Abstract
The biological role and therapeutic potential of long non-coding RNAs (lncRNAs) in chronic lymphocytic leukemia (CLL) are still open questions. Herein, we investigated the significance of the lncRNA NEAT1 in CLL. We examined NEAT1 expression in 310 newly diagnosed Binet A patients, in normal CD19+ B-cells, and other types of B-cell malignancies. Although global NEAT1 expression level was not statistically different in CLL cells compared to normal B cells, the median ratio of NEAT1_2 long isoform and global NEAT1 expression in CLL samples was significantly higher than in other groups. NEAT1_2 was more expressed in patients carrying mutated IGHV genes. Concerning cytogenetic aberrations, NEAT1_2 expression in CLL with trisomy 12 was lower with respect to patients without alterations. Although global NEAT1 expression appeared not to be associated with clinical outcome, patients with the lowest NEAT1_2 expression displayed the shortest time to first treatment; however, a multivariate regression analysis showed that the NEAT1_2 risk model was not independent from other known prognostic factors, particularly the IGHV mutational status. Overall, our data prompt future studies to investigate whether the increased amount of the long NEAT1_2 isoform detected in CLL cells may have a specific role in the pathology of the disease.
Chronic lymphocytic leukemia (CLL) has a highly heterogeneous clinical course, ranging from an indolent behavior to an aggressive disease that needs prompt treatment in almost 30% of cases. These differences have been associated with a number of markers of the leukemic cells, including chromosomal aberrations, mutational status of the Immunoglobulin heavy chain variable region genes (IGHV), TP53 inactivation, CD38 and ZAP-70 expression [1]. However, despite the availability of these markers, the disease course remains somewhat unpredictable.
In the recent years, attention has been focused on long non-coding RNA (lncRNA), which are involved in many biological processes, such as transcriptional gene regulation, cell development and differentiation. Deregulation of lncRNAs has been demonstrated to be connected with tumor formation, progression and metastasis in many types of cancers, including hematological malignancies, although the information on a potential pathogenetic role in CLL is rather limited [2,3,4,5].
In this study, we focused on nuclear paraspeckle assembly transcript 1 (NEAT1), a well-known lncRNA located on chromosome 11q13. NEAT1 is transcribed in two different isoforms: a canonically polyadenylated short transcript of 3.7 kb (NEAT1_1), and a longer non-polyadenylated transcript (NEAT1_2) of about 23 kb that includes entirely the short NEAT1_1 form. The two isoforms share a common promoter but have an alternative transcription termination site. NEAT1_2 is an indispensable structural component of paraspeckles (PSs), which are membraneless compartments of the nucleus [6]. Although their function is not fully defined, PSs are involved in stress response and influence gene expression by regulating both transcription and pre-mRNA splicing events and by holding nuclear mRNA for editing [7]. NEAT1_2 could indirectly control these events by modulating the functions of PSs upon exposure to specific stresses [8]. Concerning NEAT1_1, even if it represents the most abundant isoform found in all samples, its biological role has to be fully elucidated. Recent data strongly suggested that it could be nonfunctional [8] leading to the hypothesis that NEAT1_1 keeps the transcription of the NEAT1 locus active, guaranteeing a rapid switch to NEAT1_2 production in response to stress.
NEAT1 deregulation has been reported in many types of solid tumors, where it is often associated with a poor prognosis, and in hematological malignancies, where it appears to affect different biological processes. Specifically, the aberrant expression of PML-RARα activity is correlated with NEAT1 downregulation in acute promyelocytic leukemia, suggesting that it may contribute to the impairment of myeloid differentiation [9]. We recently reported that the expression of NEAT1 in multiple myeloma (MM) is well above the normal controls, although this deregulation does not appear to correlate with prognosis. However, the putative NEAT1 involvement in different mechanisms of cellular stress response, such as the Unfolded Protein Response (UPR) and TP53 pathways, makes it a confident candidate for a potential targeted therapy in the disease [10,11]. Moreover, the high NEAT1_1 levels observed in MM suggest the possibility of NEAT1_1 involvement in PSs unrelated functions.
Information on NEAT1 expression and its possible deregulation in CLL is still lacking. Recently, Blume et al reported that NEAT1 expression can be induced during DNA damage responses in CLL cases with an intact TP53 function [12]. To gain further information on this issue, we investigated NEAT1 expression in 310 newly diagnosed Binet A patients prospectively enrolled in an observational multicenter study (clinicaltrial.gov #NCT00917540 from January 2007 to May 2011) (Table 1) [13]. The National Cancer Institute (NCI)-sponsored Working Group guidelines were followed for diagnosis and staging [1]. Eighty four of these 310 cases, who had less than 5.0 × 106 monoclonal B lymphocytes/L in the blood, were reclassified and diagnosed as monoclonal B-lymphocytosis (MBL) in accordance with the more recent International Workshop on Chronic Lymphocytic Leukemia (IWCLL) diagnostic criteria [1]. Median follow-up time was 76 months (range, 1–130 months). Highly enriched CD19+ CLL cells were characterized for IGHV mutational status and cytogenetic alterations, including deletion of 13q (del13), 11q (del11), and 17p (del17) and trisomy of chromosome 12 (12+), as previously reported [14]. NOTCH1 mutations were also investigated as described [15].

Table 1.
Features of 310 CLL samples.
In addition, we evaluated NEAT1 expression also in other types of hematological tumors, including B-cell acute lymphoid leukemia (ALL), acute and chronic myeloid leukemia (AML and CML), MM, B cell-lymphoma cell lines, and different types of normal B-cell populations, i.e., 27 samples including normal peripheral blood B-cells (pBC) and naïve and memory B cells purified from spleen or tonsils as specified elsewhere [14]. All the statistical tests were performed using appropriate R functions setting p-value < 0.01 as cutoff for significance.
For NEAT1 determination a quantitative real-time PCR (qRT-PCR) approach was used that was capable of discriminating the NEAT1 (NEAT1_1 and NEAT1_2) global expression from that of the NEAT1_2 isoform [11]. Global NEAT1 expression was also confirmed by RNA FISH (Supplementary Figure S1).
The NEAT1 expression levels in CLL cells are shown in Figure 1A also in comparison with those found in normal B-cell populations and in the malignant cells from the other hematological tumors. NEAT1 and NEAT1_2 expression levels were not statistically different in CLL cells compared to normal B cells. No differences could be detected even when CLL cells were compared separately with either normal naïve or memory B cells, which are considered closer to CLL cells (Supplementary Figure S2) [14,16]. CLL cells expressed significantly more NEAT1 than those of the other hematological neoplasias with the exception of MM cells, which are known to express high levels of this lncRNA (Figure 1a, left panel) [17]. Next, we verified the correlation between the two NEAT1 isoforms in all the populations analyzed and, with the exception of the small group of B-lymphoma cell lines, we found that NEAT1_2 expression levels positively correlated with those of NEAT1_1 (Figure 1b). Interestingly, CLL cells expressed the highest amount of NEAT1_2 isoform compared to the other cell types (Figure 1a, right panel). In particular, the median ratio of NEAT1_2 and NEAT1 expression in CLL samples (40%) was significantly higher than in the other groups (range 5–21%, Figure 1c).
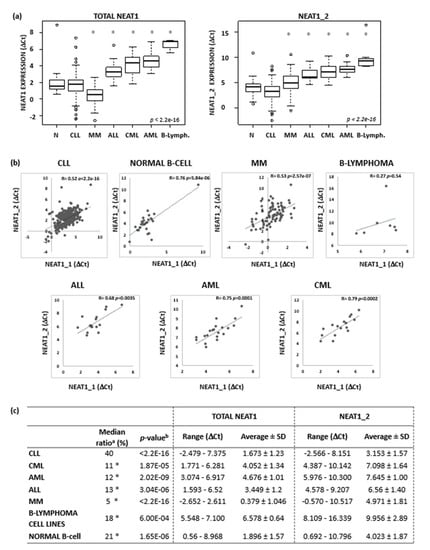
Figure 1.
NEAT1 and NEAT1_2 expression levels in normal B-cells and in B-cell malignancies. (a) Boxplots of NEAT1 and NEAT1_2 expression levels evaluated by qRT-PCR in 27 normal B-cells, 310 CLL, 82 MM, 16 ALL, 16 CML, and 20 AML samples, and 7 B-lymphoma cell lines (OCILY7, MAVER1, JEKO, MINO, SULTAN, P3HR1, and NAMALWA). Expression data are reported as Ct referred to GAPDH housekeeping gene. Significant differences versus CLL group are indicated by an asterisk (Benjamini-Hochberg adjusted Dunn’s test, p < 0.01). (b) Pearson’s correlation on NEAT1_1 (x-axis) and NEAT1_2 (y-axis) expression level expressed as Ct. NEAT1_1 expression values are inferred as described in Appendix B. Correlation coefficient and p-values are reported in each plot. (c) Ratios of NEAT1_2 and total NEAT1 expression level; range, average and standard deviation are reported for each group. a Median value of the ratios of NEAT1_2 and NEAT1 expression level evaluated for each sample in the specified subgroups. Significant differences versus CLL group are indicated by an asterisk (Benjamini-Hochberg adjusted Dunn’s test, p < 0.01). b Significant differences of NEAT1 and NEAT1_2 expression levels (Wilcoxon test).
Although, on the whole, our CLL series had a median NEAT1 expression similar to that of normal B cells, a proportion of samples showed high expression levels of global NEAT1 (Figure 1a, left panel) or NEAT1_2 (Figure 1a, right panel) long isoform. Prompted by such findings, we investigated whether differences in NEAT1 expression could correlate with other characteristics, which usually define different CLL prognostic groups. The global NEAT1 expression was comparable in CLL and in MBL cases, and there was no significant difference in IGHV-mutated (M) or -unmutated (UM) cases or between cases with different cytogenetic alterations (Figure 2a, upper panel; and Supplementary Figure S3). In contrast, the expression of the long NEAT1_2 isoform was significantly different in CLL subgroups stratified according to prognostic markers (Figure 2a). Specifically, NEAT1_2 was more expressed in the IGHV-M than in the IGHV-UM cases and in CLL cases without cytogenetic aberrations or with the 13q deletion, whereas it was significantly lower in patients with 12+ (Figure 2a, lower panel). No difference in NEAT1_2 expression was observed in the groups with or without NOTCH1 mutations (Figure 2a, upper panel) [15]. As for global NEAT1, NEAT1_1 isoform did not show any significantly differential expression in all the subgroups investigated (data not shown).
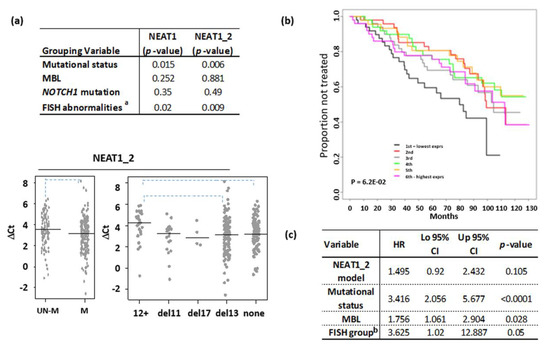
Figure 2.
NEAT1 expression level in CLL. (a) Wilcoxon test results comparing CLL subgroup defined by the indicated parameter; p-value < 0.01 was considered significant (aKruskal-Wallis test). Below, stripchart of NEAT1_2 expression in CLL subgroups defined by mutational IGVH status or the presence of the main chromosomal abnormalities detected by FISH; none = absence of FISH abnormalities (dashed line for p < 0.01, Dunn’s test). (b) Kaplan–Meier estimated curves of the six groups defined by NEAT1_2 expression levels. (c) Multivariate analysis comparing the NEAT1_2 risk model with prognostic variables or with MBL status in CLL series. b del17 or del11 CLL vs. others.
In addition, we investigated the possible association between NEAT1 and TP53 expression, based on data reporting NEAT1 as an effector of p53 protein, likely playing an important role in suppressing transformation in response to stress signals [18]. To do this, we focused our attention on cases harboring del17p in our series. Although the analysis has been limited only to the four available patients with del17p, our results showed that neither global NEAT1 nor NEAT1_2 expression levels in CLL were significantly lower than the ones detected in patients without del17p (n = 299; NEAT1 with del17p: 1.067 ± 1.013 vs without del17p 1.628 ± 1.229, p = 0.25; NEAT1_2 with del17p: 2.679 ± 0.557 vs. without del17p 3.154 ± 1.576, p = 0.34). To better characterize the TP53 status in these 4 patients, we sequenced the gene and found TP53 mutations in all samples, with a Variant Allele Frequency (VAF) higher than 95% in three cases (Supplementary Table S1). Therefore, in these 4 patients, TP53 gene appears to be completely disrupted. Overall, these results are in keeping with data by Blume et al. [12], showing that basal NEAT1 expression level is quite similar in CLL patients with a wild-type TP53 status or in those carrying TP53 alteration (i.e., mutation and/or deletion). Next, we evaluated whether mutated TP53 proteins found in our 4 patients were capable to activate NEAT1 transcription, by exploiting a yeast-based P53 functional assay (Appendix A) [19,20,21]. Firstly, a new reporter yeast strain (yLFM-NEAT1) in which the p53 response element (RE) from the NEAT1 target gene (5’-GAGCAAGCCTGGGCTTGCCA-3’) [18] controls the expression of the LUC1 reporter gene, was constructed. Whereas wild-type P53 confirmed the ability to activate transcription in yLFM-NEAT1 (Supplementary Figure S4A), all four P53 mutants encoded by the corresponding TP53 mutations failed to activate transcription in yLFM-NEAT1 (Supplementary Figure S4B). Therefore, it is possible to speculate that a significantly lower NEAT1 expression level in patients harbouring a completely inactive TP53 mutation with respect to patients without TP53 alterations is detectable only upon P53 induction by stress, as also suggested by Blume et al. [12].
Lastly, we correlated NEAT1 expression levels with time to first treatment (TTFT) as clinical outcome. To this end, patients were subdivided into sextiles based on global NEAT1 or NEAT1_2 specific expression by leukemic cells. We found that NEAT1 expression was not associated with prognosis. Patients with the lowest NEAT1_2 expression (1st sextile) displayed the shortest TTFT if compared with all the other samples (Figure 2b); however, a multivariate regression analysis showed that the NEAT1_2 risk model was not independent from other known prognostic factors, particularly the IGHV mutational status (Figure 2c).
In conclusion, our study, performed in a large and well-characterized cohort of early stage Binet A CLL patients, has provided evidence that NEAT1 expression levels are quite heterogeneous irrespectively of cytogenetic groups or clinical outcome. Based on these findings and the suggestion that the two NEAT1 transcripts may have different biological roles [8,22], it would be of interest to investigate whether the increased amount of the long NEAT1_2 isoform detected in CLL cells may have a specific role in the pathology.
Supplementary Materials
The following are available online at https://www.mdpi.com/2311-553X/6/1/11/s1, Figure S1: NEAT1 RNA FISH detection, Figure S2: NEAT1 expression level in CLL, Figure S3: Stripchart of NEAT1 expression in CLL subgroup, Figure S4: yeast-based P53 functional assay, Table S1: Molecular features of the four CLL patients showing the concomitant presence of del17p and TP53 mutation.
Author Contributions
Conceptualization, E.T., D.R and A.N.; formal analysis, D.R. and L.A.; investigation, E.T., V.F., P.M.(Paola Monti), S.F., I.S., M.C., P.M. (Paola Menichini), S.M., R.N. and R.G.; resources, G.C. and M.G.; data curation, S.F. and I.S.; writing—original draft preparation, D.R.; writing—review and editing, E.T., A.N., M.F., L.B., F.M. and G.F.; supervision, A.N. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by grants to Antonino Neri [from Associazione Italiana Ricerca sul Cancro (AIRC) (IG16722, IG10136, and the “Special Program Molecular Clinical Oncology-5 per mille” #9980, 2010/15)]; to Giovanna Cutrona and Gilberto Fronza [from the Italian Ministry of Health 5 × 1000 funds 2014, 2015, 2016, and from the Compagnia S. Paolo Turin Italy (project 2017.0526)]; to Manlio Ferrarini (the “Special Program Molecular Clinical Oncology-5 per mille” #9980 and AIRC I.G. n.14326); to Fortunato Morabito (the “Special Program Molecular Clinical Oncology-5 per mille” #9980 and AIRC and Fondazione CaRiCal co-financed Multi-Unit Regional Grant 2014 n.16695); Elisa Taiana was supported by a fellowship (#19370) from the Fondazione Italiana Ricerca sul cancro (FIRC).
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A
The new haploid S. cerevisiae yeast strain yLFM-Neat1 was generated using the delitto perfetto approach [21] by the genomic cloning of the human NEAT1 promoter P53 RE [-1458 bp from the transcription start site (TSS): 5’-GAGCAAGCCTGGGCTTGCCA-3’] [18]. The available yeast strains yLFM-P21-5’ and yLFM-mir-34a [19] were also used for comparison. The haploid strain yIG397 was used for the cloning of the TP53 mutations in a pLS-based yeast expression vector; yeast manipulations and the functional assay were performed as previously described [20].
Appendix B
Reverse transcription and quantitative PCR. Total RNA was extracted using TRIzol® Reagent (Invitrogen) according to manufacturer’s instructions. The purity and concentration of total RNA was determined by the NanoDrop 1000 spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA). The ratios of absorption (260 nm/280 nm) of all samples were between 1.8 and 2.0. cDNA was synthesized from 500 ng of total RNA with random primers using the High Capacity cDNA Reverse Transcriptase Kit (Invitrogen, Life Technologies, Carlsbad, CA, USA) according to the manufacturer’s instructions. To evaluate the expression levels of listed genes, RT-PCR was performed using SYBR green PCR Master Mix (Applied Biosystems, Foster City, CA, USA) after optimization of the primer conditions. 10 ng of reverse-transcribed RNAs were mixed with 300 nM of specific forward and reverse primers in a final volume of 10 μl. RT-PCR was performed on an Applied Biosystems StepOnePlus Real-Time PCR system for 40 cycles. Data were analyzed using the ΔCt method to measure the relative changes in each gene’s expression compared with GAPDH expression. To determine RNA levels by qPCR, the following primers were used:
| Primer Name | Sequence (5’-3’) |
| NEAT1 FW | 5’-GCCTTGTAGATGGAGCTTGC-3’ |
| NEAT1 RW | 5’-GCACAACACAATGACACCCT-3’ |
| NEAT1_2 FW | 5′-GGCCAGAGCTTTGTTGCTTC-3′ |
| NEAT1_2 RW | 5′-GGTGCGGGCACTTACTTACT-3’ |
| GAPDH FW | 5’-ACAGTCAGCCGCATCTTCTT-3’ |
| GAPDH RW | 5’-AATGAAGGGGTCATTGATGG-3’ |
RT-PCR primers efficiency. To estimate RT-PCR primers efficiency, we followed the method based on standard curve assessment, which relies on repeating the PCR reaction with known amounts of template. Ct values versus template concentration input (i.e., reverse transcribed total RNA expressed as log values) were plotted to calculate the slope. Efficiency percentage value, E (%), was defined as
in which Slope was derived from the regression curve calculated between the template log values and all the average Ct values [23,24]. In our study, we evaluated the following E (%) for each couple of primers: GAPDH 100%, NEAT1 106% and NEAT1_2 120%. Amplification values derived from RT-PCR analysis were adjusted taking into consideration the efficiency of both NEAT1 couples of primer and the adjusted results were used for all experiments. NEAT1_1 Ct expression values were inferred as the difference between adjusted fold change of NEAT1 and NEAT1_2.
E (%) = (10−1/Slope − 1) × 100
Ratio calculations. The ratios of NEAT1_2 and total NEAT1 expression level was evaluated by calculating, for each sample, the ratio in the corresponding adjusted fold change; then, the median value for each group was reported.
Statistical analysis. Conventional statistical tests were applied as reported in the manuscript using standard functions in base R package (Pearson coefficient to assess sample correlation; Wilcoxon rank-sum, Kruskal-Wallis and Dunn’s test to assess whether the samples originate from the same distribution). We used the Cox proportional hazards model to test the association between NEAT1 expression levels, assumed as continuous variables or stratified into n (up to six) equally parted groups and time to first treatment (TTFT) as clinical outcome.
References
- Hallek, M.; Cheson, B.D.; Catovsky, D.; Caligaris-Cappio, F.; Dighiero, G.; Dohner, H.; Hillmen, P.; Keating, M.; Montserrat, E.; Chiorazzi, N.; et al. iwCLL guidelines for diagnosis, indications for treatment, response assessment, and supportive management of CLL. Blood 2018, 131, 2745–2760. [Google Scholar] [CrossRef] [PubMed]
- Dahl, M.; Kristensen, L.S.; Gronbaek, K. Long Non-Coding RNAs Guide the Fine-Tuning of Gene Regulation in B-Cell Development and Malignancy. Int. J. Mol. Sci. 2018, 19, 2475. [Google Scholar] [CrossRef] [PubMed]
- Nobili, L.; Ronchetti, D.; Taiana, E.; Neri, A. Long non-coding RNAs in B-cell malignancies: A comprehensive overview. Oncotarget 2017, 8, 60605–60623. [Google Scholar] [CrossRef] [PubMed]
- Ronchetti, D.; Manzoni, M.; Agnelli, L.; Vinci, C.; Fabris, S.; Cutrona, G.; Matis, S.; Colombo, M.; Galletti, S.; Taiana, E.; et al. lncRNA profiling in early-stage chronic lymphocytic leukemia identifies transcriptional fingerprints with relevance in clinical outcome. Blood Cancer J. 2016, 6, e468. [Google Scholar] [CrossRef] [PubMed]
- Sattari, A.; Siddiqui, H.; Moshiri, F.; Ngankeu, A.; Nakamura, T.; Kipps, T.J.; Croce, C.M. Upregulation of long noncoding RNA MIAT in aggressive form of chronic lymphocytic leukemias. Oncotarget 2016, 7, 54174–54182. [Google Scholar] [CrossRef]
- Li, R.; Harvey, A.R.; Hodgetts, S.I.; Fox, A.H. Functional dissection of NEAT1 using genome editing reveals substantial localization of the NEAT1_1 isoform outside paraspeckles. RNA 2017, 23, 872–881. [Google Scholar] [CrossRef]
- Nakagawa, S.; Yamazaki, T.; Hirose, T. Molecular dissection of nuclear paraspeckles: Towards understanding the emerging world of the RNP milieu. Open Biol. 2018, 8. [Google Scholar] [CrossRef]
- Adriaens, C.; Rambow, F.; Bervoets, G.; Silla, T.; Mito, M.; Chiba, T.; Asahara, H.; Hirose, T.; Nakagawa, S.; Jensen, T.H.; et al. The long noncoding RNA NEAT1_1 is seemingly dispensable for normal tissue homeostasis and cancer cell growth. RNA 2019, 25, 1681–1695. [Google Scholar] [CrossRef]
- Zeng, C.; Xu, Y.; Xu, L.; Yu, X.; Cheng, J.; Yang, L.; Chen, S.; Li, Y. Inhibition of long non-coding RNA NEAT1 impairs myeloid differentiation in acute promyelocytic leukemia cells. BMC Cancer 2014, 14, 693. [Google Scholar] [CrossRef]
- Adriaens, C.; Standaert, L.; Barra, J.; Latil, M.; Verfaillie, A.; Kalev, P.; Boeckx, B.; Wijnhoven, P.W.; Radaelli, E.; Vermi, W.; et al. p53 induces formation of NEAT1 lncRNA-containing paraspeckles that modulate replication stress response and chemosensitivity. Nat. Med. 2016, 22, 861–868. [Google Scholar] [CrossRef]
- Taiana, E.; Ronchetti, D.; Favasuli, V.; Todoerti, K.; Manzoni, M.; Amodio, N.; Tassone, P.; Agnelli, L.; Neri, A. Long non-coding RNA NEAT1 shows high expression unrelated to molecular features and clinical outcome in multiple myeloma. Haematologica 2019, 104, e72–e76. [Google Scholar] [CrossRef] [PubMed]
- Blume, C.J.; Hotz-Wagenblatt, A.; Hullein, J.; Sellner, L.; Jethwa, A.; Stolz, T.; Slabicki, M.; Lee, K.; Sharathchandra, A.; Benner, A.; et al. p53-dependent non-coding RNA networks in chronic lymphocytic leukemia. Leukemia 2015, 29, 2015–2023. [Google Scholar] [CrossRef] [PubMed]
- Morabito, F.; Mosca, L.; Cutrona, G.; Agnelli, L.; Tuana, G.; Ferracin, M.; Zagatti, B.; Lionetti, M.; Fabris, S.; Maura, F.; et al. Clinical monoclonal B lymphocytosis versus Rai 0 chronic lymphocytic leukemia: A comparison of cellular, cytogenetic, molecular, and clinical features. Clin. Cancer Res. 2013, 19, 5890–5900. [Google Scholar] [CrossRef] [PubMed]
- Ronchetti, D.; Mosca, L.; Cutrona, G.; Tuana, G.; Gentile, M.; Fabris, S.; Agnelli, L.; Ciceri, G.; Matis, S.; Massucco, C.; et al. Small nucleolar RNAs as new biomarkers in chronic lymphocytic leukemia. BMC Med. Genom. 2013, 6, 27–37. [Google Scholar] [CrossRef]
- Lionetti, M.; Fabris, S.; Cutrona, G.; Agnelli, L.; Ciardullo, C.; Matis, S.; Ciceri, G.; Colombo, M.; Maura, F.; Mosca, L.; et al. High-throughput sequencing for the identification of NOTCH1 mutations in early stage chronic lymphocytic leukaemia: Biological and clinical implications. Br. J. Haematol. 2014, 165, 629–639. [Google Scholar] [CrossRef]
- Chiorazzi, N.; Ferrarini, M. Cellular origin(s) of chronic lymphocytic leukemia: Cautionary notes and additional considerations and possibilities. Blood 2011, 117, 1781–1791. [Google Scholar] [CrossRef]
- Ronchetti, D.; Agnelli, L.; Pietrelli, A.; Todoerti, K.; Manzoni, M.; Taiana, E.; Neri, A. A compendium of long non-coding RNAs transcriptional fingerprint in multiple myeloma. Sci. Rep. 2018, 8, 6557. [Google Scholar] [CrossRef]
- Mello, S.S.; Sinow, C.; Raj, N.; Mazur, P.K.; Bieging-Rolett, K.; Broz, D.K.; Imam, J.F.C.; Vogel, H.; Wood, L.D.; Sage, J.; et al. Neat1 is a p53-inducible lincRNA essential for transformation suppression. Genes Dev. 2017, 31, 1095–1108. [Google Scholar] [CrossRef]
- Inga, A.; Storici, F.; Darden, T.A.; Resnick, M.A. Differential transactivation by the p53 transcription factor is highly dependent on p53 level and promoter target sequence. Mol. Cell. Biol. 2002, 22, 8612–8625. [Google Scholar] [CrossRef]
- Monti, P.; Russo, D.; Bocciardi, R.; Foggetti, G.; Menichini, P.; Divizia, M.T.; Lerone, M.; Graziano, C.; Wischmeijer, A.; Viadiu, H.; et al. EEC- and ADULT-associated TP63 mutations exhibit functional heterogeneity toward P63 responsive sequences. Hum. Mutat. 2013, 34, 894–904. [Google Scholar] [CrossRef]
- Storici, F.; Resnick, M.A. Delitto perfetto targeted mutagenesis in yeast with oligonucleotides. Genet. Eng. 2003, 25, 189–207. [Google Scholar]
- Hirose, T.; Yamazaki, T.; Nakagawa, S. Molecular anatomy of the architectural NEAT1 noncoding RNA: The domains, interactors, and biogenesis pathway required to build phase-separated nuclear paraspeckles. Wiley Interdiscip. Rev. RNA 2019. [Google Scholar] [CrossRef]
- Lane, K.D.; Mu, J.; Lu, J.; Windle, S.T.; Liu, A.; Sun, P.D.; Wellems, T.E. Selection of Plasmodium falciparum cytochrome B mutants by putative PfNDH2 inhibitors. Proc. Natl. Acad. Sci. USA 2018, 115, 6285–6290. [Google Scholar] [CrossRef] [PubMed]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).