Dietary Fructose Increases the Sensitivity of Proximal Tubules to Angiotensin II in Rats Fed High-Salt Diets
Abstract
:1. Introduction
2. Materials and Methods
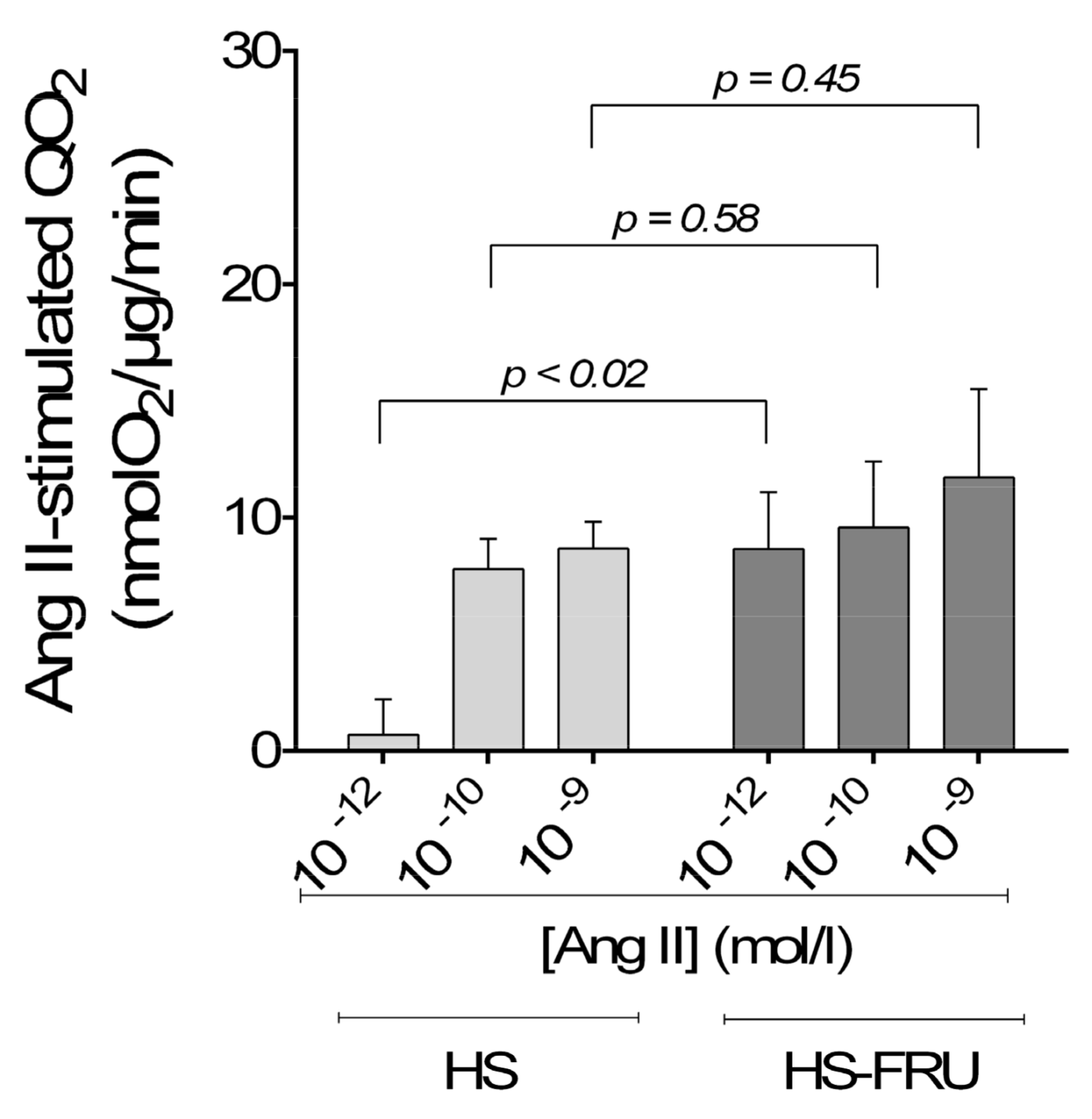
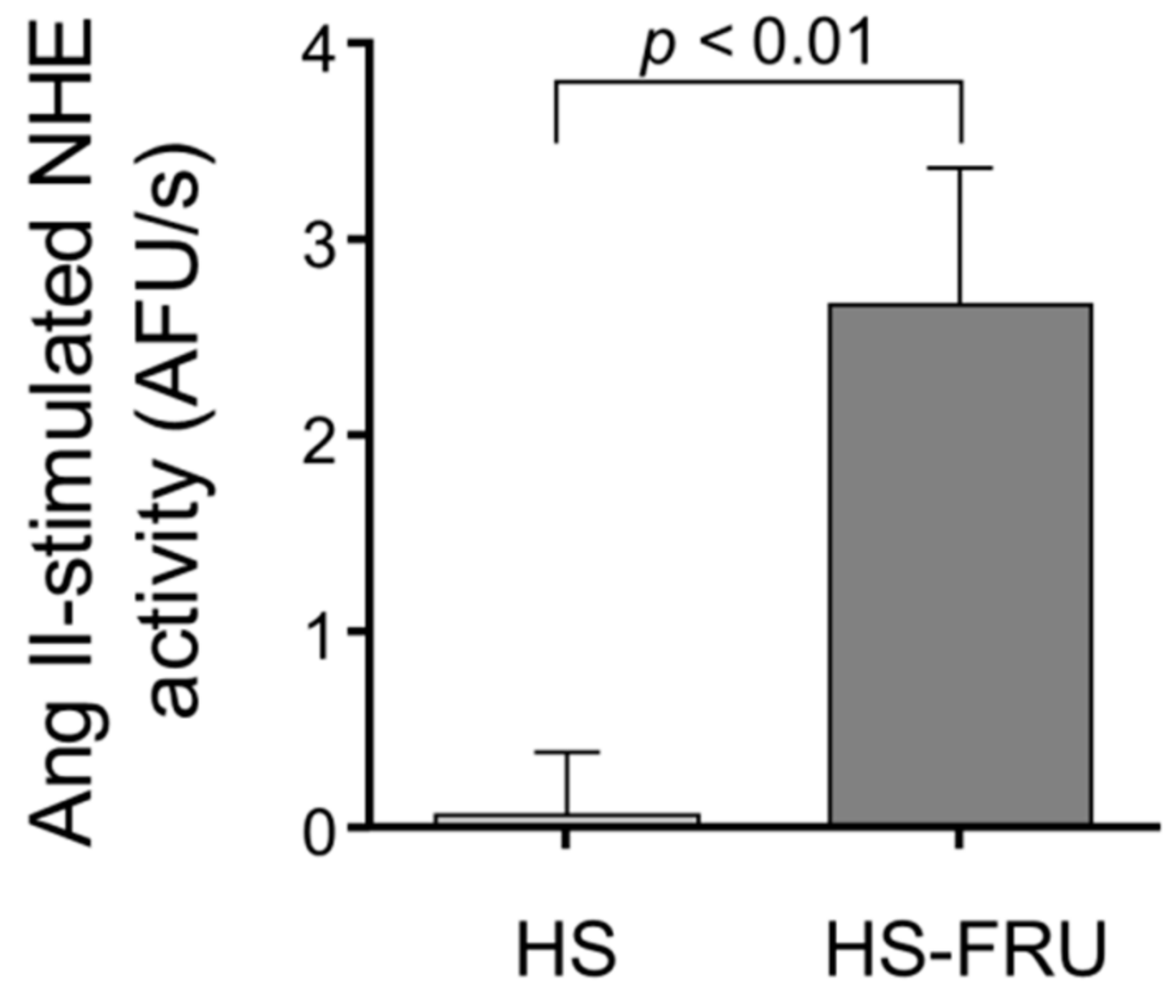
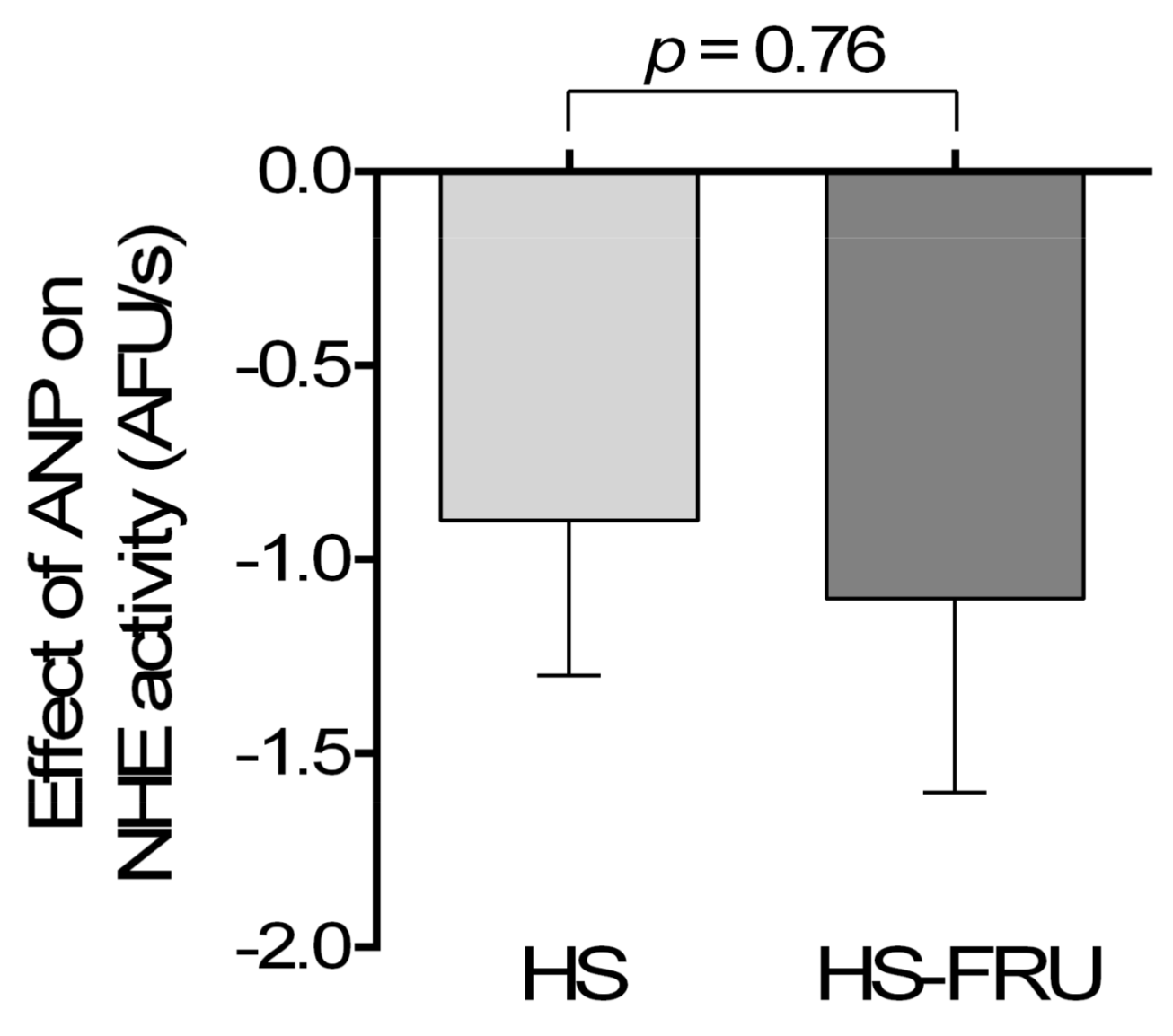
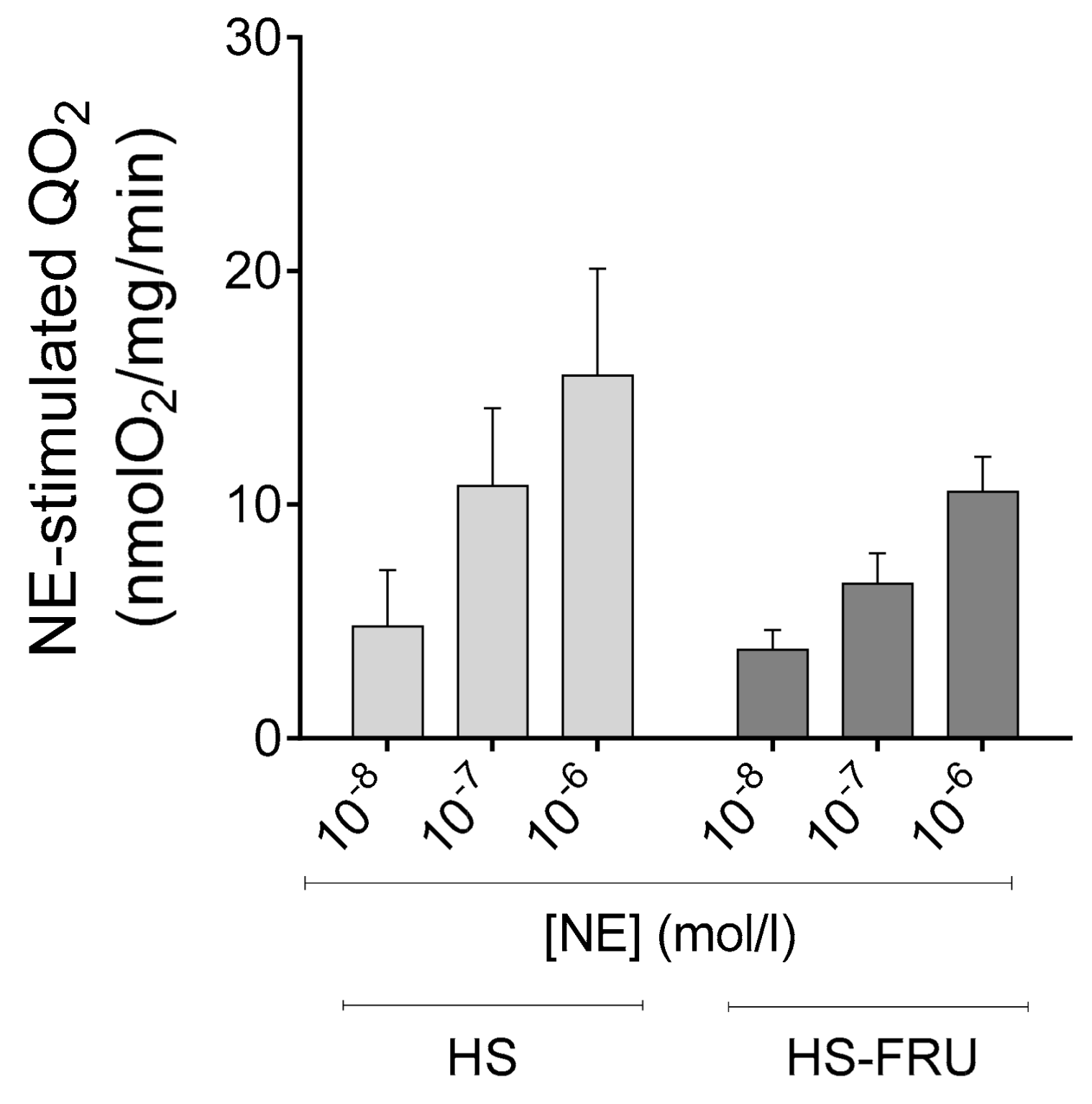
3. Results
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Cordain, L.; Eaton, S.B.; Sebastian, A.; Mann, N.; Lindeberg, S.; Watkins, B.A.; O’Keefe, J.H.; Brand-Miller, J. Origins and evolution of the western diet: Health implications for the 21st century. Am. J. Clin. Nutr. 2005, 81, 341–354. [Google Scholar] [CrossRef] [PubMed]
- Powles, J.; Fahimi, S.; Micha, R.; Khatibzadeh, S.; Shi, P.; Ezzati, M.; Engell, R.E.; Lim, S.S.; Danaei, G.; Mozaffarian, D.; et al. Global, regional and national sodium intakes in 1990 and 2010: A systematic analysis of 24 h urinary sodium excretion and dietary surveys worldwide. BMJ Open 2013, 3, e003733. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hipgrave, D.B.; Chang, S.; Li, X.; Wu, Y. Salt and sodium intake in china. JAMA 2016, 315, 703–705. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention: Sodium Fact Sheet. Available online: https://www.cdc.gov/dhdsp/data_statistics/fact_sheets/fs_sodium.htm (accessed on 8 August 2018).
- National Health and Nutrition Examination Survey (NHANES). Available online: https://www.cdc.gov/nchs/nhanes/wweia.htm (accessed on 8 August 2018).[Green Version]
- World Health Organization. Guideline: Sodium Intake for Adults and Children; World Health Organization: Geneva, Switzerland, 2012. [Google Scholar]
- Dietary Guidelines for Americans 2015–2020. Available online: https://health.gov/dietaryguidelines/2015/guidelines/ (accessed on 8 August 2018).
- Sluik, D.; Engelen, A.I.; Feskens, E.J. Fructose consumption in the netherlands: The dutch national food consumption survey 2007–2010. Eur. J. Clin. Nutr. 2015, 69, 475–481. [Google Scholar] [CrossRef] [PubMed]
- Montonen, J.; Jarvinen, R.; Knekt, P.; Heliovaara, M.; Reunanen, A. Consumption of sweetened beverages and intakes of fructose and glucose predict type 2 diabetes occurrence. J. Nutr. 2007, 137, 1447–1454. [Google Scholar] [CrossRef] [PubMed]
- Vos, M.B.; Kimmons, J.E.; Gillespie, C.; Welsh, J.; Blanck, H.M. Dietary fructose consumption among us children and adults: The third national health and nutrition examination survey. Medscape J. Med. 2008, 10, 160. [Google Scholar] [PubMed]
- Grasser, E.K.; Dulloo, A.; Montani, J.P. Cardiovascular responses to the ingestion of sugary drinks using a randomised cross-over study design: Does glucose attenuate the blood pressure-elevating effect of fructose? Br. J. Nutr. 2014, 112, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Brown, C.M.; Dulloo, A.G.; Yepuri, G.; Montani, J.P. Fructose ingestion acutely elevates blood pressure in healthy young humans. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008, 294, R730–R737. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Endo, M.Y.; Fujihara, C.; Yamazaki, C.; Kashima, H.; Eguchi, K.; Miura, A.; Fukuoka, Y.; Fukuba, Y. Acute responses of regional vascular conductance to oral ingestion of fructose in healthy young humans. J. Physiol. Anthropol. 2014, 33, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siqueira, J.H.; Mill, J.G.; Velasquez-Melendez, G.; Moreira, A.D.; Barreto, S.M.; Bensenor, I.M.; Molina, M. Sugar-sweetened soft drinks and fructose consumption are associated with hyperuricemia: Cross-sectional analysis from the brazilian longitudinal study of adult health (elsa-brasil). Nutrients 2018, 10, 981. [Google Scholar] [CrossRef] [PubMed]
- Weber, K.S.; Simon, M.C.; Strassburger, K.; Markgraf, D.F.; Buyken, A.E.; Szendroedi, J.; Mussig, K.; Roden, M.; Group, G.D.S. Habitual fructose intake relates to insulin sensitivity and fatty liver index in recent-onset type 2 diabetes patients and individuals without diabetes. Nutrients 2018, 10, 774. [Google Scholar] [CrossRef] [PubMed]
- Bahadoran, Z.; Mirmiran, P.; Tohidi, M.; Azizi, F. Longitudinal associations of high-fructose diet with cardiovascular events and potential risk factors: Tehran lipid and glucose study. Nutrients 2017, 9, 872. [Google Scholar] [CrossRef] [PubMed]
- Jalal, D.I.; Smits, G.; Johnson, R.J.; Chonchol, M. Increased fructose associates with elevated blood pressure. J. Am. Soc. Nephrol. 2010, 21, 1543–1549. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, D.S.; Peterson, K.E.; Gortmaker, S.L. Relation between consumption of sugar-sweetened drinks and childhood obesity: A prospective, observational analysis. Lancet 2001, 357, 505–508. [Google Scholar] [CrossRef]
- Nguyen, S.; Choi, H.K.; Lustig, R.H.; Hsu, C.Y. Sugar-sweetened beverages, serum uric acid, and blood pressure in adolescents. J. Pediatr. 2009, 154, 807–813. [Google Scholar] [CrossRef] [PubMed]
- Schulze, M.B.; Manson, J.E.; Ludwig, D.S.; Colditz, G.A.; Stampfer, M.J.; Willett, W.C.; Hu, F.B. Sugar-sweetened beverages, weight gain, and incidence of type 2 diabetes in young and middle-aged women. JAMA 2004, 292, 927–934. [Google Scholar] [CrossRef] [PubMed]
- China Industrial Information. Available online: https://www.chyxx.com/industry/201405/248688.html (accessed on 8 August 2018).
- National Bureau of Statistics of China. Available online: http://data.stats.gov.cn/easyquery.htm?cn=C01&zb=A0I0904&sj=2016 (accessed on 8 August 2018).
- Gordish, K.L.; Kassem, K.M.; Ortiz, P.A.; Beierwaltes, W.H. Moderate (20%) fructose-enriched diet stimulates salt-sensitive hypertension with increased salt retention and decreased renal nitric oxide. Physiol. Rep. 2017, 5, e13162. [Google Scholar] [CrossRef] [PubMed]
- Zenner, Z.P.; Gordish, K.L.; Beierwaltes, W.H. Free radical scavenging reverses fructose-induced salt-sensitive hypertension. Integr. Blood Press. Control 2018, 11, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.Y.; Boini, K.M.; Friedrich, B.; Metzger, M.; Just, L.; Osswald, H.; Wulff, P.; Kuhl, D.; Vallon, V.; Lang, F. Blunted hypertensive effect of combined fructose and high-salt diet in gene-targeted mice lacking functional serum- and glucocorticoid-inducible kinase sgk1. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006, 290, R935–R944. [Google Scholar] [CrossRef] [PubMed]
- Nishimoto, Y.; Tomida, T.; Matsui, H.; Ito, T.; Okumura, K. Decrease in renal medullary endothelial nitric oxide synthase of fructose-fed, salt-sensitive hypertensive rats. Hypertension 2002, 40, 190–194. [Google Scholar] [CrossRef] [PubMed]
- Catena, C.; Cavarape, A.; Novello, M.; Giacchetti, G.; Sechi, L.A. Insulin receptors and renal sodium handling in hypertensive fructose-fed rats. Kidney Int. 2003, 64, 2163–2171. [Google Scholar] [CrossRef] [PubMed]
- Ying, J.; Stuart, D.; Hillas, E.; Gociman, B.R.; Ramkumar, N.; Lalouel, J.M.; Kohan, D.E. Overexpression of mouse angiotensinogen in renal proximal tubule causes salt-sensitive hypertension in mice. Am. J. Hypertens. 2012, 25, 684–689. [Google Scholar] [CrossRef] [PubMed]
- Gurley, S.B.; Riquier-Brison, A.D.M.; Schnermann, J.; Sparks, M.A.; Allen, A.M.; Haase, V.H.; Snouwaert, J.N.; Le, T.H.; McDonough, A.A.; Koller, B.H.; et al. At1a angiotensin receptors in the renal proximal tubule regulate blood pressure. Cell Metab. 2011, 13, 469–475. [Google Scholar] [CrossRef] [PubMed]
- Houillier, P.; Chambrey, R.; Achard, J.M.; Froissart, M.; Poggioli, J.; Paillard, M. Signaling pathways in the biphasic effect of angiotensin ii on apical Na/H antiport activity in proximal tubule. Kidney Int. 1996, 50, 1496–1505. [Google Scholar] [CrossRef] [PubMed]
- Bharatula, M.; Hussain, T.; Lokhandwala, M.F. Angiotensin II at1 receptor/signaling mechanisms in the biphasic effect of the peptide on proximal tubular Na+, K+-atpase. Clin. Exp. Hypertens. 1998, 20, 465–480. [Google Scholar] [CrossRef] [PubMed]
- Rabie, E.M.; Heeba, G.H.; Abouzied, M.M.; Khalifa, M.M. Comparative effects of aliskiren and telmisartan in high fructose diet-induced metabolic syndrome in rats. Eur. J. Pharmacol. 2015, 760, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Shahataa, M.G.; Mostafa-Hedeab, G.; Ali, E.F.; Mahdi, E.A.; Mahmoud, F.A. Effects of telmisartan and pioglitazone on high fructose induced metabolic syndrome in rats. Can. J. Physiol. Pharmacol. 2016, 94, 907–917. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamari, Y.; Harari, A.; Shaish, A.; Peleg, E.; Sharabi, Y.; Harats, D.; Grossman, E. Effect of telmisartan, angiotensin ii receptor antagonist, on metabolic profile in fructose-induced hypertensive, hyperinsulinemic, hyperlipidemic rats. Hypertens. Res. 2008, 31, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Cid, J.; Maeso, R.; Perez-Vizcaino, F.; Cachofeiro, V.; Ruilope, L.M.; Tamargo, J.; Lahera, V. Effects of losartan on blood pressure, metabolic alterations, and vascular reactivity in the fructose-induced hypertensive rat. Hypertension 1995, 26, 1074–1078. [Google Scholar] [CrossRef] [PubMed]
- Iyer, S.N.; Katovich, M.J. Effect of acute and chronic losartan treatment on glucose tolerance and insulin sensitivity in fructose-fed rats. Am. J. Hypertens. 1996, 9, 662–668. [Google Scholar] [CrossRef]
- Iyer, S.N.; Katovich, M.J. Effect of chronic losartan potassium treatment on fructose-induced hypertension. Life Sci. 1994, 55, PL139–PL144. [Google Scholar] [CrossRef]
- Gonzalez-Vicente, A.; Cabral, P.D.; Hong, N.J.; Asirwatham, J.; Yang, N.; Berthiaume, J.M.; Dominici, F.P.; Garvin, J.L. Dietary fructose enhances the ability of low concentrations of angiotensin II to stimulate proximal tubule Na+ reabsorption. Nutrients 2017, 9, 885. [Google Scholar] [CrossRef] [PubMed]
- Ivy, J.R.; Bailey, M.A. Pressure natriuresis and the renal control of arterial blood pressure. J. Physiol. 2014, 592, 3955–3967. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Mircheff, A.K.; Hensley, C.B.; Magyar, C.E.; Warnock, D.G.; Chambrey, R.; Yip, K.P.; Marsh, D.J.; Holstein-Rathlou, N.H.; McDonough, A.A. Rapid redistribution and inhibition of renal sodium transporters during acute pressure natriuresis. Am. J. Physiol. 1996, 270, F1004–F1014. [Google Scholar] [PubMed]
- Granger, J.P.; Alexander, B.T.; Llinas, M. Mechanisms of pressure natriuresis. Curr. Hypertens. Rep. 2002, 4, 152–159. [Google Scholar] [CrossRef] [PubMed]
- McDonough, A.A.; Leong, P.K.; Yang, L.E. Mechanisms of pressure natriuresis: How blood pressure regulates renal sodium transport. Ann. N. Y. Acad. Sci. 2003, 986, 669–677. [Google Scholar] [CrossRef] [PubMed]
- Hammond, T.G.; Yusufi, A.N.; Knox, F.G.; Dousa, T.P. Administration of atrial natriuretic factor inhibits sodium-coupled transport in proximal tubules. J. Clin. Investig. 1985, 75, 1983–1989. [Google Scholar] [CrossRef] [PubMed]
- Haas, J.A.; Knox, F.G. Effect of synthetic atrial natriuretic factor on superficial and deep proximal tubule sodium reabsorption. J. Lab. Clin. Med. 1989, 113, 458–462. [Google Scholar] [PubMed]
- Shi, S.J.; Vellaichamy, E.; Chin, S.Y.; Smithies, O.; Navar, L.G.; Pandey, K.N. Natriuretic peptide receptor a mediates renal sodium excretory responses to blood volume expansion. Am. J. Physiol. Ren. Physiol. 2003, 285, F694–F702. [Google Scholar] [CrossRef] [PubMed]
- Kouyoumdzian, N.M.; Rukavina Mikusic, N.L.; Kravetz, M.C.; Lee, B.M.; Carranza, A.; Del Mauro, J.S.; Pandolfo, M.; Gironacci, M.M.; Gorzalczany, S.; Toblli, J.E.; et al. Atrial natriuretic peptide stimulates dopamine tubular transport by organic cation transporters: A novel mechanism to enhance renal sodium excretion. PLoS ONE 2016, 11, e0157487. [Google Scholar] [CrossRef] [PubMed]
- Kalinowski, L.; Szczepanska-Konkel, M.; Jankowski, M.; Angielski, S. Studies on potential involvement of protein kinase c in glomerular insensitivity to atrial natriuretic factor on low sodium intake. Med. Sci. Monit. 2001, 7, 628–634. [Google Scholar] [PubMed]
- Kalinowski, L.; Szczepanska-Konkel, M.; Pawelczyk, T.; Bizon, D.; Angielski, S. Inhibition of cgmp-phosphodiesterase restores the glomerular effects of atrial natriuretic factor in low sodium diet rats. Ren. Physiol. Biochem. 1995, 18, 254–266. [Google Scholar] [CrossRef] [PubMed]
- Cabral, P.D.; Hong, N.J.; Hye Khan, M.A.; Ortiz, P.A.; Beierwaltes, W.H.; Imig, J.D.; Garvin, J.L. Fructose stimulates Na/H exchange activity and sensitizes the proximal tubule to angiotensin II. Hypertension 2014, 63, e68–e73. [Google Scholar] [CrossRef] [PubMed]
- Myers, B.D.; Deen, W.M.; Brenner, B.M. Effects of norepinephrine and angiotensin ii on the determinants of glomerular ultrafiltration and proximal tubule fluid reabsorption in the rat. Circ. Res. 1975, 37, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Abdulla, M.H.; Sattar, M.A.; Johns, E.J.; Abdullah, N.A.; Hye Khan, M.A.; Rathore, H.A. High-fructose feeding impacts on the adrenergic control of renal haemodynamics in the rat. Br. J. Nutr. 2012, 107, 218–228. [Google Scholar] [CrossRef] [PubMed]
- Giani, J.F.; Mayer, M.A.; Munoz, M.C.; Silberman, E.A.; Hocht, C.; Taira, C.A.; Gironacci, M.M.; Turyn, D.; Dominici, F.P. Chronic infusion of angiotensin-(1-7) improves insulin resistance and hypertension induced by a high-fructose diet in rats. Am. J. Physiol. Endocrinol. Metab. 2009, 296, E262–E271. [Google Scholar] [CrossRef] [PubMed]
- Giani, J.F.; Munoz, M.C.; Mayer, M.A.; Veiras, L.C.; Arranz, C.; Taira, C.A.; Turyn, D.; Toblli, J.E.; Dominici, F.P. Angiotensin-(1-7) improves cardiac remodeling and inhibits growth-promoting pathways in the heart of fructose-fed rats. Am. J. Physiol. Heart Circ. Physiol. 2010, 298, H1003–H1013. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Vicente, A.; Garvin, J.L. Angiotensin ii-induced hypertension increases plasma membrane Na pump activity by enhancing Na entry in rat thick ascending limbs. Am. J. Physiol. Ren. Physiol. 2013, 305, F1306–F1314. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Vicente, A.; Garvin, J.L.; Hopfer, U. Transcriptome signature for dietary fructose-specific changes in rat renal cortex: A quantitative approach to physiological relevance. PLoS ONE 2018, 13, e0201293. [Google Scholar] [CrossRef] [PubMed]
- Mentzel, S.; Van Son, J.P.; De Jong, A.S.; Dijkman, H.B.; Koene, R.A.; Wetzels, J.F.; Assmann, K.J. Mouse glomerular epithelial cells in culture with features of podocytes in vivo express aminopeptidase a and angiotensinogen but not other components of the renin-angiotensin system. J. Am. Soc. Nephrol. 1997, 8, 706–719. [Google Scholar] [PubMed]
- Krieger, J.E.; Liard, J.F.; Cowley, A.W., Jr. Hemodynamics, fluid volume, and hormonal responses to chronic high-salt intake in dogs. Am. J. Physiol. 1990, 259, H1629–H1636. [Google Scholar] [CrossRef] [PubMed]
- Tank, J.E.; Moe, O.W.; Henrich, W.L. Abnormal regulation of proximal tubule renin mrna in the dahl/rapp salt-sensitive rat. Kidney Int. 1998, 54, 1608–1616. [Google Scholar] [CrossRef] [PubMed]
- Hwang, I.S.; Ho, H.; Hoffman, B.B.; Reaven, G.M. Fructose-induced insulin resistance and hypertension in rats. Hypertension 1987, 10, 512–516. [Google Scholar] [CrossRef] [PubMed]
- Delbosc, S.; Paizanis, E.; Magous, R.; Araiz, C.; Dimo, T.; Cristol, J.P.; Cros, G.; Azay, J. Involvement of oxidative stress and nadph oxidase activation in the development of cardiovascular complications in a model of insulin resistance, the fructose-fed rat. Atherosclerosis 2005, 179, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Lozada, L.G.; Tapia, E.; Jimenez, A.; Bautista, P.; Cristobal, M.; Nepomuceno, T.; Soto, V.; Avila-Casado, C.; Nakagawa, T.; Johnson, R.J.; et al. Fructose-induced metabolic syndrome is associated with glomerular hypertension and renal microvascular damage in rats. Am. J. Physiol. Ren. Physiol 2007, 292, F423–F429. [Google Scholar] [CrossRef] [PubMed]
- Rukavina Mikusic, N.L.; Kouyoumdzian, N.M.; Del Mauro, J.S.; Cao, G.; Trida, V.; Gironacci, M.M.; Puyo, A.M.; Toblli, J.E.; Fernandez, B.E.; Choi, M.R. Effects of chronic fructose overload on renal dopaminergic system: Alteration of urinary l-dopa/dopamine index correlates to hypertension and precedes kidney structural damage. J. Nutr. Biochem. 2018, 51, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Prince, P.D.; Lanzi, C.R.; Toblli, J.E.; Elesgaray, R.; Oteiza, P.I.; Fraga, C.G.; Galleano, M. Dietary (-)-epicatechin mitigates oxidative stress, no metabolism alterations, and inflammation in renal cortex from fructose-fed rats. Free Radic. Biol. Med. 2016, 90, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Salin, K.; Auer, S.K.; Rey, B.; Selman, C.; Metcalfe, N.B. Variation in the link between oxygen consumption and atp production, and its relevance for animal performance. Proc. Biol. Sci. 2015, 282, 20151028. [Google Scholar] [CrossRef] [PubMed]
- Harris, P.J. Regulation of proximal tubule function by angiotensin. Clin. Exp. Pharmacol. Physiol. 1992, 19, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Reilly, A.M.; Harris, P.J.; Williams, D.A. Biphasic effect of angiotensin II on intracellular sodium concentration in rat proximal tubules. Am. J. Physiol. 1995, 269, F374–F380. [Google Scholar] [CrossRef] [PubMed]
- Harris, P.J. Angiotensin II and atrial natriuretic factor: Cellular mechanisms in mammalian proximal tubule. Exp. Nephrol. 1994, 2, 105. [Google Scholar] [PubMed]
- John, S.W.; Krege, J.H.; Oliver, P.M.; Hagaman, J.R.; Hodgin, J.B.; Pang, S.C.; Flynn, T.G.; Smithies, O. Genetic decreases in atrial natriuretic peptide and salt-sensitive hypertension. Science 1995, 267, 679–681. [Google Scholar] [CrossRef] [PubMed]
- Oliver, P.M.; John, S.W.; Purdy, K.E.; Kim, R.; Maeda, N.; Goy, M.F.; Smithies, O. Natriuretic peptide receptor 1 expression influences blood pressures of mice in a dose-dependent manner. Proc. Natl. Acad. Sci. USA 1998, 95, 2547–2551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eitle, E.; Harris, P.J.; Morgan, T.O. Effects of atrial natriuretic factor on cyclic nucleotides in rabbit proximal tubule. Hypertension 1994, 23, 358–363. [Google Scholar] [CrossRef] [PubMed]
- Eitle, E.; Hiranyachattada, S.; Wang, H.; Harris, P.J. Inhibition of proximal tubular fluid absorption by nitric oxide and atrial natriuretic peptide in rat kidney. Am. J. Physiol. 1998, 274, C1075–C1080. [Google Scholar] [CrossRef] [PubMed]
- Gomes, G.N.; Aires, M.M. Interaction of atrial natriuretic factor and angiotensin II in proximal hco3- reabsorption. Am. J. Physiol. 1992, 262, F303–F308. [Google Scholar] [CrossRef] [PubMed]
- Garcia, N.H.; Garvin, J.L. ANF and angiotensin II interact via kinases in the proximal straight tubule. Am. J. Physiol. 1995, 268, F730–F735. [Google Scholar] [CrossRef] [PubMed]



| Bicarbonate-Buffered Physiological Saline | HEPES-Buffered Physiological Saline | K+-Free HEPES-Buffered Solution | Acid Pulse Buffer | 4× Reaction Media | 4× Reaction Media with Ouabain | |
|---|---|---|---|---|---|---|
| Concentrations in mmol/L | ||||||
| NaHCO3 | 25.0 | - | - | - | - | - |
| HEPES | - | 10.0 | 10.0 | 10.0 | - | - |
| Imidazole | - | - | - | - | 200.0 | 200.0 |
| NaCl | 114.0 | 130.0 | 130.0 | 120.0 | 320.0 | 320.0 |
| KCl | 4.0 | 4.0 | - | 4.0 | 120.0 | - |
| Na2HPO4 | 2.1 | 2.5 | 2.5 | 2.5 | - | - |
| NaH2PO4 | 0.4 | - | - | - | - | - |
| Mg SO4 | 1.2 | 1.2 | 1.2 | 1.2 | 20.0 | 20.0 |
| Ca(Lactate)2 | 2.0 | 2.0 | 2.0 | 2.0 | - | - |
| Na3Citrate | 1.0 | 1.0 | 1.0 | 1.0 | - | - |
| DL-alanine | 6.0 | 6.0 | 6.0 | 6.0 | - | - |
| Glucose | 5.5 | 5.5 | 5.5 | 5.5 | - | - |
| NH4Cl | - | - | - | 10.0 | - | - |
| EGTA | - | - | - | - | 2.0 | 2.0 |
| Na2ATP | - | - | - | - | 20.0 | 20.0 |
| NADH | - | - | - | - | 4.0 | 4.0 |
| Ascorbic Acid | - | - | - | - | 4.0 | 4.0 |
| PEP | - | - | - | - | 40.0 | 40.0 |
| Ouabain | - | - | - | - | - | 1.0 |
| HS (n = 6) | HS-FRU (n = 6) | Change | t-Test | ||||
|---|---|---|---|---|---|---|---|
| Mean | SEM | Mean | SEM | ||||
| Beginning weight | (g) | 163 | 7 | 160 | 8 | = | p = 0.77 |
| Final weight | (g) | 222 | 12 | 202 | 13 | = | p = 0.28 |
| Weight gain | (g/24 h) | 9 | 1 | 8 | 1 | = | p = 0.43 |
| Fluid intake | (mL/24 h) | 64 | 3 | 46 | 2 | ↓ | p < 0.01 |
| Food intake | (g/24 h) | 19 | 1 | 12 | 1 | ↓ | p < 0.01 |
| Caloric intake | (kcal/24 h) | 74 | 3 | 80 | 5 | = | p = 0.66 |
| Na+ intake | (mEq/24 h) | 14 | 1 | 9 | 1 | * | |
| HS (n = 6) | HS-FRU (n = 6) | Change | t-Test | ||||
|---|---|---|---|---|---|---|---|
| Mean | SEM | Mean | SEM | ||||
| Glucose | (mg/dL) | 113 | 5 | 97 | 8 | = | p = 0.12 |
| Insulin | (pmol/L) | 35 | 5 | 32 | 5 | = | p = 0.69 |
| pH | 7.38 | 0.02 | 7.34 | 0.03 | = | p = 0.21 | |
| Lactate | (mmol/L) | 0.8 | 0.1 | 1.0 | 0.1 | = | p = 0.25 |
| Na+ | (mmol/L) | 137.7 | 0.5 | 138.2 | 0.4 | = | p = 0.44 |
| K+ | (mmol/L) | 3.9 | 0.1 | 3.9 | 0.1 | = | p = 0.94 |
| Cl− | (mmol/L) | 109.3 | 0.6 | 108.6 | 0.6 | = | p = 0.49 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gonzalez-Vicente, A.; Hong, N.J.; Yang, N.; Cabral, P.D.; Berthiaume, J.M.; Dominici, F.P.; Garvin, J.L. Dietary Fructose Increases the Sensitivity of Proximal Tubules to Angiotensin II in Rats Fed High-Salt Diets. Nutrients 2018, 10, 1244. https://doi.org/10.3390/nu10091244
Gonzalez-Vicente A, Hong NJ, Yang N, Cabral PD, Berthiaume JM, Dominici FP, Garvin JL. Dietary Fructose Increases the Sensitivity of Proximal Tubules to Angiotensin II in Rats Fed High-Salt Diets. Nutrients. 2018; 10(9):1244. https://doi.org/10.3390/nu10091244
Chicago/Turabian StyleGonzalez-Vicente, Agustin, Nancy J. Hong, Nianxin Yang, Pablo D. Cabral, Jessica M. Berthiaume, Fernando P. Dominici, and Jeffrey L. Garvin. 2018. "Dietary Fructose Increases the Sensitivity of Proximal Tubules to Angiotensin II in Rats Fed High-Salt Diets" Nutrients 10, no. 9: 1244. https://doi.org/10.3390/nu10091244





