Impact of SchisandraChinensis Bee Pollen on Nonalcoholic Fatty Liver Disease and Gut Microbiota in HighFat Diet Induced Obese Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation and Identification of SCPE
2.2. Antioxidant Activities of SCPE
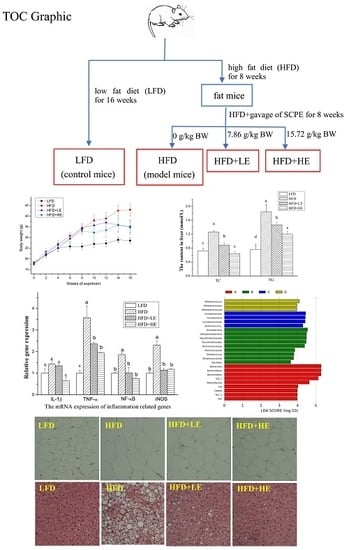
2.3. Animal Experiments
2.4. Serum and Hepatic Biochemical Analysis
2.5. Quantification of Gene Expression Analysis
2.6. Histopathological Examinations of Liver and Epididymal Adipose
2.7. Illumina Sequencing and Statistical Analysis of 16S rRNA Gene V3-V4 Region of Gut Microbiota
2.8. Statistical Analysis
3. Results
3.1. The Phenolic Compound and Antioxidant Activity of SCPE
3.2. SCPE Attenuated Obesity Induced by HFD in Mice
3.3. Effects of SCPE on TC, TG and LDL-C Levels in Serum and Liver
3.4. Effects of SCPE on Liver Damage, Oxidative Stress, Inflammation and NAFLD Formation
3.5. SCPE Consumption Modulates Gut Microbiota in Obese Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Skinner, A.C.; Ravanbakht, S.N.; Skelton, J.A.; Perrin, E.M.; Armstrong, S.C. Prevalence of obesity and severe obesity in US children, 1999–2016. Pediatrics 2018, 141, e20173459. [Google Scholar] [CrossRef] [PubMed]
- Petts, G.; Lloyd, K.; Goldin, R. Fatty liver disease. Diagn. Histopathol. 2014, 20, 102–108. [Google Scholar] [CrossRef]
- Doerstling, S.S.; O’Flanagan, C.H.; Hursting, S.D. Obesity and cancer metabolism: A perspective on interacting tumor—intrinsic and extrinsic factors. Front. Oncol. 2017, 7, 216. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhao, J.; Chu, Z.; Zhou, J. Increasing prevalence of childhood overweight and obesity in a coastal province in China. Pediatr.Obes. 2016, 11, e22–e26. [Google Scholar] [CrossRef] [PubMed]
- Skinner, A.C.; Perrin, E.M.; Skelton, J.A. Prevalence of obesity and severe obesity in US children, 1999–2014. Obesity 2016, 24, 1116–1123. [Google Scholar] [CrossRef] [PubMed]
- Ogden, C.L.; Carroll, M.D.; Lawman, H.G.; Fryar, C.D.; Kruszon-Moran, D.; Kit, B.K.; Flegal, K.M. Trends in obesity prevalence among children and adolescents in the United States, 1988–1994 through 2013–2014. JAMA 2016, 315, 2292–2299. [Google Scholar] [CrossRef] [PubMed]
- Roberts, E.A. Pediatric nonalcoholic fatty liver disease (NAFLD): A “growing” problem? Heaptology 2007, 46, 1133–1142. [Google Scholar] [CrossRef]
- Jurado-Ruiz, E.; Varela, L.M.; Luque, A.; Berná, G.; Cahuana, G.; Martinez-Force, E.; Gallego-durán, R.; Soria, B.; Roos, B.; Gómez, M.R.; et al. An extra virgin olive oil rich diet intervention ameliorates the nonalcoholic steatohepatitis induced by a high-fat “Western-type” diet in mice. Mol. Nutr. Food Res. 2017, 61, 1600549. [Google Scholar] [CrossRef]
- Wang, D.; Wei, Y.; Pagliassotti, M.J. Saturated fatty acids promote endoplasmic reticulum stress and liver injury in rats with hepatic steatosis. Endocrinology 2006, 147, 943–951. [Google Scholar] [CrossRef]
- Neuschwander-Tetri, B.A. Non-alcoholic fatty liver disease. BMC Med. 2017, 15, 45. [Google Scholar] [CrossRef]
- Sadaf, N.; Munazza, S.; Hina, S.; Misbah, M.; Maliha, S. A review of antihperlipidemic effect of synthetic phenolic compounds. Matrix Sci. Med. 2017, 1, 22–26. [Google Scholar]
- Chen, H.; Sang, S. Biotransformation of tea polyphenols by gut microbiota. J. Funct. Foods 2014, 7, 26–42. [Google Scholar] [CrossRef]
- Boursier, J.; Mueller, O.; Barret, M.; Machado, M.; Fizannc, L.; Araujo-Perez, F.; Guy, C.D.; Seed, P.C.; Rawls, J.F.; David, L.A.; et al. The severity of nonalcoholic fatty liver disease is associated with gut dysbiosis and shift in the metabolic function of the gut microbiota. Hepatology 2016, 63, 764–775. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, G.; Xie, M.; Dai, Z.; Wang, P.; Ye, H.; Zeng, X.; Sun, Y. Kudingcha and Fuzhuan brick tea prevent obesity and modulate gut microbiota in high-fat diet fed mice. Mol. Nutr. Food Res. 2018, 62, 1700485. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Juaristi, M.; Martínez-López, S.; Sarria, B.; Bravo, L.; Mateos, R. Absorption and metabolism of yerba mate phenolic compounds in humans. Food Chem. 2018, 240, 1028–1038. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Chen, Z.; Guo, H.; He, D.; Zhao, D.; Wang, Z.; Zhang, W.; Liao, L.; Zhang, C.; Ni, L. The modulatory effect of infusions of green tea, oolong tea, and black tea on gut microbiota in high-fat-induced obese mice. Food Funct. 2016, 7, 4869–4879. [Google Scholar] [CrossRef]
- Neyrinck, A.M.; Hée, V.F.V.; Bindels, L.B.; Backer, F.D.; Cani, P.D.; Delzenne, N.M. Polyphenol-rich extract of pomegranate peel alleviates tissue inflammation and hypercholesterolaemia in high-fat diet-induced obese mice: Potential implication of the gut microbiota. Br. J. Nutr. 2013, 109, 802–809. [Google Scholar] [CrossRef]
- Baldwin, J.; Collins, B.; Wolf, P.G.; Martinez, K.; Shen, W.; Chuang, C.; Zhong, W.; Cooney, P.; Cockrell, C.; Chang, E.; et al. Table grape consumption reduces adiposity and markers of hepatic lipogenesis and alters gut microbiota in butter fat-fed mice. J. Nutr. Biochem. 2016, 27, 123–135. [Google Scholar] [CrossRef] [Green Version]
- Su, D.; Zhang, R.; Hou, F.; Chi, J.; Huang, F.; Yan, S.; Liu, L.; Deng, Y.; Wei, Z.; Zhang, M. Lychee pulp phenolics ameliorate hepatic lipid accumulation by reducing miR-33 and miR-122 expression in mice fed a high-fat diet. Food Funct. 2017, 8, 808–815. [Google Scholar] [CrossRef]
- Ai, J.; Wang, Y.P.; Li, C.Y.; Yan, T.L.; Guo, X.W. Pollen shape and pollination character of Schisandrachinensis. J. Jilin Agric. Univ. 2007, 29, 293–297. [Google Scholar]
- Sun, C.R. Pollen morphology of the Schisandraceae and its systematic significance. ActaPhytotax. Sin. 2000, 38, 437–445. [Google Scholar]
- Cheng, N.; Ren, N.; Gao, H.; Lei, X.; Zheng, J.; Cao, W. Antioxidant and hepatoprotective effects of Schisandrachinensis pollen extract on CCl4-induced acute liver damage in mice. Food Chem.Toxicol. 2013, 55, 234–240. [Google Scholar] [CrossRef] [PubMed]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C.L.W.T. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Benzie, F.F.; Strain, J.J. The ferric reducing ability of plasma as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Rajini, P.S. Freee radical scavenging activity of an aqueous extract of potato peel. Food Chem. 2004, 85, 611–616. [Google Scholar] [CrossRef]
- Wu, T.; Yin, J.; Zhang, G.; Long, H.; Zheng, X. Mulberry and cherry anthocyanin consumption prevents oxidative stress and inflammation in diet-induced obese mice. Mol. Nutr. Food Res. 2016, 60, 687–694. [Google Scholar] [CrossRef] [PubMed]
- Sim, W.; Park, S.; Lee, K.; Je, Y.; Yin, H.; Choi, Y.; Sung, S.H.; Park, S.; Park, H.; Shin, K.J.; et al. LXR-α antagonist meso-dihydroguaiaretic acid attenuates high-fat diet-induced nonalcoholic fatty liver. Biochem. Pharmacol. 2014, 90, 414–424. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2-△△Ct method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Liu, G.; Bei, J.; Liang, L.; Yu, G.; Li, L.; Li, Q. Stachyose improves inflammation through modulating gut microbiota of high-fat diet/streptozotocin-induced type 2 diabetes in rats. Mol. Nutr. Food Res. 2018, 62, 1700954. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Kuczynski, J.J.; Stombaugh, K.; Bittinger, F.D.; Bushman, E.K.; Costello, N. QIIME allows analysis of high-throughputcommunity sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef]
- Shim, J.J.; Kim, J.W.; Oh, C.H.; Lee, Y.R.; Lee, J.S.; Park, S.Y.; Kim, B.H.; Oh, I.H. Serum alanine aminotransferase level and liver-related mortality in patients with chronic hepatitis B: A large national cohort study. Liver Int. 2018, 38, 1751–1759. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Sánchez, A.; Madrigal-Santillán, E.; Bautista, M.; Esquivel-Soto, J.; Morales-González, Á.; Esquivel-Chirino, C.; Durante-Montiel, I.; Sánchez-Rivera, G.; Valadez-Vega, C.; Morales-González, J.A. Inflammation, oxidative stress, and obesity. Int. J. Mol. Sci. 2011, 12, 3117–3132. [Google Scholar]
- Chen, Q.; Wang, T.; Li, J.; Wang, S.; Qiu, F.; Yu, H.; Zhang, Y.; Wang, T. Effects of natural products on fructose-induced nonalcoholic fatty liver disease (NAFLD). Nutrients 2017, 9, 96. [Google Scholar] [CrossRef] [PubMed]
- Dandona, P.; Aljada, A.; Bandyopadhyay, A. Inflammation: The link between insulin resistance, obesity and diabetes. TrendsImmunol. 2004, 25, 4–7. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, M.; Ho, C.; Guo, X.; Wu, Z.; Weng, P.; Yan, M. Metagenomics analysis of gut microbiota modulatory effect of green tea polyphenols by high fat diet-induced obesity mice model. Funct. Foods 2018, 46, 268–277. [Google Scholar] [CrossRef]
- Amirkhizi, F.; Siassi, F.; Minaie, S.; Djalali, M.; Rahimi, A.; Chamari, M. Is obesity associated with increased plasma lipid peroxidación and oxidative stress in women. Arya Atheroscler. 2007, 2, 189–192. [Google Scholar]
- Neuschwander-Tetri, B.A.; Caldwell, S.H. Nonalcoholic steatohepatitis: Summary of an AASLD Single Topic Conference. Hepatology 2003, 37, 1202–1219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, Y.; Wang, C.C.N.; Chang, H.; Chu, F.; Hsu, Y.; Cheng, W.; Ma, W.; Chen, C.; Wan, L.; Lim, Y. Ursoli acid, a novel liver X receptor α (LXRα) antagonist inhibiting ligand-induced nonalcoholic fatty liver and drug-induced lipogenesis. J. Agric. Food Chem. 2018, 66, 11647–11662. [Google Scholar] [CrossRef]
- Higuchi, N.; Kato, M.; Shundo, Y.; Tajiri, H.; Tanaka, M.; Yamashita, N.; Kohjima, M.; Kotoh, K.; Nakamuta, M.; Takayanagi, R.; et al. Liver X receptor in cooperation with SREBP-1c is a major lipid synthesis regulator in nonalcoholic fatty liver disease. Hepatol. Res. 2008, 38, 1122–1129. [Google Scholar] [CrossRef]
- Sikaris, K. The clinical biochemistry of obesity. Clin. Biochem. Rev. 2004, 25, 165–181. [Google Scholar]
- Fonseca-Alaniz, M.H.; Takada, J.; Alonso-Vale, M.I.; Lima, F.B. Adipose tissue as an endocrine organ: From theory to practice. J. Pediatr. 2007, 83, S192–S203. [Google Scholar] [CrossRef]
- Alwahsh, S.M.; Xu, M.; Schultze, F.C.; Wilting, J.; Mihm, S.; Raddatz, D.; Ramadori, G. Combination of alcohol and fructose exacerbates metabolic imbalance in terms of hepatic damage, dyslipidemia, and insulin resistance in rats. PLoS ONE 2014, 9, e104220. [Google Scholar] [CrossRef] [PubMed]
- Stender, S.; Kozlitina, J.; Nordestgarrd, B.G.; Tybjærg-Hansen, A.; Hobbs, H.H.; Cohen, J.C. Adiposity amplifies the genetic risk of fatty liver disease conferred by multiple loci. Nat. Genet. 2017, 49, 842–847. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ulusoy, E.; Kolayli, S. Phenolic composition and antioxidant properties of anzer bee pollen. J. Food Biochem. 2014, 38, 73–82. [Google Scholar] [CrossRef]
- Jin, T.; Saravanakumar, K.; Wang, M. In vitro and in vivo antioxidant properties of water and methanol extracts of linden bee pollen. Biocatal. Agric. Biotech. 2018, 13, 186–189. [Google Scholar] [CrossRef]
- Nicholson, J.K.; Holmes, E.; Kinross, J.; Burcelin, R.; Gibson, G.; Jia, W.; Pettersson, S. Host-gut microbiota metabolic interactions. Science 2012, 336, 1262–1267. [Google Scholar] [CrossRef] [PubMed]
- Vaiserman, A.M.; Koliada, A.K.; Marotta, F. Gut microbiota: A player in aging and a target for anti-aging intervention. Ageing Res. Rev. 2017, 35, 36–45. [Google Scholar] [CrossRef]
- Espín, J.C.; González-Sarrías, A.; Tomás-Barberán, F.A. The gut microbiota: A key factor in the therapeutic effects of (poly)phenols. Biochem.Pharmacol. 2017, 139, 82–93. [Google Scholar] [CrossRef]
- Marchesi, J.R.; Adams, D.H.; Fava, F.; Hermes, G.D.A.; Hirschfield, G.M.; Hold, G.; Quraishi, M.N.; Kinross, J.; Smidt, H.; Tuohy, K.M.; et al. Hart, The gut microbiota and host health: A new clinical frontier. Gut 2016, 65, 330–339. [Google Scholar] [CrossRef]
- Hervert-Hernández, D.; Goñi, I. Dietary polyphenols and human gut microbiota: A review. Food Rev. Int. 2011, 27, 154–169. [Google Scholar] [CrossRef]
- Cardona, F.; Andrés-Lacueva, C.; Tulipani, S.; Tinahones, F.J.; Queipo-Ortuño, M. Benefits of polyphenols on gut microbiota and implications in human health. J. Nutr.Biochem. 2013, 24, 1415–1422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aura, A.M. Microbial metabolism of dietary phenolic compounds in the colon. Phytochem. Rev. 2008, 7, 407–429. [Google Scholar] [CrossRef]
- Rechner, A.R.; Smith, M.A.; Kuhnle, G.; Gibson, G.R.; Debnam, E.S.; Srai, S.K.S.; Moore, K.P.; Rice-Evans, C.A. Colonic metabolism of dietary polyphenol: Influence of structure on microbial fermentation products. Free Radic. Biol. 2004, 36, 212–225. [Google Scholar] [CrossRef]
- Selma, M.A.; Espín, J.C.; Tomás-Barberán, F.A. Interaction between phenolics and gut microbiota: Role in human health. J. Agric. Food Chem. 2009, 57, 6485–6501. [Google Scholar] [CrossRef] [PubMed]







| No. | Compounds | tR (min) | Exptl (m/z) | Calcd (m/z) | Fragment Ions (m/z) | Content (mg/g) |
|---|---|---|---|---|---|---|
| 1 | 2,3-Dihydroxybenzoic acid | 2.26 | 153.0193 | 153.0193 | 135.0 (5), 117.0 (10), 109.0 (10), 91 (5), 73.0 (5) | 0.0111 |
| 2 | 4-Hydroxybenzoic acid | 2.72 | 137.0244 | 137.0244 | 119.0 (50), 93.0 (10), 75.0 (5) | - |
| 3 | 2,4-Dihydroxybenzoic Acid | 3.20 | 153.0193 | 153.0193 | 135.0 (50), 117.0 (10), 109.0 (50), 91 (5), 73.0 (5) | 0.0673 |
| 4 | 5-Methoxysalicylic acid | 3.28 | 167.0349 | 167.035 | 149.0 (20), 123.0 (10) | 0.0176 |
| 5 | Rutin | 3.49 | 609.1462 | 609.1461 | 301.0 (100) | 0.5375 |
| 6 | Kaempferol 3-O-β-rutinoside | 3.62 | 593.1512 | 593.1512 | 285.0 (10) | - |
| 7 | Quercetin 3-β-D-glucoside | 3.65 | 463.0883 | 463.0882 | 301.1 (10), 179.1(10) | - |
| 8 | 4-Hydroxycoumarin | 3.90 | 161.0245 | 161.0244 | 143.0 (40), 133.0 (10), 117.0 (20), 115.0 (5), 99.0 (5) | - |
| 9 | Naringenin | 4.84 | 271.0612 | 271.0612 | 271.1 (40), 165.0 (40) | 1.8934 |
| 10 | Apigenin | 4.88 | 269.0455 | 269.0455 | 151.0 (15) | - |
| 11 | Chrysin | 5.73 | 253.0506 | 253.0506 | 151.1 (25) | 0.5627 |
| 12 | Isoliquiritigenin | 5.77 | 255.0663 | 255.0663 | 119.0 (20) | 0.2830 |
| TPC (mg GA/g) | TFC (mg rutin/g) | DPPH Scavenging Activity IC50 (mg /mL) | FRAP (mgTrolox/g) | Ferrous Ion-Chelating Activity (mg Na2EDTA/g) |
|---|---|---|---|---|
| 101.83 ± 0.01 | 73.22 ± 0.04 | 0.74 ± 0.05 | 297.80 ± 0.92 | 44.82 ± 0.60 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, N.; Chen, S.; Liu, X.; Zhao, H.; Cao, W. Impact of SchisandraChinensis Bee Pollen on Nonalcoholic Fatty Liver Disease and Gut Microbiota in HighFat Diet Induced Obese Mice. Nutrients 2019, 11, 346. https://doi.org/10.3390/nu11020346
Cheng N, Chen S, Liu X, Zhao H, Cao W. Impact of SchisandraChinensis Bee Pollen on Nonalcoholic Fatty Liver Disease and Gut Microbiota in HighFat Diet Induced Obese Mice. Nutrients. 2019; 11(2):346. https://doi.org/10.3390/nu11020346
Chicago/Turabian StyleCheng, Ni, Sinan Chen, Xinyan Liu, Haoan Zhao, and Wei Cao. 2019. "Impact of SchisandraChinensis Bee Pollen on Nonalcoholic Fatty Liver Disease and Gut Microbiota in HighFat Diet Induced Obese Mice" Nutrients 11, no. 2: 346. https://doi.org/10.3390/nu11020346