Sulfur-Doped Reduced Graphene Oxide for Enhanced Sodium Ion Pseudocapacitance
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
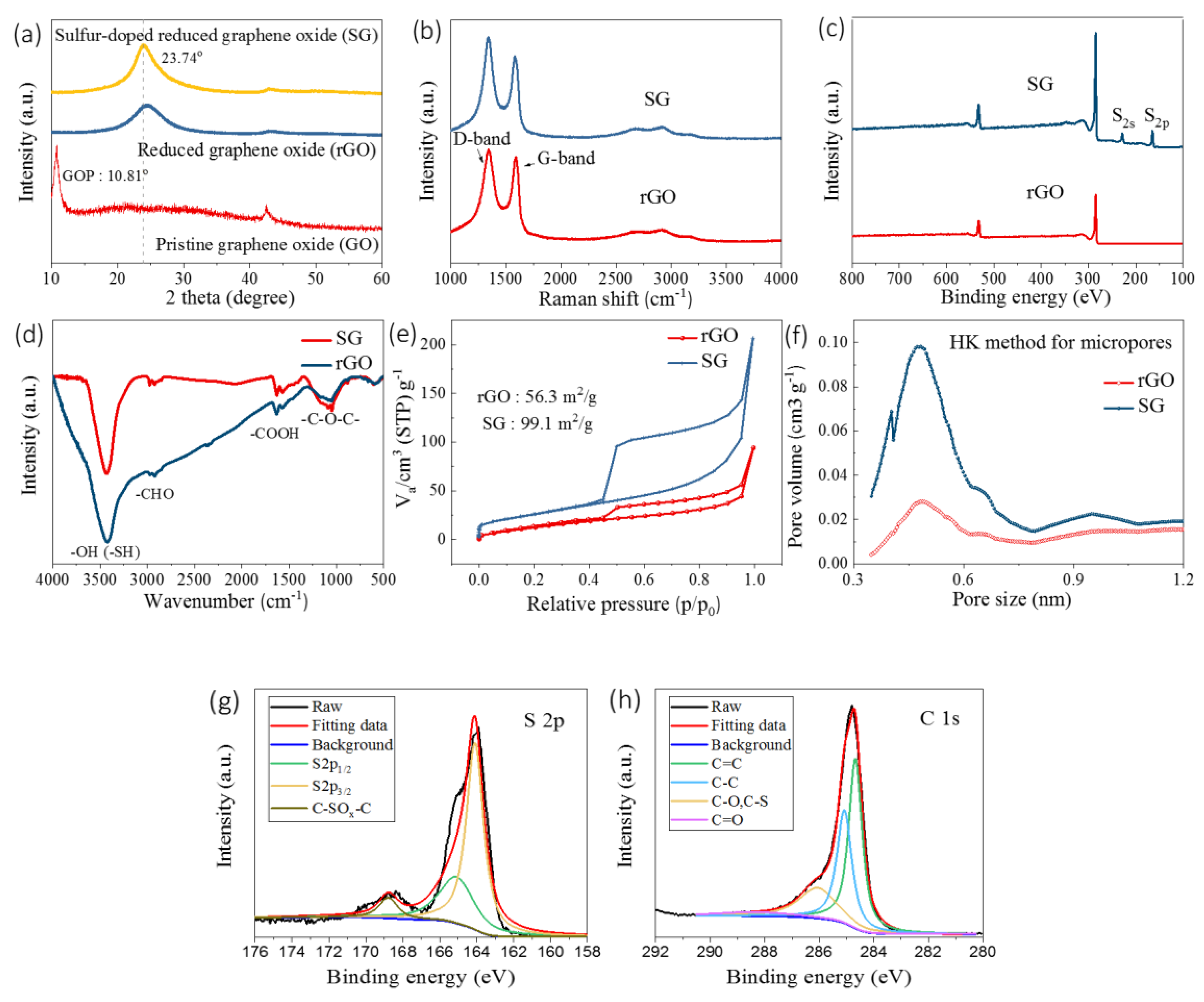
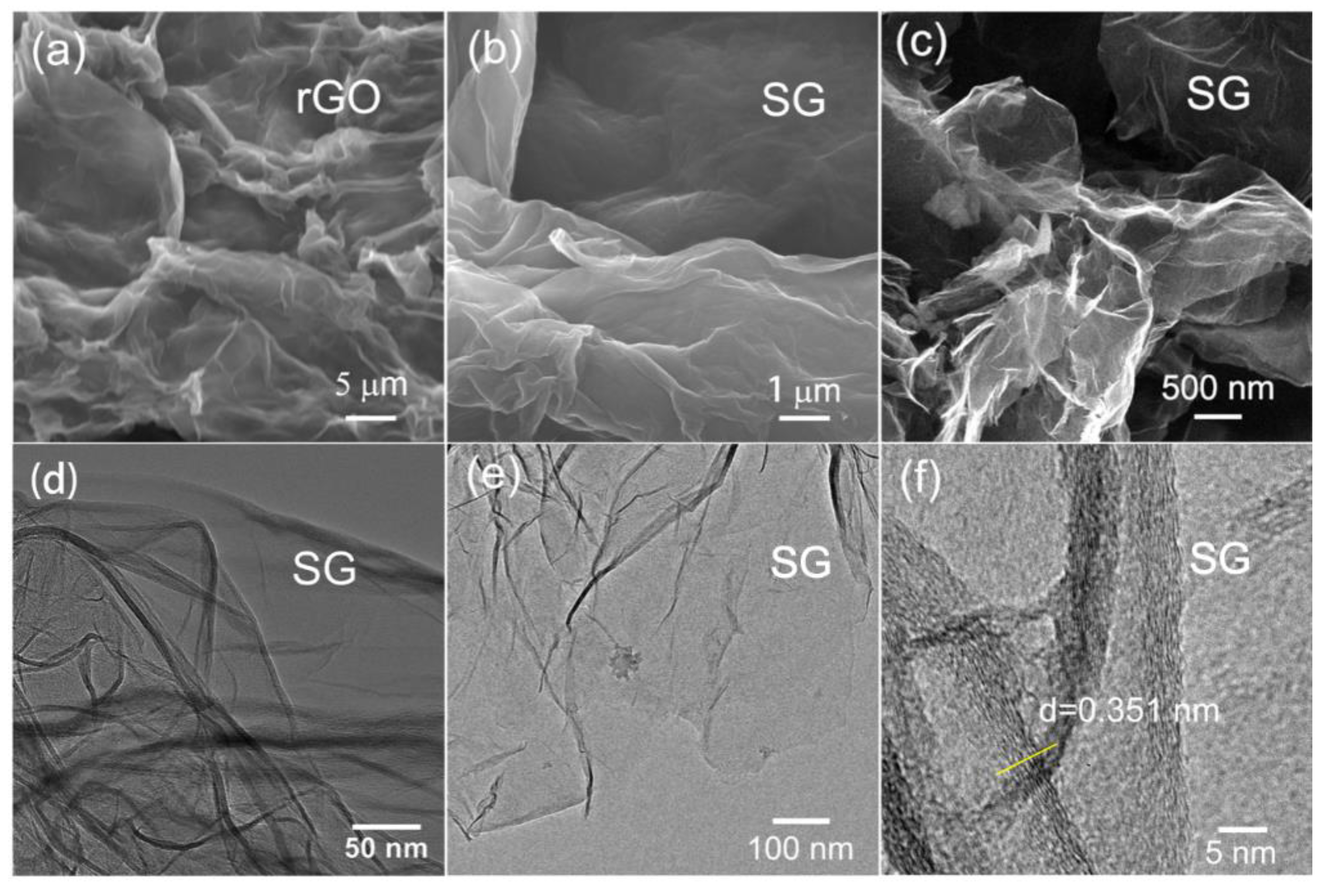
3.1. Morphology and Structural Characterization
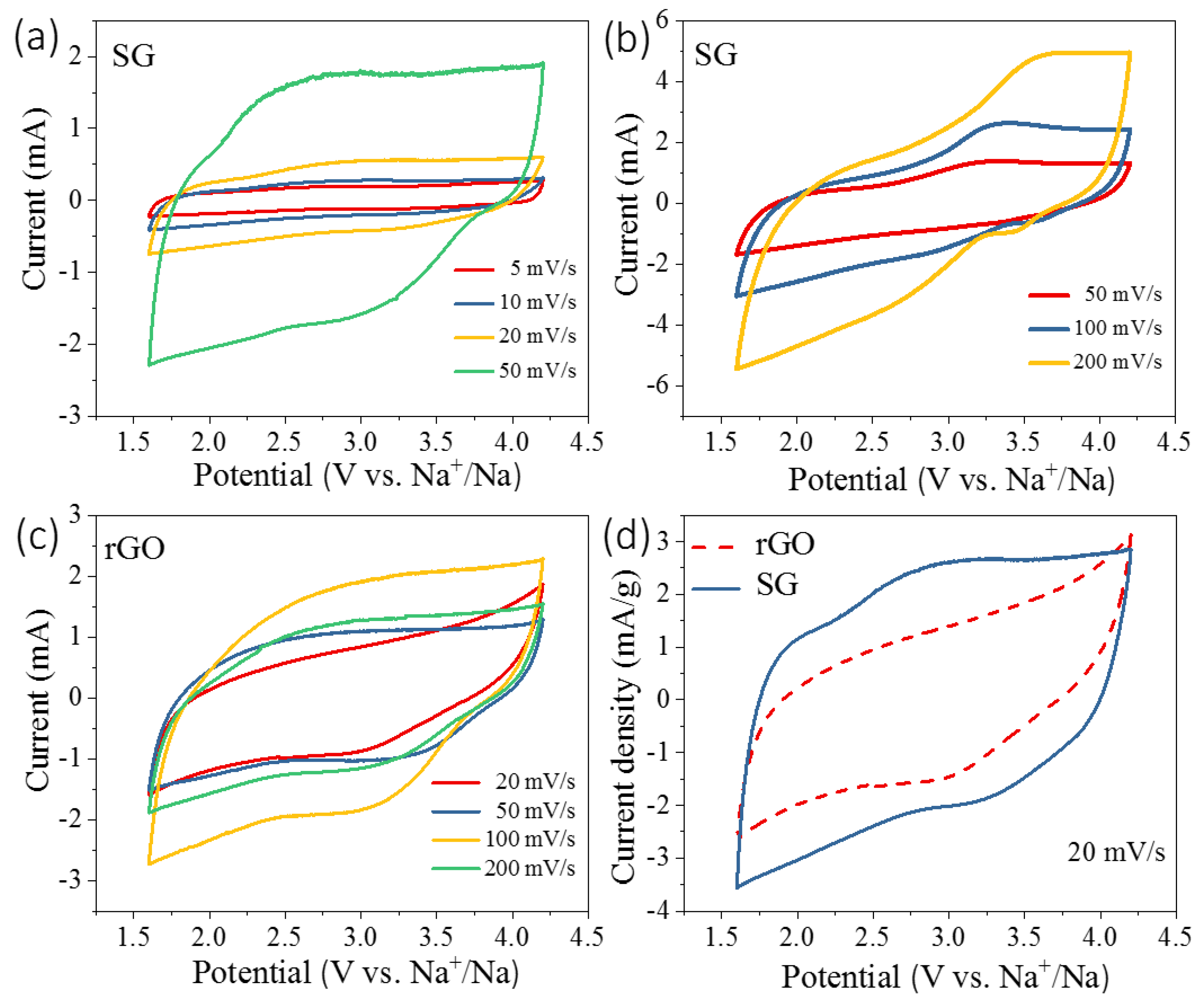
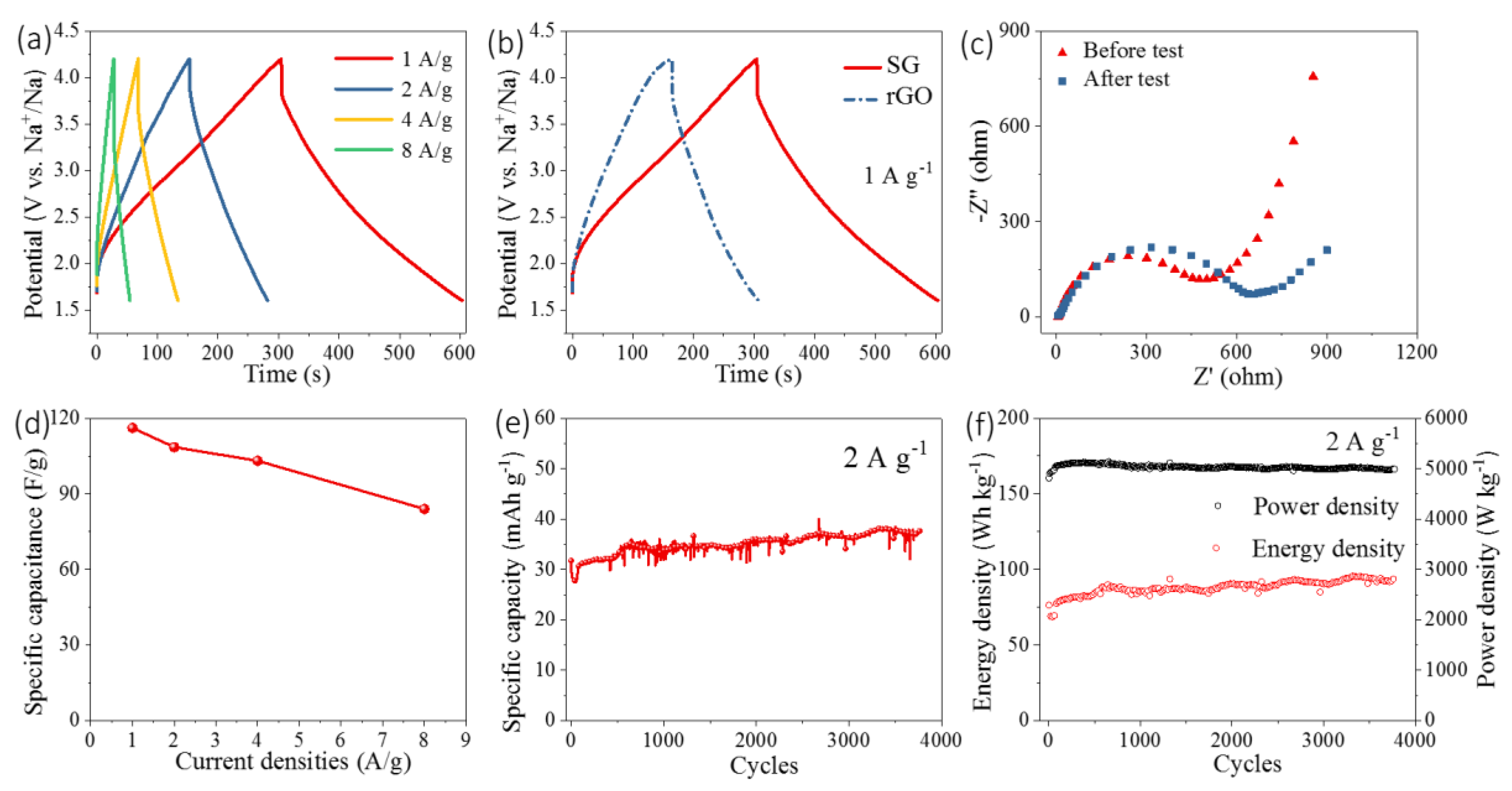
3.2. Electrochemical Properties
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hou, P.Y.; Chu, G.; Gao, J.; Zhang, Y.T.; Zhang, L.Q. Li-ion batteries: Phase transition. Chin. Phys. B 2016, 25, 11. [Google Scholar] [CrossRef]
- Etacheri, V.; Marom, R.; Elazari, R.; Salitra, G.; Aurbach, D. Challenges in the development of advanced Li-ion batteries: A review. Energy Environ. Sci. 2011, 4, 3243–3262. [Google Scholar] [CrossRef]
- Simon, P.; Gogotsi, Y. Materials for electrochemical capacitors. Nat. Mater. 2008, 7, 845–854. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.L.; Wang, Q.; Zhu, X.T.; Jiang, F.Y. Three-Dimensional SnS Decorated Carbon Nano-Networks as Anode Materials for Lithium and Sodium Ion Batteries. Nanomaterials 2018, 8, 9. [Google Scholar]
- Zhou, Y.L.; Zhu, Q.; Tian, J.; Jiang, F.Y. TiO2 Nanobelt@ Co9S8 Composites as Promising Anode Materials for Lithium and Sodium Ion Batteries. Nanomaterials 2017, 7, 11. [Google Scholar] [CrossRef] [PubMed]
- Bella, F.; Muñoz-García, A.B.; Meligrana, G.; Lamberti, A.; Destro, M.; Pavone, M.; Gerbaldi, C. Unveiling the controversial mechanism of reversible Na storage in TiO2 nanotube arrays: Amorphous versus anatase TiO2. Nano Res. 2017, 10, 2891–2903. [Google Scholar] [CrossRef]
- Bella, F.; Muñoz-García, A.B.; Coloò, F.; Meligrana, G.; Lamberti, A.; Destro, M.; Pavone, M.; Gerbaldi, C. Combined Structural, Chemometric, and Electrochemical Investigation of Vertically Aligned TiO2 Nanotubes for Na-ion Batteries. ACS Omega 2018, 3, 8440–8450. [Google Scholar] [CrossRef]
- Liu, F.S.; Sun, X.X.; Liu, Y.T.; Song, X.Y.; Gao, J.; Qin, G.H. TiO2 nanorods confined in porous V2O5 nanobelts and interconnected carbon channels for sodium ion batteries. Appl. Surf. Sci. 2019, 473, 873–884. [Google Scholar] [CrossRef]
- Luo, J.; Zheng, J.; Nai, J.; Jin, C.; Yuan, H.; Sheng, O.; Liu, Y.; Fang, R.; Zhang, W.; Huang, H.; et al. Atomic Sulfur Covalently Engineered Interlayers of Ti3C2 MXene for Ultra-Fast Sodium-Ion Storage by Enhanced Pseudocapacitance. Adv. Funct. Mater. 2019, 29, 10. [Google Scholar] [CrossRef]
- Zheng, G.X.; Chen, M.H.; Yin, J.H.; Zhang, H.R.; Liang, X.Q.; Zhang, J.W. Metal Organic Frameworks Derived Nano Materials for Energy Storage Application. Int. J. Electrochem. Sci. 2019, 14, 2345–2362. [Google Scholar] [CrossRef]
- Ding, J.; Wang, H.; Li, Z.; Cui, K.; Karpuzov, D.; Tan, X.; Kohandehghan, A.; Mitlin, D. Peanut shell hybrid sodium ion capacitor with extreme energy-power rivals lithium ion capacitors. Energy Environ. Sci. 2015, 8, 941–955. [Google Scholar] [CrossRef]
- Que, L.F.; Yu, F.D.; He, K.W.; Wang, Z.B.; Gu, D.M. Robust and Conductive Na2Ti2O5-x Nanowire Arrays for High Performance Flexible Sodium-Ion Capacitor. Chem. Mater. 2017, 29, 9133–9141. [Google Scholar] [CrossRef]
- Aravindan, V.; Gnanaraj, J.; Lee, Y.S.; Madhavi, S. Insertion-Type Electrodes for Nonaqueous Li-Ion Capacitors. Chem Rev. 2014, 114, 11619–11635. [Google Scholar] [CrossRef]
- Zhong, C.; Deng, Y.D.; Hu, W.B.; Qiao, J.L.; Zhang, L.; Zhang, J.J. A review of electrolyte materials and compositions for electrochemical supercapacitors. Chem. Soc. Rev. 2015, 44, 7484–7539. [Google Scholar] [CrossRef]
- Dall’Agnese, Y.; Taberna, P.L.; Gogotsi, Y.; Simon, P. Two-Dimensional Vanadium Carbide (MXene) as Positive Electrode for Sodium-Ion Capacitors. J. Phys. Chem. Lett. 2015, 6, 2305–2309. [Google Scholar] [CrossRef] [Green Version]
- Marcano, D.C.; Kosynkin, D.V.; Berlin, J.M.; Sinitskii, A.; Sun, Z.; Slesarev, A.S.; Alemany, L.B.; Lu, W.; Tour, J.M. Improved Synthesis of Graphene Oxide (vol 4, pg 4806, 2010). ACS Nano 2018, 12, 2078. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.S.; Ren, W.C.; Xu, L.; Li, F.; Cheng, H.M. Doped Graphene Sheets As Anode Materials with Superhigh Rate and Large Capacity for Lithium Ion Batteries. ACS Nano 2011, 5, 5463–5471. [Google Scholar] [CrossRef]
- Yang, Z.; Yao, Z.; Li, G.; Fang, G.; Nie, H.; Liu, Z.; Zhou, X.; Chen, X.A.; Huang, S. Sulfur-Doped Graphene as an Efficient Metal-free Cathode Catalyst for Oxygen Reduction. ACS Nano 2012, 6, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.Z.; Mahmood, N.; Yin, H.; Liu, F.; Hou, Y.L. Synthesis of Phosphorus-Doped Graphene and its Multifunctional Applications for Oxygen Reduction Reaction and Lithium Ion Batteries. Adv. Mater. 2013, 25, 4932–4937. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.L.; Zhou, R.; Zhao, X.S. Graphene-based materials as supercapacitor electrodes. J. Mater. Chem. 2010, 20, 5983–5992. [Google Scholar] [CrossRef]
- Cha, H.A.; Jeong, H.M.; Kang, J.K. Nitrogen-doped open pore channeled graphene facilitating electrochemical performance of TiO2 nanoparticles as an anode material for sodium ion batteries. J. Mater. Chem. A 2014, 2, 5182–5186. [Google Scholar] [CrossRef]
- Park, S.; Ruoff, R.S. Chemical methods for the production of graphenes. Nat. Nanotechnol. 2009, 4, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.R.; Outlaw, R.A.; Holloway, B.C. Graphene Double-Layer Capacitor with ac Line-Filtering Performance. Science 2010, 329, 1637–1639. [Google Scholar] [CrossRef]
- Voiry, D.; Yang, J.; Kupferberg, J.; Fullon, R.; Lee, C.; Jeong, H.Y.; Shin, H.S.; Chhowalla, M. High-quality graphene via microwave reduction of solution-exfoliated graphene oxide. Science 2016, 353, 1413–1416. [Google Scholar] [CrossRef] [Green Version]
- Stankovich, S.; Dikin, D.A.; Piner, R.D.; Kohlhaas, K.A.; Kleinhammes, A.; Jia, Y.; Wu, Y.; Nguyen, S.T.; Ruoff, R.S. Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide. Carbon 2007, 45, 1558–1565. [Google Scholar] [CrossRef]
- Dreyer, D.R.; Park, S.; Bielawski, C.W.; Ruoff, R.S. The chemistry of graphene oxide. Chem. Soc. Rev. 2010, 39, 228–240. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.S.; Zhao, Y.; Jang, H.; Lee, S.Y.; Kim, J.M.; Kim, K.S.; Ahn, J.H.; Kim, P.; Choi, J.Y.; Hong, B.H. Large-scale pattern growth of graphene films for stretchable transparent electrodes. Nature 2009, 457, 706–710. [Google Scholar] [CrossRef]
- Stankovich, S.; Dikin, D.A.; Dommett, G.H.; Kohlhaas, K.M.; Zimney, E.J.; Stach, E.A.; Piner, R.D.; Nguyen, S.T.; Ruoff, R.S. Graphene-based composite materials. Nature 2006, 442, 282–286. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, F.; Yang, X.; Long, G.; Wu, Y.; Zhang, T.; Leng, K.; Huang, Y.; Ma, Y.; Yu, A.; et al. Porous 3D graphene-based bulk materials with exceptional high surface area and excellent conductivity for supercapacitors. Sci. Rep. 2013, 3, 9. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Li, H.; Long, J.; He, J.; Zhang, J.; Zheng, H.; Gou, X. Nitrogen and Phosphorus Dual-doped Porous Carbon Nanosheets for Efficient Oxygen Reduction in Both Alkaline and Acidic Media. ChemCatChem 2018, 10, 4038–4046. [Google Scholar] [CrossRef]
- Tuček, J.; Błoński, P.; Sofer, Z.; Šimek, P.; Petr, M.; Pumera, M.; Otyepka, M.; Zbořil, R. Sulfur Doping Induces Strong Ferromagnetic Ordering in Graphene: Effect of Concentration and Substitution Mechanism. Adv. Mater. 2016, 28, 5045–5053. [Google Scholar] [CrossRef]
- Sheng, Z.H.; Shao, L.; Chen, J.J.; Bao, W.J.; Wang, F.B.; Xia, X.H. Catalyst-Free Synthesis of Nitrogen-Doped Graphene via Thermal Annealing Graphite Oxide with Melamine and Its Excellent Electrocatalysis. ACS Nano 2011, 5, 4350–4358. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Shao, Y.Y.; Matson, D.W.; Li, J.H.; Lin, Y.H. Nitrogen-Doped Graphene and Its Application in Electrochemical Biosensing. ACS Nano 2010, 4, 1790–1798. [Google Scholar] [CrossRef]
- Liang, J.; Jiao, Y.; Jaroniec, M.; Qiao, S.Z. Sulfur and Nitrogen Dual-Doped Mesoporous Graphene Electrocatalyst for Oxygen Reduction with Synergistically Enhanced Performance. Angew. Chem. Int. Ed. 2012, 51, 11496–11500. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Qi, X.Y.; Boey, F.; Zhang, H. Graphene-based composites. Chem. Soc. Rev. 2012, 41, 666–686. [Google Scholar] [CrossRef]
- Sun, L.; Wang, L.; Tian, C.; Tan, T.; Xie, Y.; Shi, K.; Li, M.; Fu, H. Nitrogen-doped graphene with high nitrogen level via a one-step hydrothermal reaction of graphene oxide with urea for superior capacitive energy storage. RSC Adv. 2012, 2, 4498–4506. [Google Scholar] [CrossRef]
- Reddy, A.L.M.; Srivastava, A.; Gowda, S.R.; Gullapalli, H.; Dubey, M.; Ajayan, P.M. Synthesis Of Nitrogen-Doped Graphene Films For Lithium Battery Application. ACS Nano 2010, 4, 6337–6342. [Google Scholar] [CrossRef]
- Su, Z.; Wei, Z.X.; Lai, C.; Deng, H.Q.; Liu, Z.X.; Ma, J.M. Robust pseudo-capacitive Li-I-2 battery enabled by catalytic, adsorptive N-doped graphene interlayer. Energy Storage Mater. 2018, 14, 129–135. [Google Scholar] [CrossRef]
- Ban, F.Y.; Jayabal, S.; Lim, H.N.; Lee, H.W.; Huang, N.M. Synthesis of nitrogen-doped reduced graphene oxide-multiwalled carbon nanotube composite on nickel foam as electrode for high-performance supercapacitor. Ceram. Int. 2017, 43, 20–27. [Google Scholar] [CrossRef]
- Li, D.; Chen, L.; Chen, L.; Sun, Q.; Zhu, M.; Zhang, Y.; Liu, Y.; Liang, Z.; Si, P.; Lou, J.; et al. Potassium gluconate-derived N/S Co-doped carbon nanosheets as superior electrode materials for supercapacitors and sodium-ion batteries. J. Power Sources 2019, 414, 308–316. [Google Scholar] [CrossRef]
- Hu, M.; Zhou, H.; Gan, X.; Yang, L.; Huang, Z.H.; Wang, D.W.; Kang, F.; Lv, R. Ultrahigh rate sodium ion storage with nitrogen-doped expanded graphite oxide in ether-based electrolyte. J. Mater. Chem. A 2018, 6, 1582–1589. [Google Scholar] [CrossRef]
- Wen, Y.Y.; Wang, B.; Huang, C.C.; Wang, L.Z.; Hulicova-Jurcakova, D. Synthesis of Phosphorus-Doped Graphene and its Wide Potential Window in Aqueous Supercapacitors. Chem. Eur. J. 2015, 21, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Martins, T.B.; Miwa, R.H.; da Silva, A.J.R.; Fazzio, A. Electronic and transport properties of boron-doped graphene nanoribbons. Phys. Rev. Lett. 2007, 98, 4. [Google Scholar] [CrossRef]
- Sheng, Z.H.; Gao, H.L.; Bao, W.J.; Wang, F.B.; Xia, X.H. Synthesis of boron doped graphene for oxygen reduction reaction in fuel cells. J. Mater. Chem. 2012, 22, 390–395. [Google Scholar] [CrossRef]
- Cui, D.; Li, H.; Li, M.; Li, C.; Qian, L.; Zhou, B.; Yang, B. Boron-Doped Graphene Directly Grown on Boron-Doped Diamond for High-Voltage Aqueous Supercapacitors. ACS Appl. Energy Mater. 2019, 2, 1526–1536. [Google Scholar] [CrossRef]
- Azadeh, M.S.S.; Kokabi, A.; Hosseini, M.; Fardmanesh, M. Tunable bandgap opening in the proposed structure of silicon-doped graphene. Micro Nano Lett. 2011, 6, 582–585. [Google Scholar] [CrossRef]
- Houmad, M.; Zaari, H.; Benyoussef, A.; El Kenz, A.; Ez-Zahraouy, H. Optical conductivity enhancement and band gap opening with silicon doped graphene. Carbon 2015, 94, 1021–1027. [Google Scholar] [CrossRef]
- Goyenola, C.; Gueorguiev, G.K.; Stafstrom, S.; Hultman, L. Fullerene-like CSx: A first-principles study of synthetic growth. Chem. Phys. Lett. 2011, 506, 86–91. [Google Scholar] [CrossRef] [Green Version]
- Stobinski, L.; Lesiak, B.; Malolepszy, A.; Mazurkiewicz, M.; Mierzwa, B.; Zemek, J.; Jiricek, P.; Bieloshapka, I. Graphene oxide and reduced graphene oxide studied by the XRD, TEM and electron spectroscopy methods. J. Electron Spectrosc. Relat. Phenom. 2014, 195, 145–154. [Google Scholar]
- Hu, M.X.; Zhang, H.W.; Yang, L.; Lv, R.T. Ultrahigh rate sodium-ion storage of SnS/SnS2 heterostructures anchored on S-doped reduced graphene oxide by ion-assisted growth. Carbon 2019, 143, 21–29. [Google Scholar] [CrossRef]
- Zhang, B.X.; Gao, H.; Li, X.L. Synthesis and optical properties of nitrogen and sulfur co-doped graphene quantum dots. New J. Chem. 2014, 38, 4615–4621. [Google Scholar] [CrossRef]
- Wu, Z.S.; Tan, Y.Z.; Zheng, S.; Wang, S.; Parvez, K.; Qin, J.; Shi, X.; Sun, C.; Bao, X.; Feng, X.; et al. Bottom-Up Fabrication of Sulfur-Doped Graphene Films Derived from Sulfur-Annulated Nanographene for Ultrahigh Volumetric Capacitance Micro-Supercapacitors. J. Am. Chem. Soc. 2017, 139, 4506–4512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, S.K.; Lee, H.; Choi, M.S.; Suh, D.H.; Nakhanivej, P.; Park, H.S. Straightforward and controllable synthesis of heteroatom-doped carbon dots and nanoporous carbons for surface-confined energy and chemical storage. Energy Storage Mater. 2018, 12, 331–340. [Google Scholar] [CrossRef]
- Hao, J.; Meng, T.; Shu, D.; Song, X.; Cheng, H.; Li, B.; Zhou, X.; Zhang, F.; Li, Z.; He, C. Synthesis of three dimensional N&S co-doped rGO foam with high capacity and long cycling stability for supercapacitors. J. Colloid Interface Sci. 2019, 537, 57–65. [Google Scholar] [PubMed]
- Chen, X.A.; Chen, X.; Xu, X.; Yang, Z.; Liu, Z.; Zhang, L.; Xu, X.; Chen, Y.; Huang, S. Sulfur-doped porous reduced graphene oxide hollow nanosphere frameworks as metal-free electrocatalysts for oxygen reduction reaction and as supercapacitor electrode materials. Nanoscale 2014, 6, 13740–13747. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Hu, M.; Ai, D.; Zhang, H.; Huang, Z.-H.; Lv, R.; Kang, F. Sulfur-Doped Reduced Graphene Oxide for Enhanced Sodium Ion Pseudocapacitance. Nanomaterials 2019, 9, 752. https://doi.org/10.3390/nano9050752
Wang Y, Hu M, Ai D, Zhang H, Huang Z-H, Lv R, Kang F. Sulfur-Doped Reduced Graphene Oxide for Enhanced Sodium Ion Pseudocapacitance. Nanomaterials. 2019; 9(5):752. https://doi.org/10.3390/nano9050752
Chicago/Turabian StyleWang, Yiting, Mingxiang Hu, Desheng Ai, Hongwei Zhang, Zheng-Hong Huang, Ruitao Lv, and Feiyu Kang. 2019. "Sulfur-Doped Reduced Graphene Oxide for Enhanced Sodium Ion Pseudocapacitance" Nanomaterials 9, no. 5: 752. https://doi.org/10.3390/nano9050752



