Fermented Dessert with Whey, Ingredients from the Peel of Jabuticaba (Myrciaria cauliflora) and an Indigenous Culture of Lactobacillus plantarum: Composition, Microbial Viability, Antioxidant Capacity and Sensory Features
Abstract
:1. Introduction
2. Materials and Methods
2.1. Obtaining the Jabuticaba Fruits
2.2. Production of the Ingredients Using the Jabuticaba Peels
2.2.1. Syrup and Jam
2.2.2. Hydroethanolic Extract
2.3. Processing of Cheese to Obtain Whey
2.4. Recovery of the Potentially Probiotic Indigenous Culture of Lactobacillus plantarum CNPC 003
2.5. Production of Fermented Dairy Desserts
2.6. Storage and Sampling Periods
2.7. Determination of the Mean Composition of Fermented Dairy Desserts
2.8. Physicochemical Parameters and Viability of Starter Cultures and Adjuvants
2.9. Extraction of Phenolics for Analysis of Total Phenolic Content and Antioxidant Capacity
2.10. Total Phenolic Analysis
2.11. DPPH Assay and Antioxidant Capacity Calculation
2.12. Instrumental Determination of Texture of Fermented Dairy Desserts
2.13. Sensory Evaluation of Fermented Dairy Desserts
2.14. Statistical Analysis
3. Results
3.1. Mean Composition of Fermented Dairy Desserts
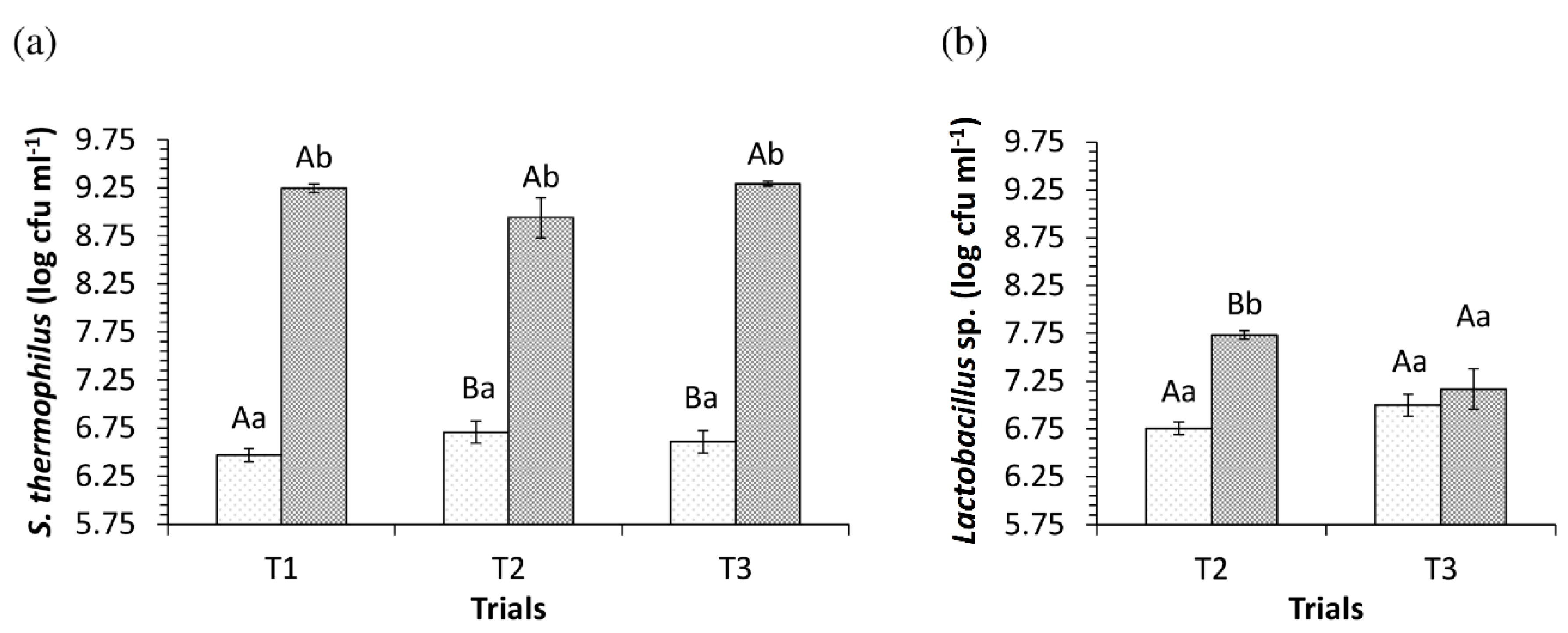
3.2. pH Values and Viability of Starter and Adjuvant Cultures in the Dairy Bases during the Fermentation Process
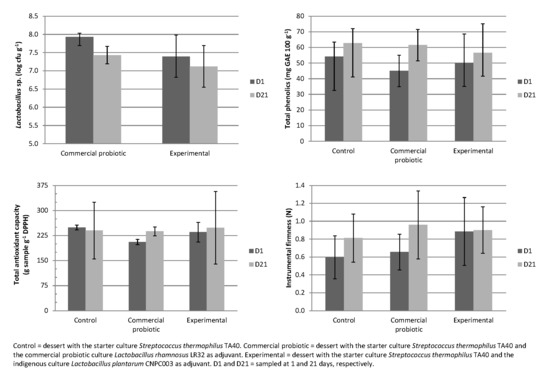
3.3. Physicochemical Parameters and Viability of Starter and Adjuvant Cultures during the Storage of the Dairy Desserts with the Ingredients of Jabuticaba Peel
3.4. Total Phenolic Compounds and Antioxidant Capacity of Dairy Desserts with the Ingredients of Jabuticaba Peel
3.5. Instrumental Texture of Fermented Dairy Desserts
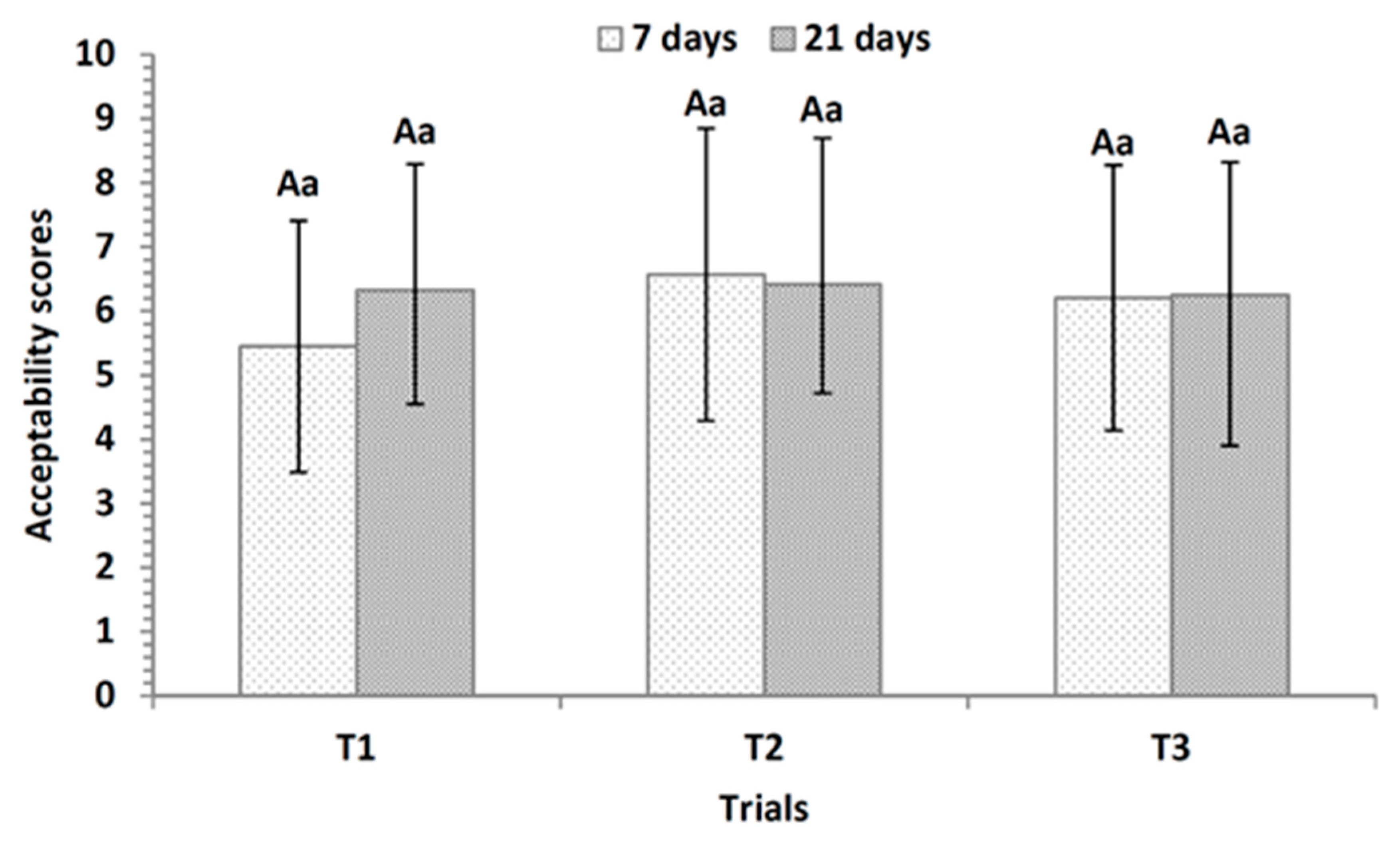
3.6. Sensory Evaluation of Fermented Dairy Desserts
4. Discussion
6. Conclusions
Author contributions
Acknowledgments
Conflicts of Interest
References
- Siegrist, M.; Shi, J.; Giusto, A.; Hartmann, C. Worlds apart: Consumer acceptance of functional foods and beverages in Germany and China. Appetite 2015, 92, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Lima, J.R.; Locatelli, G.O.; Finkler, L.; Luna-Finkler, C.L. Incorporation of encapsulated Lactobacillus casei into type curd cheese. Rev. Ciênc. Saúde 2014, 7, 27–34. (In Portuguese) [Google Scholar] [CrossRef]
- Hill, C.; Reid, F.G.G.; Gibson, R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, J.H.; Salminen, S.; Calder, P.C.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Vinderola, G.; Capellini, B.; Villarreal, F.; Suárez, V.; Quiberone, A.; Reinhemer, J. Usefulness of a set of simple in vitro tests for the screening and identification of probiotic candidate strains for dairy use. LWT-Food Sci. Technol. 2007, 41, 1678–1688. [Google Scholar] [CrossRef]
- Abreu, L.R. Identification and characterization of the probiotic potential of bacteria isolated from goat cheese. Master’s Thesis, Universidade Federal do Ceará, Sobral, Brazil, 2015. (In Portuguese). [Google Scholar]
- Caldeira, L.A.; Ferrao, S.P.B.; Fernandes, S.A.A.; Magnavita, A.P.A.; Santos, T.D.R. Development of strawberry-flavored milk drink using different yoghurt levels and whey obtained from buffalo’s milk. Ciênc. Rural 2010, 40, 2193–2198. [Google Scholar] [CrossRef]
- Siqueira, A.M.O.; Machado, E.C.L.; Samfor, T.L.M. Dairy beverage containing cheese whey and fruit. Ciênc. Rural 2013, 43, 1693–1700. (In Portuguese) [Google Scholar] [CrossRef]
- Morales, P.; Barros, L.; Dias, M.I.; Santos-Buelga, C.; Ferreira, I.C.F.R.; Asquieri, E.R.; Berrios, J.J. Non-fermented and fermented jabuticaba (Myrciaria cauliflora Mart.) pomaces as valuable sources of functional ingredients. Food Chem. 2016, 208, 220–227. [Google Scholar] [Green Version]
- Silva, M.C.; De Souza, V.B.; Thomazini, M.; Da Silva, E.R.; Smaniotto, T.; Carvalho, R.A.; Genovese, M.I.; Favaro-Trindade, C.S. Use of the jabuticaba (Myrciaria cauliflora) depulping residue to produce a natural pigment powder with functional properties. LWT-Food Sci. Technol. 2014, 55, 203–209. [Google Scholar]
- Zago, M.F.C.; Caliari, M.; Soares Júnior, M.S.; Campos, M.R.H.; Batista, J.E.R. Jabuticaba peel in the production of cookies for school food: Technological and sensory aspects. Ciênc. Agrotec. 2015, 39, 624–633. [Google Scholar] [CrossRef]
- Leite-Legatti, A.V.; Batista, A.G.; Dragano, N.R.V.; Marques, A.C.; Malta, L.G.; Riccio, M.F.; Eberlin, M.N.; Machado, A.R.T.; Carvalho-Silva, L.B.; Ruiz, A.L.T.G.; et al. Jaboticaba peel: Antioxidant compounds, antiproliferative and antimutagenic activities. Food Res. Int. 2012, 49, 596–603. [Google Scholar] [CrossRef]
- Alezandro, M.R.; Granato, D.; Genovese, M.I. Jaboticaba (Myrciaria jaboticaba (Vell.) Berg), a Brazilian grape-like fruit, improves plasma lipid profile in streptozotocin-mediated oxidative stress in diabetic rats. Food Res. Int. 2013, 54, 650–659. [Google Scholar] [CrossRef]
- Leite, A.V.; Malta, L.G.; Riccio, M.F.; Eberlin, M.N.; Pastore, G.M.; Maróstica Júnior, M.R. Antioxidant potential of rat plasma by administration of freeze-dried jaboticaba peel (Myrciaria jaboticaba Vell Berg). J. Agric. Food Chem. 2011, 59, 2277–2283. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, K.M.; Oliveira, I.C.; Lopes, M.A.C.; Cruz, A.P.G.; Buriti, F.C.A.; Cabral, L.M. Addition of grape pomace extract to probiotic fermented goat milk: The effect on phenolic content, probiotic viability and sensory acceptability. J. Sci. Food Agric. 2017, 97, 1108–1115. [Google Scholar] [CrossRef] [PubMed]
- Florentino, E.R. Production of Coalho Cheese with Pasteurized Milk; UEPB: Campina Grande, Brazil, 1997. (In Portuguese) [Google Scholar]
- Instituto Adolfo Lutz. Physicochemical Methods for Food Analysis, 4th ed.; IAL: São Paulo, Brazil, 2008. [Google Scholar]
- Folch, J.; Less, M.; Stanley, S.A. Simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [PubMed]
- AOAC International. Official Methods of Analysis, 18th ed.; AOAC International: Gaithersburg, MD, USA, 2010. [Google Scholar]
- Food and Agriculture Organization of the United Nations. Food Energy: Methods of Analysis and Conversion Factors. Report of a Technical Workshop; Food and Nutrition Paper Volume 77; FAO: Rome, Italy, 2003. [Google Scholar]
- Buriti, F.C.A.; Freitas, S.C.; Egito, A.S.; dos Santos, K.M.O. Effects of tropical fruit pulps and partially hydrolysed galactomannan from Caesalpinia pulcherrima seeds on the dietary fibre content, probiotic viability, texture and sensory features of goat dairy beverages. LWT-Food Sci. Technol. 2014, 59, 196–203. [Google Scholar] [CrossRef]
- Karaaslan, M.; Ozden, M.; Vardin, H.; Turkoglu, H. Phenolic fortification of yogurt using grape and callus extracts. LWT-Food Sci. Technol. 2011, 44, 1065–1072. [Google Scholar] [CrossRef]
- Rufino, M.S.M.; Alves, R.E.; Brito, E.S.; Morais, S.M.; Sampaio, C.G.; Pérez-Jiménez, J.; Saura-Calixto, F.D. Metodologia Científica: Determinação da Atividade Antioxidante Total em Frutas Pela Captura do Radical Livre DPPH; EMBRAPA: Fortaleza, Brazil, 2008. Comunicado Técnico Volume 127. (In Portuguese) [Google Scholar]
- Villanueva, N.D.M.; Da Silva, M.A.A.P. Comparative performance of the nine-point hedonic, hybrid and self-adjusting scales in the generation of internal preference maps. Food Qual. Prefer. 2009, 20, 1–12. [Google Scholar] [CrossRef]
- Giarnetti, M.; Paradiso, V.M.; Caponio, F.; Summo, C.; Pasqualone, A. Fat replacement in shortbread cookies using an emulsion filled gel based on inulin and extra virgin olive oil. LWT-Food Sci. Technol. 2015, 63, 339–345. [Google Scholar] [CrossRef]
- Codex Alimentarius. Codex Alimentarius Guidelines for Use of Nutrition and Health Claims CAC/GL 23-1997; Codex Alimentarius: Rome, Italy, 2013; Available online: http://www.codexalimentarius.net/download/standards/351/CXG_023e_u.pdf (accessed on 8 May 2018).
- ANVISA. Resolution RDC No. 54 from November 12th, 2012. Technical regulation on complementary nutrition information. Off. J. Union 2012, 219, 122–126. (In Portuguese) [Google Scholar]
- ANVISA. Resolution RDC No. 359 from December 23rd, 2003. Technical regulation on serving sizes with nutrition facts purposes for packed foods. Off. J. Union 2003, 251, 28–32. (In Portuguese) [Google Scholar]
- World Health Organization; Food and Agriculture Organization of the United Nations; United Nations University. Protein and Amino Acid Requirements in Human Nutrition: Report of a Joint WHO/FAO/UNU Expert Consultation; World Health Organization: Geneva, Switzerland, 2007. [Google Scholar]
- Rufino, J.S.; Nascimento, K.P.; Ribeiro, D.S.; Chinelate, G.C.B. Preparation of fermented milk drink flavored honey. Rev. Bras. Agroecol. 2015, 5, 42–48. [Google Scholar]
- Pelikánová, J.; Liptáková, D.; Valí, Ľ. Suitability of lactic acid bacteria for fermentation of maize and amaranth. J. Food Nutr. Res. 2015, 54, 354–364. [Google Scholar]
- Georgieva, R.; Iliev, I.; Haertlé, T.; Chobert, J.; Isanova, I.; Danova, S. Technological properties of candidate probiotic Lactobacillus plantarum strains. Int. Dairy J. 2009, 19, 696–702. [Google Scholar] [CrossRef]
- Settachaimongkon, S.; Van Valenberg, H.J.F.; Gazi, I.; Nout, M.J.; Van Hooijdonk, T.C.M.; Zwietering, M.H.; Smid, E.J. Influence of Lactobacillus plantarum WCFS1 on post-acidification, metabolite formation and survival of starter bacteria in set-yoghurt. Food Microbiol. 2016, 59, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Pereira, E.P.R.; Faria, J.A.F.; Cavalcanti, R.N.; Garcia, R.K.A.; Silva, R.; Esmerino, E.A.; Cappato, L.P.; Arellano, D.B.; Raices, R.S.L.; Silva, M.C.; et al. Oxidative stress in probiotic Petit Suisse: Is the jabuticaba skin extract a potential option? Food Res. Int. 2016, 81, 149–156. [Google Scholar] [CrossRef]
- Armelle, L.; Ntsame Affane, A.; Glen, P.; Fox, A.B.; Gunnar, O.; Sigge, A.; Marena, M.A.; Trevor, J.B. Simultaneous prediction of acidity parameters (pH and titratable acidity) in Kefir using near infrared reflectance spectroscopy. Int. Dairy J. 2011, 21, 896–900. [Google Scholar]
- De Souza Pereira, A.M.; Silva, G.S.; Almeida, R.; Salles, H.O.; dos Santos, K.M.O.; Florentino, E.R.; Alonso Buriti, F.C. Instrumental texture and sensory evaluation of fermented dairy beverages processed with reconstituted goat whey powder and a co-culture of Streptococcus thermophilus and Lactobacillus casei. Mljekarstvo 2018, 68, 21–29. [Google Scholar] [CrossRef]
- Brazil. Ministry of Agriculture, Livestock and Food Supply. Normative Instruction No. 46 from October 23rd, 2007. Technical regulation on the identity and quality of fermented milks. Off. J. Union 2007, 205, 4–7. (In Portuguese) [Google Scholar]
- Sidira, M.; Santarmaki, V.; Kiourtzidis, M.; Argyri, A.A.; Papadopoulou, O.S.; Chorianopoulos, N.; Tassou, C.; Kaloutsas, S.; Galanis, A.; Kourkotas, Y. Evaluation of immobilized Lactobacillus plantarum 2035 on whey protein as adjunct probiotic culture in yoghurt production. LWT-Food Sci. Technol. 2017, 75, 137–146. [Google Scholar] [CrossRef]
- Buriti, F.C.A.; Saad, S.M.I. Chilled milk-based desserts as emerging probiotic and prebiotic products. Crit. Rev. Food Sci. Nutr. 2014, 54, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, H.; Curiel, J.A.; Landete, J.M.; de las Rivas, B.; de Felipe, F.L.; Gómez-Cordovés, C.; Mancheño, J.M.; Muñoz, R. Food phenolics and lactic acid bacteria. Int. J. Food Microbiol. 2009, 132, 79–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pádua, H.C.; Silva, A.P.; Souza, D.G.; Moura, L.C.; Plácido, G.R.; Couto, G.V.L.; Caliari, M. Yogurt flavored banana (Musa AAB, subgrupo prata) flour enriched with the bark of jabuticaba (Myrciaria jabuticaba (vell.) Berg. Glob. Sci. Technol. 2017, 10, 89–104. [Google Scholar]
- Shori, A.B. Antioxidant activity and viability of lactic acid bacteria in soybean-yogurt made from cow and camel milk. J. Taibah Univ. Sci. 2013, 7, 1–17. [Google Scholar] [CrossRef]
- Caleja, C.; Barros, L.; Antonio, A.; Carocho, M.; Oliveira, M.; Ferreira, I. Fortification of yogurts with different antioxidant preservatives: A comparative study between natural and synthetic additives. Food Chem. 2016, 210, 262–268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costa, M.P.; Frasao, B.S.; Silva, A.C.O.; Freitas, M.Q.; Franco, R.M.; Conte-Junior, C.A. Cupuassu (Theobroma grandiflorum) pulp, probiotic, and prebiotic: Influence on color, apparent viscosity, and texture of goat milk yogurts. J. Dairy Sci. 2015, 98, 5995–6003. [Google Scholar] [CrossRef] [PubMed]
- Szczesniak, A.S. Texture is a sensory property. Food Qual. Prefer. 2002, 13, 215–225. [Google Scholar] [CrossRef] [Green Version]
- Bebejová, A.; Capla, J.; Zajác, P.; Belej, L.; Curlej, J.; Vietoris, V. Determination of textural properties of different kinds of ketchups of two different rates under different conditions of storage for the determination of their consumal quality. Potravinarstvo Slovak J. Food Sci. 2014, 8, 23–32. [Google Scholar]
- Ettinger, M.L.; Keller, R.D.; Duizer, L.M. Characterizing commercial pureed foods: Sensory, nutritional, and textural analysis. J. Nutr. Gerontol. Geriatr. 2014, 33, 179–197. [Google Scholar] [CrossRef] [PubMed]
- Guggisberg, D.; Cuthbert-Steven, J.; Piccinali, P.; Bütikofer, U.; Eberhard, P. Rheological, microstructural and sensory characterization of low-fat and whole milk set yogurt as influenced by inulin addition. Int. Dairy J. 2009, 19, 107–115. [Google Scholar] [CrossRef]
- Buriti, F.C.A.; Cardarelli, H.A.; Saad, S.M.I. Influence of Lactobacillus paracasei and inulin on instrumental texture and sensory evaluation of fresh cream cheese. Revista Brasileira de Ciências Farmacêuticas 2008, 44, 75–84. [Google Scholar] [CrossRef]
- Bayarri, S.; Carbonell, I.; Barrios, E.; Costell, E. Impact of sensory differences on consumer acceptability of yoghurt and yoghurt-like products. Int. Dairy J. 2011, 21, 111–118. [Google Scholar] [CrossRef] [Green Version]
- Dias, M.L.L.A.; Salgado, S.M.; Guerra, N.B.; Livera, A.V.S.; Andrade, S.A.C.; Ximenes, G.N.C. Phisicochemical, sensory, and microbiological evaluation and development of symbiotic fermented drink. Food Sci. Technol. 2013, 33, 805–811. [Google Scholar] [CrossRef] [Green Version]
- Torskangerpoll, K.; Andersen, O.M. Colour stability of anthocyanins in aqueous solutions at various pH values. Food Chem. 2005, 89, 427–440. [Google Scholar] [CrossRef]

| Trials | ||||
|---|---|---|---|---|
| Product Step | Ingredient (g) | T1 | T2 | T3 |
| Whey | 840.0 | 840.0 | 840.0 | |
| Sucrose | 80.0 | 80.0 | 80.0 | |
| Dairy base | Milk powder | 80.0 | 80.0 | 80.0 |
| Streptococcus thermophilus TA40 | 0.03 | 0.03 | 0.03 | |
| Lactobacillus rhamnosus LR32 | − | 0.2 | − | |
| Lactobacillus plantarum CNPC003 | − | − | * | |
| Sum | 1000.03 | 1000.23 | 1000.03 ** | |
| Ingredient (g) | ||||
| Fermented dairy base | 900.0 | 900.0 | 900.0 | |
| Jabuticaba peel syrup | 57.655 | 57.655 | 57.655 | |
| Dessert mixture | Hydroethanolic extract of jabuticaba peel | 20.0 | 20.0 | 20.0 |
| Pectin | 17.5 | 17.5 | 17.5 | |
| Lactic acid | 4.8 | 4.8 | 4.8 | |
| Cochineal carmine dye | 0.450 | 0.450 | 0.450 | |
| Sum | 1000.405 | 1000.405 | 1000.405 | |
| Ingredient (g) | ||||
| Final product | Dessert mixture | 75.0 | 75.0 | 75.0 |
| Jabuticaba peel jam | 12.5 | 12.5 | 12.5 | |
| Sum | 87.5 | 87.5 | 87.5 |
| Trials | |||
|---|---|---|---|
| Parameter | T1 | T2 | T3 |
| Total solids (g 100 g−1) | 28.90 ± 2.74 A | 26.49 ± 4.98 A | 29.66 ± 8.92 A |
| Ash—FW (g 100 g−1) | 0.922 ± 0.225 A | 1.02 ± 0.72 A | 0.874 ± 0.132A |
| Ash—DM (g 100 g−1) | 3.16 ± 0.56 A | 3.75 ± 2.49 A | 3.35 ± 1.69 A |
| Fat—FW (g 100 g−1) | 0.302 ± 0.145 A | 0.320 ± 0.145 A | 0.410 ± 0.169 A |
| Fat—DM (g 100 g−1) | 1.09 ± 0.62 A | 1.33 ± 0.82 A | 1.69 ± 1.30 A |
| Protein—FW (g 100 g−1) | 2.31 ± 0.33 A | 2.29 ± 0.36 A | 2.33 ± 0.42 A |
| Protein—DM (g 100 g−1) | 8.01 ± 1.09 A | 8.77 ± 1.33 A | 9.03 ± 5.17 A |
| Total carbohydrate—FW (g 100 g−1) | 25.37 ± 2.57 A | 22.86 ± 4.61 A | 26.04 ± 8.82 A |
| Total carbohydrate—DM (g 100 g−1) | 87.74 ± 1.19 A | 86.15 ± 3.16 A | 85.92 ± 7.99 A |
| Time | Trial | |||
|---|---|---|---|---|
| Parameter | (days) | T1 | T2 | T3 |
| 1 | 3.88 ± 0.10 Aa | 3.87 ± 0.07 Aa | 3.97 ± 0.08 Aa | |
| pH | 7 | 3.86 ± 0.18 Aa | 3.83 ± 0.10 Aa | 3.91 ± 0.12 Aa |
| 14 | 3.87 ± 0.18 Aa | 3.90 ± 0.11 Aa | 3.97 ± 0.10 Aa | |
| 21 | 3.89 ± 0.11 Aa | 3.87 ± 0.06 Aa | 3.96 ± 0.10 Aa | |
| 1 | 1.11 ± 0.12 Aa | 0.99 ± 0.17 Aa | 1.06 ± 0.14 Aa | |
| Titratable acidity | 7 | 1.13 ± 0.20 Aa | 1.12 ± 0.18 Aa | 1.11 ± 0.20 Aa |
| (lactic acid 100 g−1) | 14 | 1.06 ± 0.15 Aa | 1.03 ± 0.26 Aa | 1.02 ± 1.17 Aa |
| 21 | 1.03 ± 0.30 Aa | 0.99 ± 0.23 Aa | 0.95 ± 0.26 Aa | |
| 1 | 9.26 ± 0.21 Ac | 9.07 ± 0.19 Ab | 9.39 ± 0.55 Ab | |
| S. thermophilus | 7 | 8.97 ± 0.03 Ab | 8.92 ± 0.10 Aa | 8.99 ± 0.16 Aa |
| (log CFU g−1) | 14 | 8.94 ± 0.16 Aab | 8.92 ± 0.12 Aa | 8.93 ± 0.22 Aa |
| 21 | 8.90 ± 0.06 Aa | 8.89 ± 0.02 Aa | 8.96 ± 0.20 Aa | |
| 1 | n.a. | 7.93 ± 0.10 Bc | 7.39 ± 0.59 Ac | |
| Lactobacillus sp. | 7 | n.a. | 8.02 ± 0.31 Bc | 7.20 ± 0.52 Ab |
| (log CFU g−1) | 14 | n.a. | 7.70 ± 0.12 Bb | 7.01 ± 0.42 Aa |
| 21 | n.a. | 7.43 ± 0.24 Aa | 7.12 ± 0.57 Aab |
| Parameters | Trial | Time (days) | |||
|---|---|---|---|---|---|
| 1 | 7 | 14 | 21 | ||
| Total phenolics (mg GAE 100 g−1) | T1 | 54.10 ± 9.27 Aa | 64.52 ± 19.23 Aa | 59.09 ± 15.35 Aa | 62.77 ± 21.60 Aa |
| T2 | 45.08 ± 9.97 Aa | 58.36 ± 20.34 Aa | 56.09 ± 14.09 Aa | 61.60 ± 10.14 Aa | |
| T3 | 50.03 ± 18.63 Aa | 56.71 ± 12.78 Aa | 68.93 ± 11.65 Aa | 56.56 ± 14.92 Aa | |
| Inhibition of radicals DPPH (%) * | T1 | 36.92 ± 5.74 Aa | 35.31 ± 10.80 Aa | 34.96 ± 12.03 Aa | 42.34 ± 5.18 Aa |
| T2 | 44.84 ± 2.35 Aa | 43.32 ± 1.40 Aa | 32.60 ± 0.78 Aa | 35.66 ± 9.06 Aa | |
| T3 | 40.45 ± 2.30 Aa | 39.08 ± 0.99 Aa | 33.75 ± 2.08 Aa | 35.50 ± 1.46 Aa | |
| EC50 (g sample L−1 sol. DPPH 100 µM) | T1 | 5.30 ± 0.10 Aa | 6.32 ± 0.78 Aa | 5.76 ± 1.04 Aa | 5.61 ± 1.48 Aa |
| T2 | 4.94 ± 0.54 Aa | 4.54 ± 0.53 Aa | 4.22 ± 0.82 Aa | 6.15 ± 0.52 Aa | |
| T3 | 4.60 ± 0.27 Aa | 4.49 ± 0.27 Aa | 5.27 ± 0.18 Aa | 5.46 ± 2.34 Aa | |
| Total antioxidant capacity (g sample g−1 DPPH) | T1 | 248.88 ± 7.39 Aa | 258.20 ± 5.47 Aa | 229.11 ± 21.97 Aa | 239.76 ± 84.74 Aa |
| T2 | 205.56 ± 8.03 Aa | 207.10 ± 10.23 Aa | 165.77 ± 48.07 Aa | 237.36 ± 13.39 Aa | |
| T3 | 234.87 ± 29.23 Aa | 210.27 ± 21.51 Aa | 253.78 ± 8.52 Aa | 248.20 ± 108.85 Aa | |
| Parameters | Treatments | Time (days) | |||
|---|---|---|---|---|---|
| 1 | 7 | 14 | 21 | ||
| Firmness (N) | T1 | 0.596 ± 0.24 Aa | 1.26 ± 0.37 Ab | 0.662 ± 0.23 Aa | 0.811 ± 0.27 Aa |
| T2 | 0.655 ± 0.20 Aa | 0.813 ± 0.21 Aa | 0.836 ± 0.28 Aa | 0.958 ± 0.38 Aa | |
| T3 | 0.885 ± 0.38 Aa | 1.01 ± 0.22 Aa | 0.770 ± 0.14 Aa | 0.90 ± 0.26 Aa | |
| Consistency (N × s) | T1 | 8.35 ±3.97 Aa | 12.55 ± 5.89 Ab | 9.70 ± 4.68 Aa | 11.35 ± 4.05 Ab |
| T2 | 9.41 ± 3.13 Aab | 12.74 ± 4.96 Ab | 10.87± 4.03 Ab | 8.79 ± 5.79 Aa | |
| T3 | 9.41 ± 4.01 Aa | 13.46 ± 2.44 Ab | 12.82 ±2.82 Aab | 11.17 ± 2.67 Aa | |
| Cohesiveness (N) | T1 | 0.515 ± 0.24 Ba | 0.838 ± 0.11 Cb | 0.435 ± 0.47 Aa | 0.555 ± 0.29 Aa |
| T2 | 0.338 ± 0.09 Aa | 0.516± 0.19 Ab | 0.499 ± 0.19 Ab | 0.574 ± 0.34 Ab | |
| T3 | 0.505 ± 0.20 Ba | 0.689 ± 0.09 Bb | 0.560 ± 0.17 Aab | 0.604± 0.09 Aab | |
| Viscosity index (N × s) | T1 | 0.499 ± 0.33 Aa | 0.884 ± 0.57 Abc | 0.769 ± 0.27 Ab | 1.015 ± 0.39 Ac |
| T2 | 0.624 ± 0.26 Aa | 0.869 ± 0.38 Aa | 0.801 ± 0.34 Aa | 0.811 ± 0.41 Aa | |
| T3 | 1.186 ± 0.32 Bb | 1.290 ± 0.12 Ab | 0.978 ± 0.23 Aa | 0.953 ± 0.11 Aa | |
| Trial | Ranking | Time (days) | Attributes Cited | Total Citations n (%) | |||
|---|---|---|---|---|---|---|---|
| Flavor n (%) | Texture n (%) | Appearance n (%) | Color n (%) | ||||
| T1 | Most | 7 | 9 (26) | 13 (37) | 2 (6) | 11 (31) | 35 (100) |
| appreciated | 21 | 11 (31) | 7 (20) | 5 (14) | 12 (34) | 35 (100) | |
| Less | 7 | 20 (57) | 3 (9) | 4 (11) | 8 (23) | 35 (100) | |
| appreciated | 21 | 19 (56) | 3 (9) | 5 (15) | 7 (21) | 34 (100) | |
| T2 | Most | 7 | 10 (29) | 10 (29) | 6 (17) | 9 (26) | 35 (100) |
| appreciated | 21 | 8 (22) | 10 (28) | 6 (17) | 12 (33) | 36 (100) | |
| Less | 7 | 16 (46) | 7 (20) | 2 (6) | 10 (29) | 35 (100) | |
| appreciated | 21 | 21 (60) | 5 (14) | 3 (9) | 6 (17) | 35 (100) | |
| T3 | Most | 7 | 9 (26) | 10 (29) | 3 (9) | 13 (37) | 35 (100) |
| appreciated | 21 | 8 (23) | 7 (20) | 6 (17) | 14 (40) | 35 (100) | |
| Less | 7 | 22 (61) | 4 (11) | 7 (19) | 3 (8) | 36 (100) | |
| appreciated | 21 | 16 (46) | 11 (31) | 2 (6) | 6 (17) | 35 (100) | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almeida Neta, M.C.; Rocha de Queiroga, A.P.; Almeida, R.L.J.; Caetano Soares, A.; Marinho Gonçalves, J.; Soares Fernandes, S.; De Sousa, M.C.; Olbrich dos Santos, K.M.; Alonso Buriti, F.C.; Rolim Florentino, E. Fermented Dessert with Whey, Ingredients from the Peel of Jabuticaba (Myrciaria cauliflora) and an Indigenous Culture of Lactobacillus plantarum: Composition, Microbial Viability, Antioxidant Capacity and Sensory Features. Nutrients 2018, 10, 1214. https://doi.org/10.3390/nu10091214
Almeida Neta MC, Rocha de Queiroga AP, Almeida RLJ, Caetano Soares A, Marinho Gonçalves J, Soares Fernandes S, De Sousa MC, Olbrich dos Santos KM, Alonso Buriti FC, Rolim Florentino E. Fermented Dessert with Whey, Ingredients from the Peel of Jabuticaba (Myrciaria cauliflora) and an Indigenous Culture of Lactobacillus plantarum: Composition, Microbial Viability, Antioxidant Capacity and Sensory Features. Nutrients. 2018; 10(9):1214. https://doi.org/10.3390/nu10091214
Chicago/Turabian StyleAlmeida Neta, Maria Carmélia, Anna Paula Rocha de Queiroga, Raphael Lucas Jacinto Almeida, Anderson Caetano Soares, Jade Marinho Gonçalves, Suenia Soares Fernandes, Marina Cínthia De Sousa, Karina Maria Olbrich dos Santos, Flávia Carolina Alonso Buriti, and Eliane Rolim Florentino. 2018. "Fermented Dessert with Whey, Ingredients from the Peel of Jabuticaba (Myrciaria cauliflora) and an Indigenous Culture of Lactobacillus plantarum: Composition, Microbial Viability, Antioxidant Capacity and Sensory Features" Nutrients 10, no. 9: 1214. https://doi.org/10.3390/nu10091214