [25], Finegoldia magna [26], Lactobacillus mucosae [27], Slackia equolifaciens [28], Slackia isoflavoniconvertens [28], and Streptococcus intermedius [25].
2.5. Statistical Analysis
Statistical analyses of the clinical data were performed using the Statistical Package for the Social Science (SPSS) version 20.0 (SPSS Inc., Chicago, IL, USA) and the R software (R Foundation for Statistical Computing, version R-3.4.3). Categorical variables are shown as frequencies and percentages, and, continuous variables are shown as the mean with the standard deviation or the median with the interquartile range. The categorical variables were compared using the Chi-square test and the continuous variables were compared using the Student’s t-test or Mann-Whitney U-test. A p-value of less than 0.05 was considered significant for all the tests. Alpha-diversity was evaluated using Shannon index and chao1 while beta-diversity was evaluated by principal components analysis and statistically analyzed using permutation multivariate analysis of variance.
2.6. Ethics Statement
This study was performed in accordance with the ethical standards of the Declaration of Helsinki, and approved by the ethics committee at Hirosaki University Medical Ethics Committee (2014-377). All patients provided written informed consent for this study.
3. Results
Among the 1044 subjects, 458 (44%) were identified as the equol producers (Table 1). Although gender was not different between the equol producers and non-producers, there was a significant difference between the ages. Alpha-diversity analysis showed a higher gut microbiota complexity in the equol producers compared to those in non-producers (Figure 2). The gut microbiome was also significantly different between of equol the equol producers and non-producers by principal component analysis (p < 0.001) (Figure 3). Figure 4 shows the difference of the relative abundances of gut microbiota between the equol producers and non-producers at the phyla and genera level.

Table 1.
Characteristics of equol producer and non-producer.
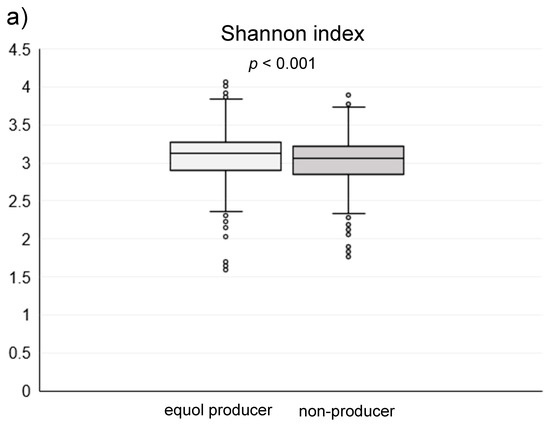
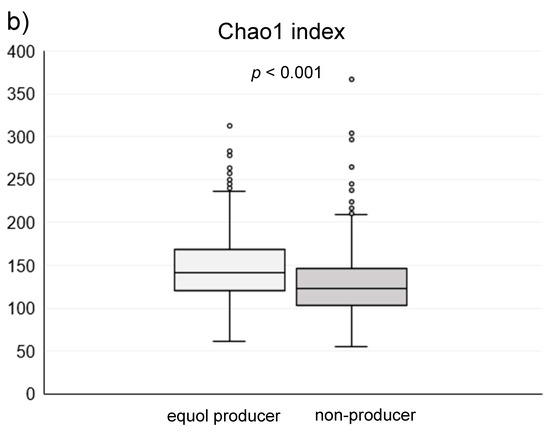
Figure 2.
(a) The Shannon index and (b) Chao1 index of the gut microbiota in the equol producers and non-producers.
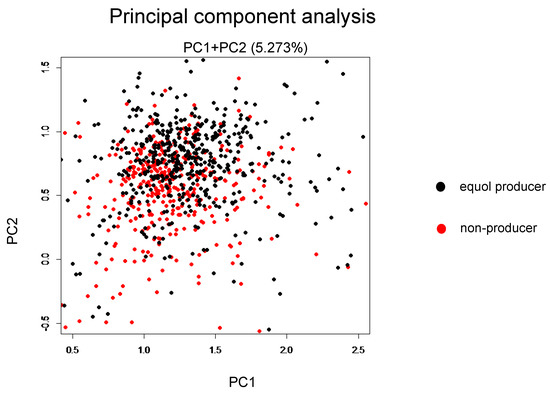
Figure 3.
Principal component analysis between the equol producers and non-producers.
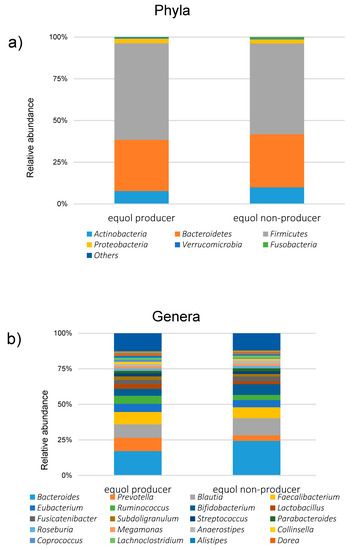
Figure 4.
The relative abundances of different gut microbiota between the equol producers and the non-producers (a) at the phyla level and (b) genera level.
The proportion of the equol producers was increased with the age and more than half of the subjects in older than 60 years were the equol producers (Figure 5). Furthermore, the daily intake of daidzein was also higher in the equol-producers. The median intake of daidzein based on the BDHQ was 15.0 mg/day in the equol producers and was 12.8 mg/day in the non-producers (p = 0.004) (Table 1). The daily intake of daidzein was also increased with the age (Figure 6).
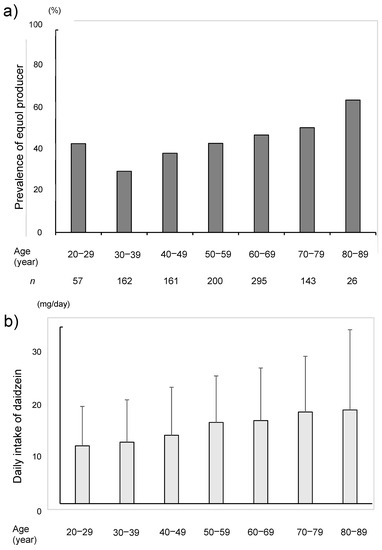
Figure 5.
The rate of equol producer (a) and the daily intake of daidzein (b) among the seven age groups and by decades.
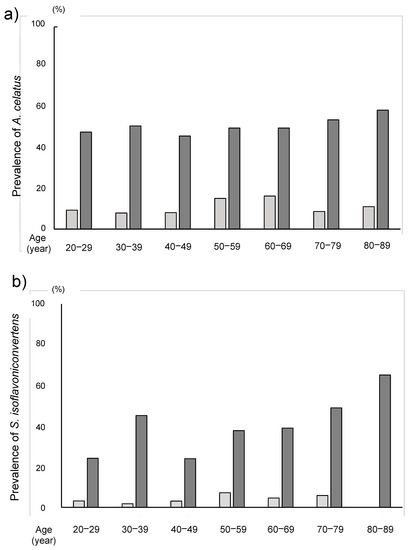
Figure 6.
The prevalence of (a) Asaccharobacter celatus and (b) Slackia isoflavoniconvertens among the seven age groups by decades.
The relative abundance of A. celatus and S. isoflavoniconvertens was significantly higher in the equol producers than those in the equol non-producers (Table 2). The prevalence of A. equolifaciens, A. celatus, B. ovatus, S. equolifaciens and S. isoflavoniconvertens in equol producer was significantly higher in the equol producers than in the non-producers (Table 3). In the equol producers, the prevalence of A. celatus and S. isoflavoniconvertens was 50.2% and 38.9%, respectively, while these values were only 11.8% and 4.3% in non-producers, respectively in the non-produces. The analyses among different age-groups by decades revealed that the prevalence of A. celatus and S. isoflavoniconvertens in the equol producers were significantly higher than that in the non-producers in all the decade-groups (Figure 4). Furthermore, in the equol producers only, the relative abundance of S. isoflavoniconvertens tended to increase with the age.

Table 2.
Relative abundance of species (percentage of the total bacterial 16sRNA).

Table 3.
Prevalence of intestinal bacteria species producing equol.
4. Discussion
The current study demonstrated that not only the equol producers, but also the non-producers possess the bacteria that convert daidzein to equol. However, there were some differences in the structure of gut microbiota and the prevalence of bacteria that convert daidzein to equol. Particularly, the abundance of A. celatus and S. isoflavoniconvertens were significantly different between the equol producers and non-producers. Moreover, the daily daidzein intake was significantly higher in the equol producers as compared the non-producers.
This is the first study to investigate the differences in the intestinal bacteria, which produce equol from daidzein, comparing between the equol producers and the non-producers in a mass-survey including more than a thousand subjects. In this study, the prevalence of the equol producers was more than 40%, consistent with a previous Japanese study [3]. Moreover, the equol producers were significantly older than the non-producers. Similar to our study, some previous studies have also reported that the prevalence of the equol producers among the younger male subjects to be significantly lower than that among the older male subjects [29,30]. Our data revealed that both the prevalence of the equol producers and the daily intake of daidzein calculated based on the BDHQ increase with the age. In the younger Japanese generation, the consumption of soy products has been decreasing with the increase of Western food intake. Decreased intake of daidzein might be associated with lower prevalence of the equol producers among the younger subjects.
Previous studies have suggested that the consumption of isoflavone daidzein does not convert an equol non-producer to an equol producer [13,14]. This concept has been widely accepted, although this has been shown for a relatively shorter duration of daidzein consumption (less than two months) [12,13,14]. In contrast, in an observational study, 18% of the non-producers changed to equol producers after the consumption of isoflavones for years [15]. Another study revealed that 16 weeks exposure to the soy isoflavones changed 8 of 20 (40%) equol non-producers to come equol producers [11]. In addition, a randomized crossover study with a 6-month exposure to soy foods also revealed that 16% of 79 pre-menopausal women acquired the ability to produce equol [31]. Therefore, the duration of the consumption of isoflavone daidzein seems to be important in the acquisition of the ability to produce equol. However, one study on postmenopausal women demonstrated the changes in the fecal bacterial community that contribute to equol production in the non-producers after only one week of isoflavones consumption [32]. The present study showed that some non-producers possess the several bacteria that contribute to equol production. Therefore, among the equol non-producers, the subjects who possessed specific intestinal bacteria in their gut microbiota can transform into equol-producers even after a short period of isoflavone consumption.
Although the bacteria which convert daidzein to equol existed in the most equol non-producer, there were differences in the complexity of the gut microbiota and prevalence of those bacteria between the equol producers and non-producers. Especially, both the relative abundance and the prevalence of A. celatus and S. isoflavoniconvertens were significantly higher in the equol producers than those in the non-producers. The daily daidzein intake based on the BDHQ analysis was increased with the age. Similarly, in the equol producers, the prevalence of A. celatus and S. isoflavoniconvertens increased with age. Therefore, daidzein intake would increase these two bacterial species and this increase would play a role in the development of the ability to produce equol.
Several limitations of this study need to be mentioned. Firstly, this study assessed the bacterial species using 16S ribosomal RNA gene sequences, but not by metagenome analysis. Therefore, the strains or functions of the species were not investigated. Second, since the mass-survey was performed in one day, the intake of soy foods for a few days prior to the mass-survey might have influenced the urine equol concentration. However, the BDHQ analysis was performed based on the record of a month prior to the survey, and thus, only 3% of the subjects were recognized as not having consumed daidzein.
In conclusion, our study revealed that not only the equol producers, but also the non-producers possess the bacteria that convert daidzein to equol. Most people in this area located in north Japan possessed the gut microbiota that have been recognized to take part in equol production. However, a higher gut microbiota complexity was observed in the equol producers. Moreover, isoflavone daidzein intake may be associated with the equol production status; particularly, the abundance of A. celatus and S. isoflavoniconvertens in the gut microbiota may play a significant role in equol production.
Author Contributions
C.I., T.S., K.I., Y.Y. and S.N. played a role in planning and/or conducting the study. C.I. and T.S. interpreted the data and drafted the manuscript. Y.Y., H.S. and S.F. played a role in reviewing and revising the manuscript. All authors approved the final version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
This study was based on the Iwaki Health Promotion Project as a project by Hirosaki University Graduate School of Medicine, in collaboration with Aomori Heath Evaluation and Promotion Center and Hirosaki City Office, Department of Health Promotion.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Setchell, K.D.R.; Borriello, S.P.; Hulme, P.; Kirk, D.N.; Axelson, M. Nonsteroidal estrogens of dietary origin: Possible roles in hormone-dependent disease. Am. J. Clin. Nutr. 1984, 40, 569–578. [Google Scholar] [CrossRef] [PubMed]
- Goodman, M.T.; Shvetsov, Y.B.; Wilkens, L.R.; Franke, A.A.; Le Marchand, L.; Kakazu, K.K.; Nomura, A.M.; Henderson, B.E.; Kolonel, L.N. Urinary phytoestrogen excretion and postmenopausal breast cancer risk: The multiethnic cohort study. Cancer Prev. Res. 2009, 2, 887–894. [Google Scholar] [CrossRef] [PubMed]
- Akaza, H.; Miyanaga, N.; Takashima, N.; Naito, S.; Hirao, Y.; Tsukamoto, T.; Fujioka, T.; Mori, M.; Kim, W.J.; Song, J.M.; et al. Comparisons of percent equol producers between prostate cancer patients and controls: Case-controlled studies of isoflavones in Japanese, Korean and American residents. Jpn. J. Clin. Oncol. 2004, 34, 86–89. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Spitznagel, E.L.; Bosland, M.C. Soy consumption and colorectal cancer risk in humans: A metaanalysis. Cancer Epidemiol. Biomark. Prev. 2010, 19, 148–158. [Google Scholar] [CrossRef] [PubMed]
- Lydeking-Olsen, E.; Beck-Jensen, J.E.; Setchell, K.D.R.; Holm-Jensen, T. Soymilk or progesterone for prevention of bone loss—A 2 year randomized, placebo-controlled trial. Eur. J. Nutr. 2004, 43, 246–257. [Google Scholar] [CrossRef] [PubMed]
- Cassidy, A. Dietary phyto-oestrogens: Molecular mechanisms, bioavailability and importance to menopausal health. Nutr. Res. Rev. 2005, 18, 183–201. [Google Scholar] [CrossRef] [PubMed]
- Rowland, I.R.; Wiseman, H.; Sanders, T.A.; Adlercreutz, H.; Bowey, E.A. Interindividual variation in metabolism of soy isoflavones and lignans: Influence of habitual diet on equol production by the gut microflora. Nutr. Cancer 2000, 36, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, C.; Frankenfeld, C.L.; Lampe, J.W. Gut bacterial metabolism of the soy isoflavone daidzein: Exploring the relevance to human health. Exp. Biol. Med. 2005, 230, 155–170. [Google Scholar] [CrossRef]
- Lampe, J.W.; Karr, S.C.; Hutchins, A.M.; Slavin, J.L. Urinary equol excretion with a soy challenge: Influence of habitual diet. Proc. Soc. Exp. Biol. Med. 1998, 217, 335–339. [Google Scholar] [CrossRef] [PubMed]
- Song, K.B.; Atkinson, C.; Frankenfeld, C.L.; Jokela, T.; Wähälä, K.; Thomas, W.K.; Lampe, J.W. Prevalence of daidzein-metabolizing phenotypes differs between Caucasian and Korean American women and girls. J. Nutr. 2006, 136, 1347–1351. [Google Scholar] [CrossRef] [PubMed]
- Ko, T.F.; Tsai, H.S.; Lin, S.M.; Liu, C.D.; Learn, S.P.; Chiou, R.Y. GC-MS determined distribution of urinary equol producers as affected by age, gender, and repeated ingestions of soymilk. J. Food Sci. 2010, 75, H306–H310. [Google Scholar] [CrossRef] [PubMed]
- Setchell, K.D.; Faughnan, M.S.; Avades, T.; Zimmer-Nechemias, L.; Brown, N.M.; Wolfe, B.E.; Brashear, W.T.; Desai, P.; Oldfield, M.F.; Botting, N.P.; et al. Comparing the pharmacokinetics of daidzein and genistein with the use of 13C-labeled tracers in premenopausal women. Am. J. Clin. Nutr. 2003, 77, 411–419. [Google Scholar] [CrossRef] [PubMed]
- Védrine, N.; Mathey, J.; Morand, C.; Brandolini, M.; Davicco, M.J.; Guy, L.; Rémésy, C.; Coxam, V.; Manach, C. One-month exposure to soy isoflavones did not induce the ability to produce equol in postmenopausal women. Eur. J. Clin. Nutr. 2006, 60, 1039–1045. [Google Scholar] [CrossRef] [PubMed]
- Mathey, J.; Lamothe, V.; Coxam, V.; Potier, M.; Sauvant, P.; Bennetau-Pelissero, C. Concentrations of isoflavones in plasma and urine of post-menopausal women chronically ingesting high quantities of soy isoflavones. J. Pharm. Biomed. Anal. 2006, 41, 957–965. [Google Scholar] [CrossRef] [PubMed]
- Frankenfeld, C.L.; Atkinson, C.; Thomas, W.K.; Gonzalez, A.; Jokela, T.; Wähälä, K.; Schwartz, S.M.; Li, S.S.; Lampe, J.W. High concordance of daidzein-metabolizing phenotypes in individuals measured 1 to 3 years apart. Br. J. Nutr. 2005, 94, 873–876. [Google Scholar] [CrossRef] [PubMed]
- Lundh, T.J.; Pettersson, H.; Kiessling, K.H. Liquid chromatographic determination of the estrogens daidzein, formononetin, coumestrol, and equol in bovine blood plasma and urine. J. Assoc. Off. Anal. Chem. 1988, 71, 938–941. [Google Scholar] [PubMed]
- Setchell, K.D.; Cole, S.J. Method of defining equol-producer status and its frequency among vegetarians. J. Nutr. 2006, 136, 2188–2193. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, S.; Yanagibori, R.; Amano, K. Self-administered diet history questionnaire developed for health education: A relative validation of the test-version by comparison with 3-day diet record in women. J. Epidemiol. 1998, 8, 203–215. [Google Scholar] [CrossRef] [PubMed]
- Okubo, H.; Sasaki, S.; Rafamantanantsoa, H.H.; Ishikawa-Takata, K.; Okazaki, H.; Tabata, I. Validation of self-reported energy intake by a self-administered diet history questionnaire using the doubly labeled water method in 140 Japanese adults. Eur. J. Clin. Nutr. 2008, 62, 1343–1350. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, S.; Honda, S.; Murakami, K.; Sasaki, S.; Okubo, H.; Hirota, N.; Notsu, A.; Fukui, M.; Date, C. Both comprehensive and brief self-administered diet history questionnaires satisfactorily rank nutrient intakes in Japanese adults. J. Epidemiol. 2012, 22, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, S.; Tomita, J.; Nishioka, K.; Hisada, T.; Nishijima, M. Development of a prokaryotic universal primer for simultaneous analysis of Bacteria and Archaea using next-generation sequencing. PLoS ONE 2014, 9, e105592. [Google Scholar] [CrossRef] [PubMed]
- Hisada, T.; Endoh, K.; Kuriki, K. Inter- and intra-individual variations in seasonal and daily stabilities of the human gut microbiota in Japanese. Arch. Microbiol. 2015, 197, 919–934. [Google Scholar] [CrossRef] [PubMed]
- Maruo, T.; Sakamoto, M.; Ito, C.; Toda, T.; Benno, Y. Adlercreutzia equolifaciens gen. nov., sp. nov., an equol-producing bacterium isolated from human faeces, and emended description of the genus Eggerthella. Int. J. Syst. Evol. Microbiol. 2008, 58, 1221–1227. [Google Scholar] [CrossRef] [PubMed]
- Minamida, K.; Ota, K.; Nishimukai, M.; Tanaka, M.; Abe, A.; Sone, T.; Tomita, F.; Hara, H.; Asano, K. Asaccharobacter celatus gen. nov., sp. nov., isolated from rat caecum. Int. J. Syst. Evol. Microbiol. 2008, 58, 1238–1240. [Google Scholar] [CrossRef] [PubMed]
- Ueno, T.; Uchiyama, S. Identification of the specific intestinal bacteria capable of metabolising soy isoflavone to equol. Ann. Nutr. Metab. 2002, 45, 114. [Google Scholar]
- Decroos, K.; Vanhemmens, S.; Cattoir, S.; Boon, N.; Verstraete, W. Isolation and characterization of an equol-producing mixed microbial culture from a human faecal sample and its activity under gastrointestinal conditions. Arch. Microbiol. 2005, 183, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.S.; Kitahara, M.; Sakamoto, M.; Hattori, M.; Benno, Y. Slackia equolifaciens sp. nov., a human intestinal bacterium capable of producing equol. Int. J. Syst. Evol. Microbiol. 2010, 60, 1721–1740. [Google Scholar] [CrossRef] [PubMed]
- Matthies, A.; Blaut, M.; Braune, A. Isolation of a human intestinal bacterium capable of daidzein and genistein conversion. Appl. Environ. Microbiol. 2009, 75, 1740–1744. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, K.; Tanaka, M.; Hirao, Y.; Nagata, Y.; Mori, M.; Miyanaga, N.; Akaza, H.; Kim, W.J. Age-stratified serum levels of isoflavones and proportion of equol producers in Japanese and Korean healthy men. Prostate Cancer Prostatic Dis. 2008, 11, 252–257. [Google Scholar] [CrossRef] [PubMed]
- Ueno, T.; Abiru, Y.; Uchiyama, S.; Ishimi, Y. Distribution of 24-h urinary equol excretion as an indicator of the physiological range in healthy Japanese equol excretors. J. Funct. Foods 2014, 7, 129–135. [Google Scholar] [CrossRef]
- Franke, A.A.; Lai, J.F.; Pagano, I.; Morimoto, Y.; Maskarinec, G. Equol production changes over time in pre-menopausal women. Br. J. Nutr. 2012, 107, 1201–1206. [Google Scholar] [CrossRef] [PubMed]
- Nakatsu, C.H.; Armstrong, A.; Clavijo, A.P.; Martin, B.R.; Barnes, S.; Weaver, C.M. Fecal bacterial community changes associated with isoflavone metabolites in postmenopausal women after soy bar consumption. PLoS ONE 2014, 9, e108924. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).