1. Introduction
Breastfed infants have a growth pattern different from formula fed infants. They grow more rapidly during the first 1–2 months (mo) and then more slowly—both weight gain and linear growth—in the first years [
1,
2,
3]. There are also differences in body composition as breastfed infants accrue more fat than formula fed infants during the first 6 mo [
4]. However, there is emerging evidence that human milk (HM) composition has an effect on growth, and some of the differences in growth within breastfed infants can be explained by differences in milk composition [
5,
6]. Recently there is emerging evidence that concentrations of the two hormones leptin and adiponectin in breast milk are related to appetite and infant growth [
5,
7].
Some infants experience excessive weight gain (EWG) during the period they are exclusively breastfed (EBF) and have a marked catch-down when complementary foods are introduced, suggesting that the EWG is caused by breastfeeding [
8,
9,
10]. Studies investigating this group of infants are sparse and little is known about causes for the rapid weight gain and potential consequences. In the two case reports [
8,
9] it is suggested that a high protein intake is the cause. In an observational cohort study of 73 infants with EWG during EBF the growth pattern was different from the case reports because growth velocity continued to be high, also after complementary foods were introduced [
11].
There is convincing evidence from several meta-analyses that a high weight gain during infancy, especially the first six months, is associated with an increased risk of overweight, obesity, and metabolic complications later in life [
12,
13,
14,
15]. However, most of these meta-analyses did not analyze the risk according to early feeding method. There is some evidence that breastfeeding reduces the risk of later overweight and obesity [
16], but it is highlighted that observed associations could be due to residual confounding or reverse causation [
17,
18]. In a prospective population-based birth cohort study including 3000 children, overweight in infancy (≥1 standard deviation (SD) body-mass-index (BMI)-for-age (BAZ)) was associated with increased odds of childhood overweight regardless of feeding mode [
19].
Although the case reports suggest that at least some breastfed infants with EWG show catch-down growth when other foods are introduced, it is important to examine if these infants are at risk of later obesity and metabolic complications. A small exploratory cohort of infants with EWG during EBF was established in order to identify predictors and consequences of the EWG and a cohort with normal weight gain was recruited for comparison.
2. Materials and Methods
2.1. Subjects and Recruitment
Participants were part of an ongoing prospective observational cohort study, the SKOT III cohort, consisting of two groups; a high weight gain group (HW-group) and a normal weight gain group (NW-group).
EBF infants considered to have excessively high weight-for-age were referred via Danish health workers who had been informed about the study through postal letters and social media from August 2015 to October 2016. High weight was defined as infants aged 4–6 mo having a weight-for-age z-score (WAZ) >2.00. The infants were qualified for enrollment to the HW-group if they had an increment of at least +1 SDS in WAZ within the first 5 mo postpartum. The NW-group was recruited through the National Civil Registry. Families from the Copenhagen area (n = 805) with infants born between 15 July 2016 and 31 August 2016 were invited by postal invitation to participate. Inclusion criteria for the NW-group were infants aged 4–6 mo with a WAZ between −1.0 and +1.0 SDS. Other inclusion criteria were similar for both groups: exclusively or fully breastfed (defined as receiving HM as their primary energy intake but were allowed to have water, vitamins, minerals and a maximum of one meal of formula per week) for at least 4 mo. At first visit the infants were to be 5–6½ mo old and have breastfeeding as the primary energy source not receiving more than two meals of solid food per day. Both HW- and NW-group mother-infant dyads were examined at infant’s age 5–6½ mo and 9 mo ± 2 weeks, and the HW-group further at 18 mo ± 4 weeks.
The study protocol was approved by the Regional Ethical Committee of the Capital Region of Denmark in accordance with the Helsinki declaration (H-15008948) and the Data Protection Agency (2015-57-0117 & 2015-57-0116) and written informed consent was obtained from parents.
2.2. Anthropometry
All anthropometric measurements at the three visits were carried out at the Department of Nutrition, Exercise and Sports, University of Copenhagen, Denmark. Weight and length from birth to 5 mo measured by health nurses and general practitioners, were reported by the parents and used for assessing recruitment criteria.
The weight of naked infants was measured using a digital pediatric scale giving an average of 40 measurements recorded to the nearest gram (Tanita BD 815 MA digital baby scale, Tanita Corporation, Tokyo, Japan). The following anthropometric measurements were performed in triplicate and the average was used: recumbent length to the nearest 0.1 cm (447 Infantronic Digital Infantometer, QuickMedical, Seattle, WA, USA), mid-upper-arm, head, lower leg, recumbent waist and thorax circumferences to the nearest millimeter (nonflexible Lasso-O tape measure, Child Growth Foundation, London, UK), and triceps and subscapular skinfold thickness to the nearest 0.1 mm (Harpenden skinfold caliper, Chasmors Ltd., London, UK). Sex and age specific
z-scores; WAZ, length-for-age
z-score (LAZ), BAZ, triceps skinfold-for-age (TSFZ) and subscapular skinfold-for-age (SSFZ) were obtained using the software WHO Anthro [
20].
Mothers’ pre-pregnancy weight, pregnancy weight gain, and the fathers’ weight and height were self-reported, while the mothers’ weight and height were measured at all three visits (weight: Tanita digital medical scale, WB-100MA, Tanita Corporation, Tokyo, Japan; height: Static digital height measurer, 235 Heightronic Digital Stadiometer, Issaquah, WA, USA).
2.3. Body Composition
Body composition was measured using a tetrapolar Bioelectrical Impedance Analyzer (BIA) Quantum III (RJL Systems, Clinton Township, Detroit, MI, USA) which uses single frequency (50 kHz) between right hand and right foot to measure whole body resistance. Prediction of fat free mass (FFM) for each infant was completed using an equation developed by Lingwood et al. [
21]. Details on BIA measurement and equation are included in
Appendix A1; Supplemental method description.
2.4. Measurements of 24-h Infant Milk Intake
Mothers were instructed to use an electronic baby weighing scale (Tanita BD 815 MA, Tanita Corporation, Tokyo, Japan) for weighing their infants to measure the 24 h milk intake at 5 mo. For a period of 72 h mothers weighed their infants before and after each breastfeeding-session (each feed) without change in clothing or diaper. Calculation of intake in grams was done by subtracting weight of the infant before the feed from the weight after the feed. In cases where test weighing was not completed for all feeds, the intake was estimated using an average of intake per feed calculated from the mother’s registration. The first feed after the 72 h period was used as the end point for registration period, and in cases where these data were missing it was estimated from the three previous days. A corrected 24 h milk intake for each infant was determined by dividing the intervals in hours and multiplying by 24. No correction for infant insensible water loss was made, and therefore the milk intake is likely to be underestimated by 3–10% [
22,
23]. Feeding frequency and duration of each feed was derived from the test weighing report. Energy intake from foods and drinks other than HM was calculated using Dankost (Dankost version 3000, Dankost Ltd., Copenhagen, Denmark).
2.5. Human Milk Collection and Analysis
At the 5 and 9 mo visits the mothers were asked to collect 10 mL foremilk and 10 mL hind milk from either breast and on a second time point to complete expression of both breasts. Samples were collected in their homes in disposable bottles (2 × 80 mL/2 × 250 mL) using a manual breast pump (Type Harmony™, Medela AG, Baar, Switzerland) and stored at −20 °C. The samples were transported in a bag with an ice pack and stored at −80 °C.
HM analysis were carried out in one mixed sample of a full expression of right and left breast. Macronutrient and energy content was analyzed by mid-infrared spectroscopy using the HM analyzer (MIRIS HMA; Miris AB, Uppsala, Sweden). The analysis was performed according to manufacturer’s instructions. The milk was heated to 40 °C and homogenized by ultrasonic processing before injected into the system.
Biochemical analyses of milk leptin, adiponectin, lysozyme, sIgA and lactoferrin were carried out at the University of Western Australia. Milk adiponectin concentration was determined by Enzyme-Linked Immunosorbent Assay (ELISA) kits (RD191023100, BioVendor-Laboratorni medicina a.s., Brno, Czech Republic). Milk leptin (m-leptin) concentration was determined by the R&D systems leptin DuoSet ELISA kit (DY398, R&D Systems Inc., Minneapolis, MN, USA). The recovery of a known amount of leptin added to the milk samples was 97 ± 3.0% (
n = 4). The detection limit of this assay was 0.016 ng/mL. Milk lactoferrin and secretory immunoglobulin A (sIgA) were measured with a sandwich ELISA method as previously described by Czank et al. [
24]. Lysozyme concentration was measured with a modified turbidimetric assay [
25]. Details on the methods are included in
Appendix A2; Supplemental method description.
2.6. Blood Sampling and Analysis
Venous blood samples (10 mL) were taken at 5 and 9 mo visits. Local anesthetic patches (EMLA, AstraZeneca AB, Södertälje, Sweden) and instructions were given to the parents before first visit. If possible, infants were fasting 2 h before blood samples were taken and the time, amount and composition of last meal were recorded. Methods for blood analyses are described in
Appendix A3; Supplemental method description.
2.7. Feeding Patterns and Other Information
Information on parental characteristics and feeding practice was collected via interviews and questionnaires at 5 and 9 mo visits for both groups and at 18 mo visit for the HW-group.
Appetite traits were measured at the 5 mo visit using the Baby Eating Behavior Questionnaire (BEBQ) [
26]. BEBQ includes 4 appetite traits; food responsiveness (6 items), enjoyment of food (4 items), satiety responsiveness (3 items), slowness in eating (4 items) and general appetite (1 item). All items were scored on a 5-point Likert scale ranging from never (1) to always (5). High mean score signify a greater enjoyment of food, higher food responsiveness, slow eating, greater satiety responsiveness and larger general appetite.
2.8. Statistical Analysis
Statistical analyses were carried out using STATATM12 (Stata Corporation, College Station, TX, USA). Significance was defined as p-value < 0.05. Parametric descriptive results are presented as means and SD, or mean and range. Independent t-test was used to test differences between the characteristics of the two study groups. Differences between groups for the anthropometric measures and blood parameters adjusted for sex (glucose, insulin, leptin, and Insulin-like Growth Factor-1 (IGF-I) and fasting time (glucose and insulin) were analyzed by general linear models. Non-normally distributed variables are presented as medians with interquartile range (IQR: 25th and 75th percentile), and Mann-Whitney U test used for comparing the study groups. Categorical variables are presented as number and percentage and comparisons were performed using Fisher’s exact test. Associations between the different macronutrients, between macronutrients/hormones and WAZ, BAZ and LAZ change, and between mothers BMI and milk composition were investigated by linear regression, for the groups separately and combined.
4. Discussion
We identified a group of breastfed infants who had a special growth pattern with EWG during the first 5 mo of EBF and catch-down during the following months, after beginning complementary foods. This growth pattern suggests that a key cause of the high weight gain should be found in factors related to HM intake and composition. There was surprisingly no difference in HM intake at the age of 5 mo between the groups. However, leptin content was significantly lower in the milk the HW-group received, while s-leptin was significantly higher in the HW-group compared to the NW-group. This suggests that leptin intake and metabolism could contribute in part to EWG in these infants.
The weight gain of infants in the HW-group was excessive. The mean weight gain per week was about 60% higher than in the NW-group, which had a weight gain close to the median weight gain in the WHO growth standard [
20]. Catch-up growth is often defined as an increase in WAZ > 0.67
z-scores [
15,
27] and the mean increase in the HW-group from birth to the 5 mo visit was 2.5 times as high (1.71
z-scores). Interestingly, the two groups had almost the same gain in weight and length from the 5 mo to the 9 mo visit; a gain not very different from the median in the WHO growth standard velocities [
20]. From the 9 to the 18 mo visit there was a continued decrease in WAZ and BAZ in the HW group, but to what degree this will continue and reach the normal range is not known. As outlined in the introduction, there is convincing evidence that early weight gain is associated with a higher risk of later overweight and obesity [
15,
27,
28,
29,
30]. However, some studies show that breastfeeding has a modifying effect on the risk [
31], while other studies do not [
19]. The risk of later overweight and obesity in EBF infants with EWG is still unknown.
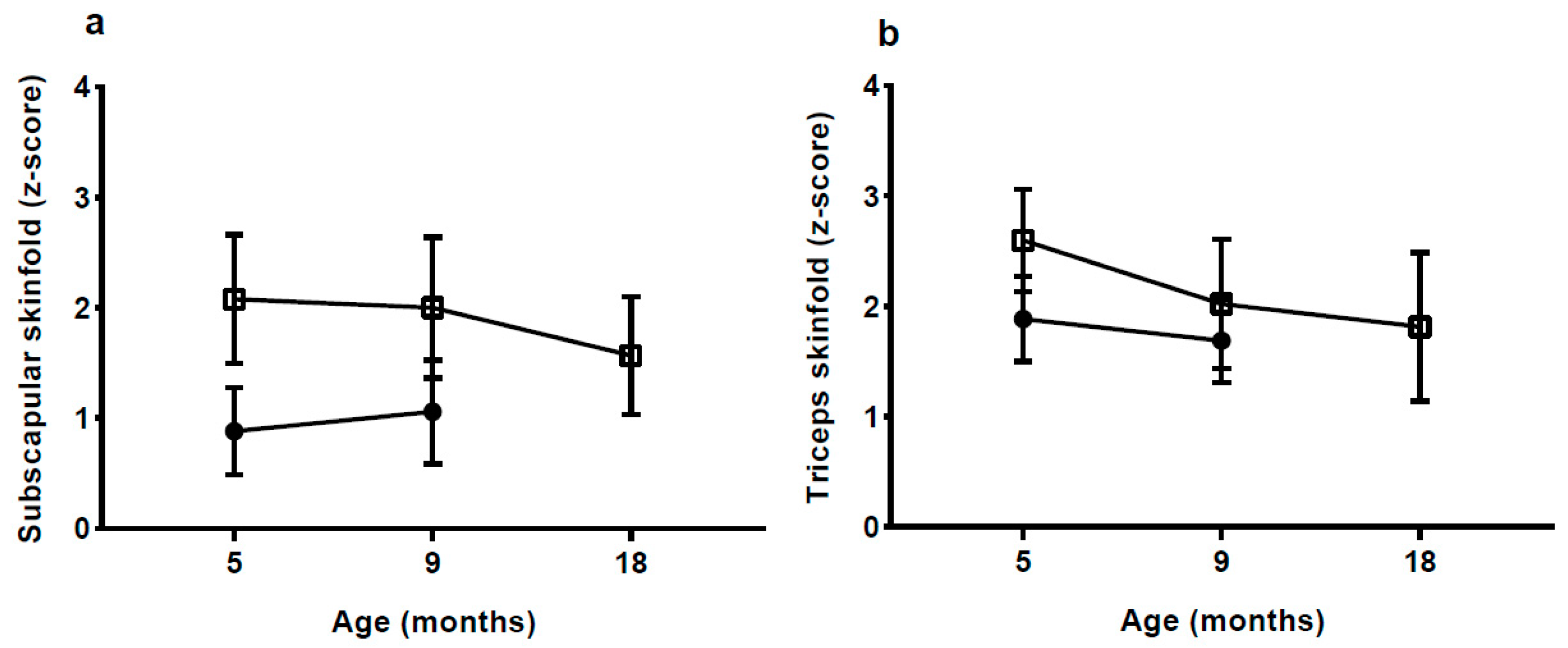
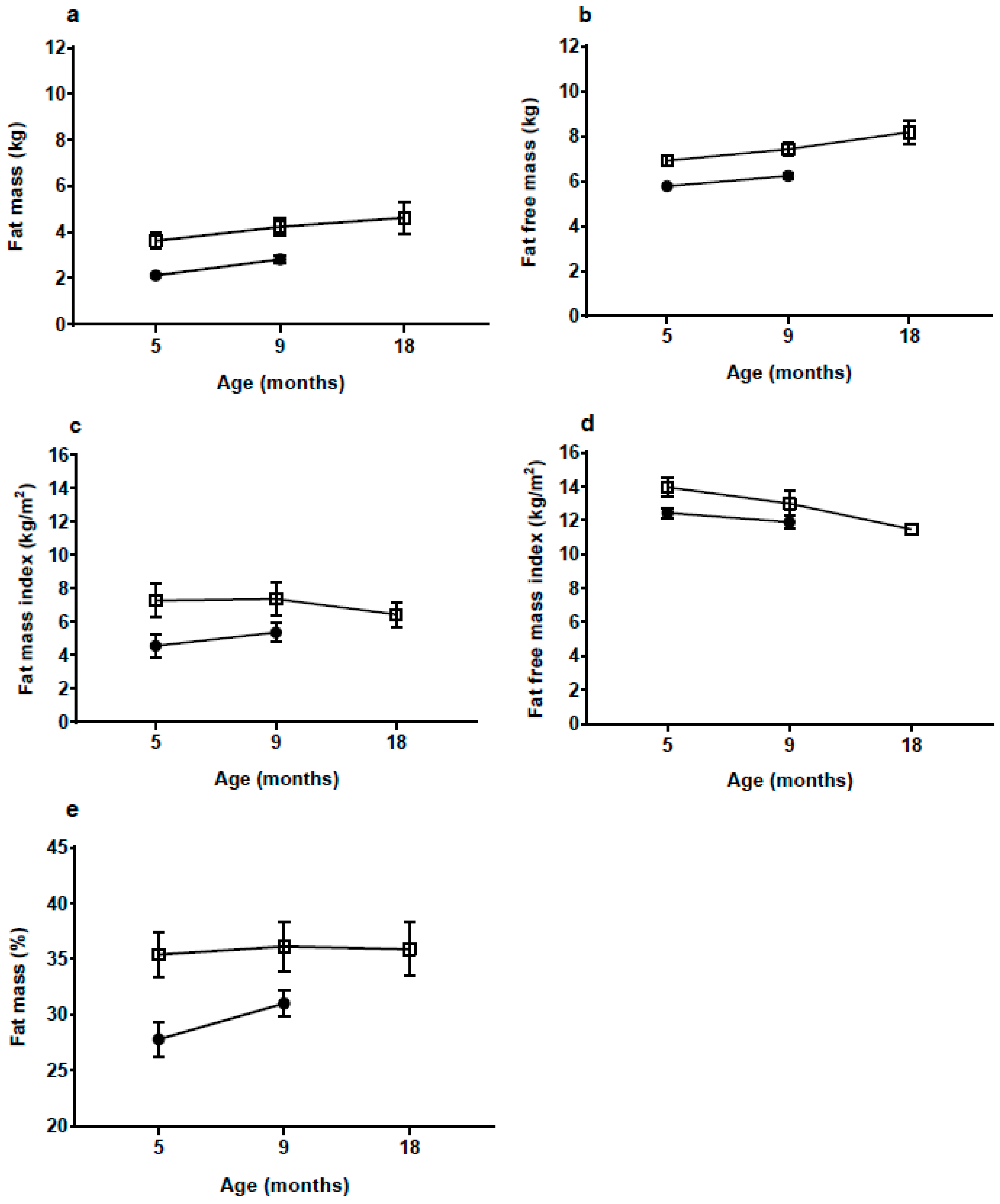
With a body FM of 3.6 kg at 5 mo, which is 70% more than the NW-group, the rate of fat deposition from birth to 5 mo must have been much greater in the HW-group. Although there was some decrease in BMI and skinfold
z-scores from 5 to 18 mo in the HW group, %FM and fat mass index (FMI) continued to be high up to 18 mo, whereas the difference in fat-free mass index appeared to be decreasing. The medium-term effects of EWG therefore appear to manifest in both elevated length and adiposity. Links between accelerated linear growth and adiposity have been reported previously in the early post-natal period [
32] and might indicate growth restriction early in pregnancy followed by the development of catch-up in late fetal life and infancy [
33], resulting in ‘overshoot’ by early childhood of both length and adiposity.
The significant higher birth weight in the HW-group might also be a result of a different intra-uterine environment, partly because of the higher maternal pre-pregnancy weight in the HW-group. However, we find it unlikely that these differences between the groups could have a significant role in explaining the very large differences in post-natal growth between the groups.
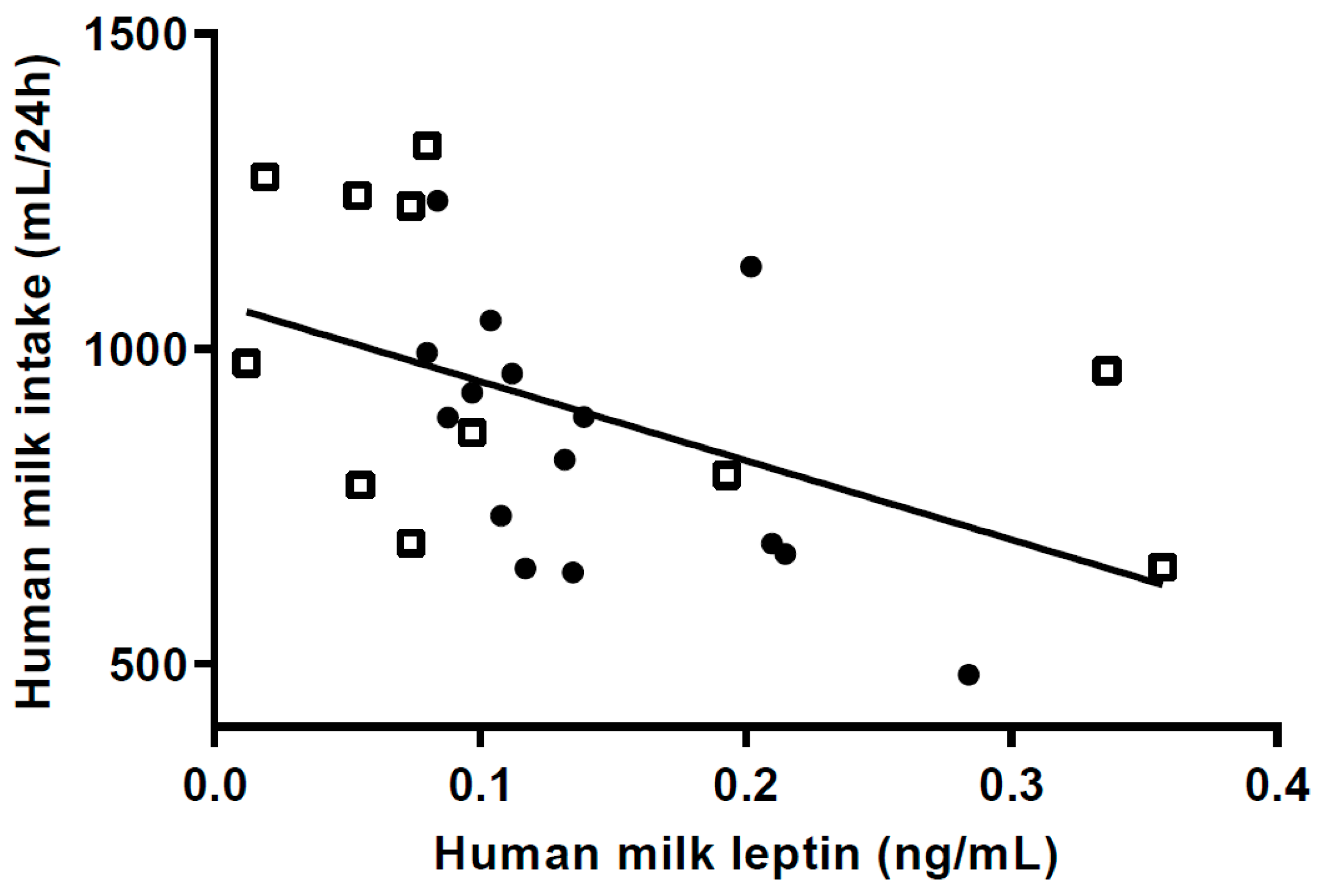
HM intake is a strong driver of infant growth [
34,
35]. Therefore it was surprising, that milk intake was not significantly higher in the HW-group. The reason for this is likely to be the timing of measuring milk intake, at 5–6 mo, when infant growth had started to decline and complementary feeding had begun for most infants. However, though far from significant (
p = 0.19), the intake was 15% higher in the HW-group. If there has been a difference of this magnitude throughout the first 5 mo it is likely to have an effect on growth. From 5 to 9 mo weight gain in the two groups was almost identical, and therefore the energy requirements might not have been different when milk intake was measured. Ideally, measurement of milk volume should have been carried out earlier e.g., between 1 and 4 mo of age when growth velocity was considerably higher; however, recruitment precluded this. Another reason that we did not find a significant difference could be the limited power in the study. In three of the cases reported earlier, 24 h milk intake at age 4 to 5.5 mo was high; 1123, 1132 and 1421 mL [
9,
10]. Mothers in the HW-group rated their infants as having higher appetite and being less sensitive to internal cues of satiety, which could reflect a higher milk intake earlier, but this is speculative.
Leptin influences the regulation of appetite by inducing satiety [
36] providing a rationale for low m-leptin levels to be associated with increased milk intake in breastfed infants [
7,
36,
37]. While transfer of m-leptin to infant plasma is one pathway by which leptin may regulate appetite control another is the presence of gastric leptin receptors which would more likely induce a more rapid infant response [
7].
Lower levels of m-leptin content have been associated with greater infant length at 1 mo postpartum, and FM at 6 mo in EBF infants [
38], although the association between milk leptin and growth is by no means conclusive [
7]. In our study, M-leptin content in the HW-group was 38% lower than in the NW-group, and higher m-leptin was associated with lower milk intake at 5 mo, supporting the speculation that HW infants consumed more milk in the first 4 months post-partum.
Low m-leptin levels may lead to higher milk intakes as a result of greater suckling pressure (frequency) in the HW-gain group. This phenomenon is generally associated with maternal malnutrition and infant catch-up growth [
39,
40] reducing milk leptin content so as to lower infant satiety and promote higher milk transfer. However increased sucking frequency has also been shown in the 3 month old infants of obese mothers [
41]. Moreover, appetite has a strong genetic basis [
41], which could account for correlations between maternal BMI and infant appetite. These factors could explain the substantial catch-up overshoot if the mother is in fact well-nourished and able to provide more milk [
42,
43]. Further these combined findings support the assumption that the EWG and increased accrual of fat in our HW-group could be in part driven by a high intake of HM during the months where there was rapid weight gain, despite our failure to show differences in milk intake. While we found a positive association of maternal BMI with m-leptin, as reported by others [
44], this included all of the participants. Comparing between groups, the HW-group mothers had significantly lower m-leptin than the NW-group, despite having higher average BMI. This again suggests some facultative response in milk composition in the HW-group, potentially mediated by elevated infant suckling and/or genetic factors.
Mean infant s-leptin values were markedly higher in the HW-group at both 5 and 9 mo. This can be explained at least partly by the higher FM in the HW-group, as s-leptin is mainly produced by fat tissue [
36,
37]. Within the whole sample, we also observed strong correlations between 5 mo s-leptin values and infant weight and FM. This is consistent with other studies reporting associations between s-leptin and BMI and %FM in early childhood [
45,
46,
47].
A small group of infants have been shown to have very high leptin levels during the first 6 mo, with a value for the 90th percentile about four times as high as the median (11.86 vs. 2.81 ng/mL) [
48]. Our values show comparable differences between the HW- and the NW-group (7.77 vs. 3.70 ng/mL). Recently, three single nucleotide polymorphisms (SNPs) have been shown to influence s-leptin levels in infants below 6 mo [
49]. Thus, we speculate that the very high s-leptin levels in our HW-group might have a genetic basis [
49]; however, this needs to be confirmed.
Leptin is considered an anorexigenic hormone but it has been suggested that during early infancy some infants are likely to develop leptin resistance. It has been suggested, based on studies with mice, that high s-leptin levels, during an early postnatal leptin surge, can influence the hypothalamus and thus increase appetite and thereby growth [
50,
51,
52]. The very high s-leptin values in some 0–6 mo old infants [
48], and the very high values in our HW-group could reflect that some infants have a postnatal leptin surge. However, this is speculative.
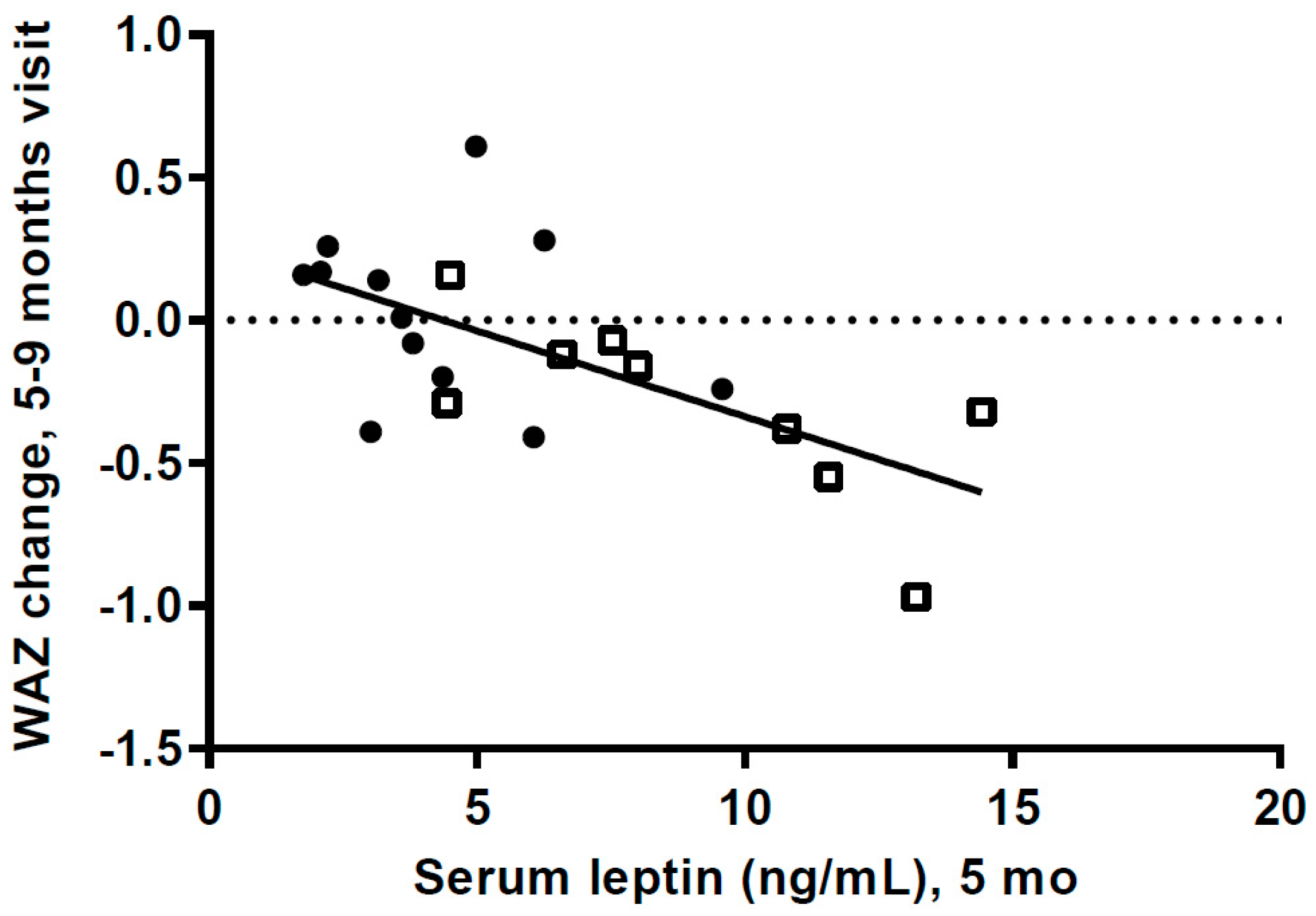
We do not know the s-leptin levels of the HW infants when weight gain was very high during the first postnatal months. However, if leptin levels were very high and leptin resistance had developed it could have an effect on milk intake and growth. The high s-leptin levels at 5 mo could also be a result of the high FM. Our data, showing a highly significant negative association between s-leptin levels at 5 mo and weight gain from 5 to 9 mo, could be a result of decreased leptin resistance when other foods were introduced, with s-leptin then having an anorexigenic effect.
Overall, it is remarkable that in this relatively small cohort there were significant differences in both m-leptin and s-leptin levels, suggesting that leptin could have a role in the excessive weight gain in the HW group. However, the potential mechanism we have discussed here are speculative and there is a need for larger studies to investigate the potential role of leptin in excessive weight gain in exclusively breastfed infants.
Other potential factors that could have an effect on the high weight gain in the HW-group are the effect of milk composition on the microbiota. Both HM oligosaccharides (HMO) and leptin have been shown to be independently associated with breastfed infants gut bacteria, which could have an effect on intestinal energy harvest [
5,
53,
54]. It has been shown that HMO can influence weight gain and body fat accretion, most likely through an effect on the microbiota [
55]. Differences in early energy metabolism and expenditure between the groups could also influence weight gain. Genes or epigenetics might also play a role, e.g., some SNPs have been shown to influence s-leptin levels [
49].
A limitation is that it was not possible to identify, recruit, and arrange clinical visits before the age of 5 to 6 mo, by which time growth velocity was decreasing and some infants had started on complementary foods. If we had been able to measure milk intake when growth velocity was considerably higher, assessment of milk intake, milk composition and blood would most likely have given a better understanding of why these infants experience such a high weight gain. Furthermore, the power of the study was not very high due to the relatively small number of infants included. We have made multiple comparisons, but as the number of infants in the groups was small and as we have had an explorative approach, we have not controlled for this.
Aside from these limitations, this study is the first to bring valuable knowledge for future studies designed to investigate the causes of EWG in exclusively breastfed infants further. A strength of the study is, that it is the first cohort of breastfed infants with EWG during the first 6 mo followed by catch-down, which has been followed longitudinally with detailed measurements of growth, body composition, HM intake and blood samples.