Biocompatible Gold Nanoparticles Ameliorate Retinoic Acid-Induced Cell Death and Induce Differentiation in F9 Teratocarcinoma Stem Cells
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis and Characterization of AuNPs Using Luteolin
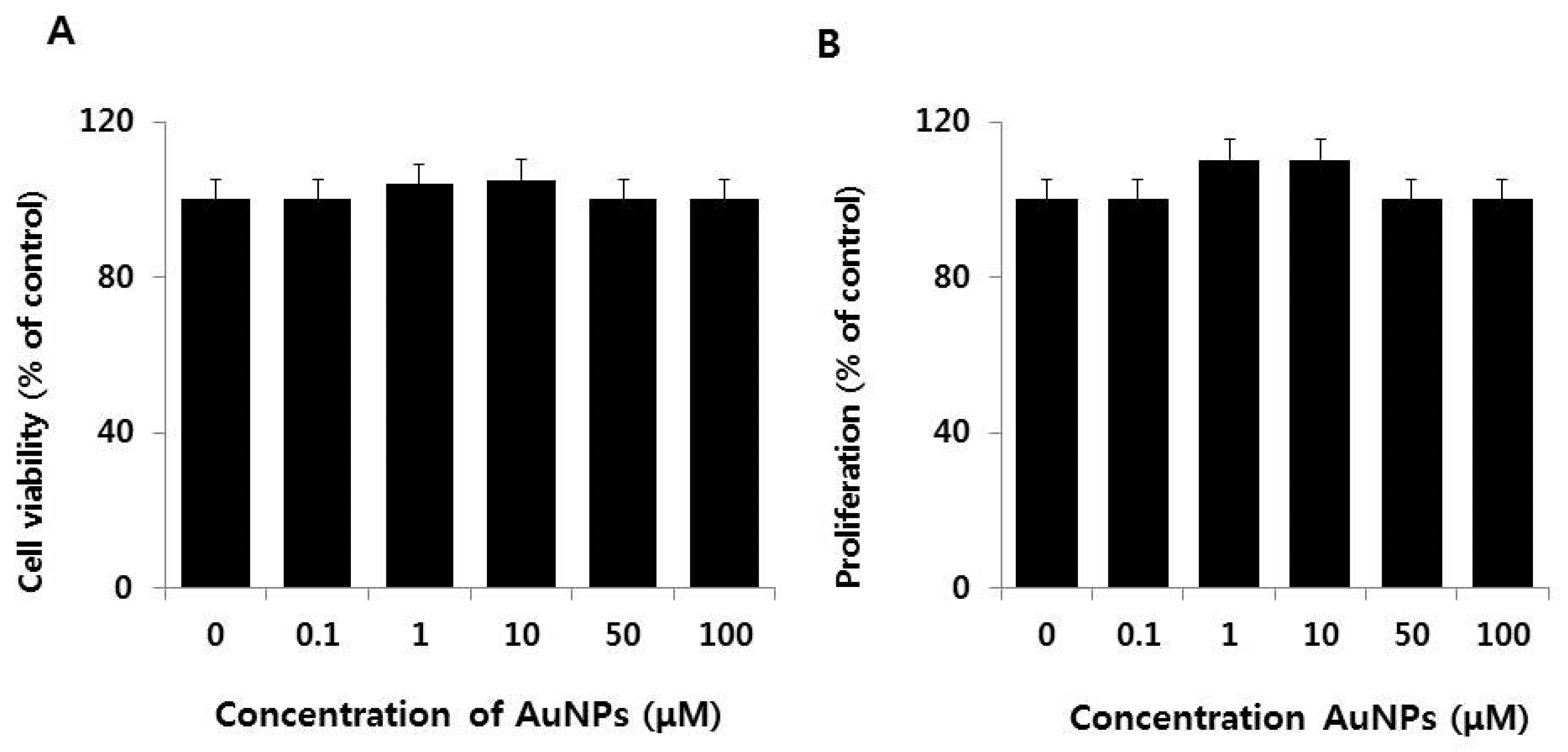
2.2. Dose-Dependent Effect of AuNPs on Cell Viability and Cell Proliferation of F9 Cells
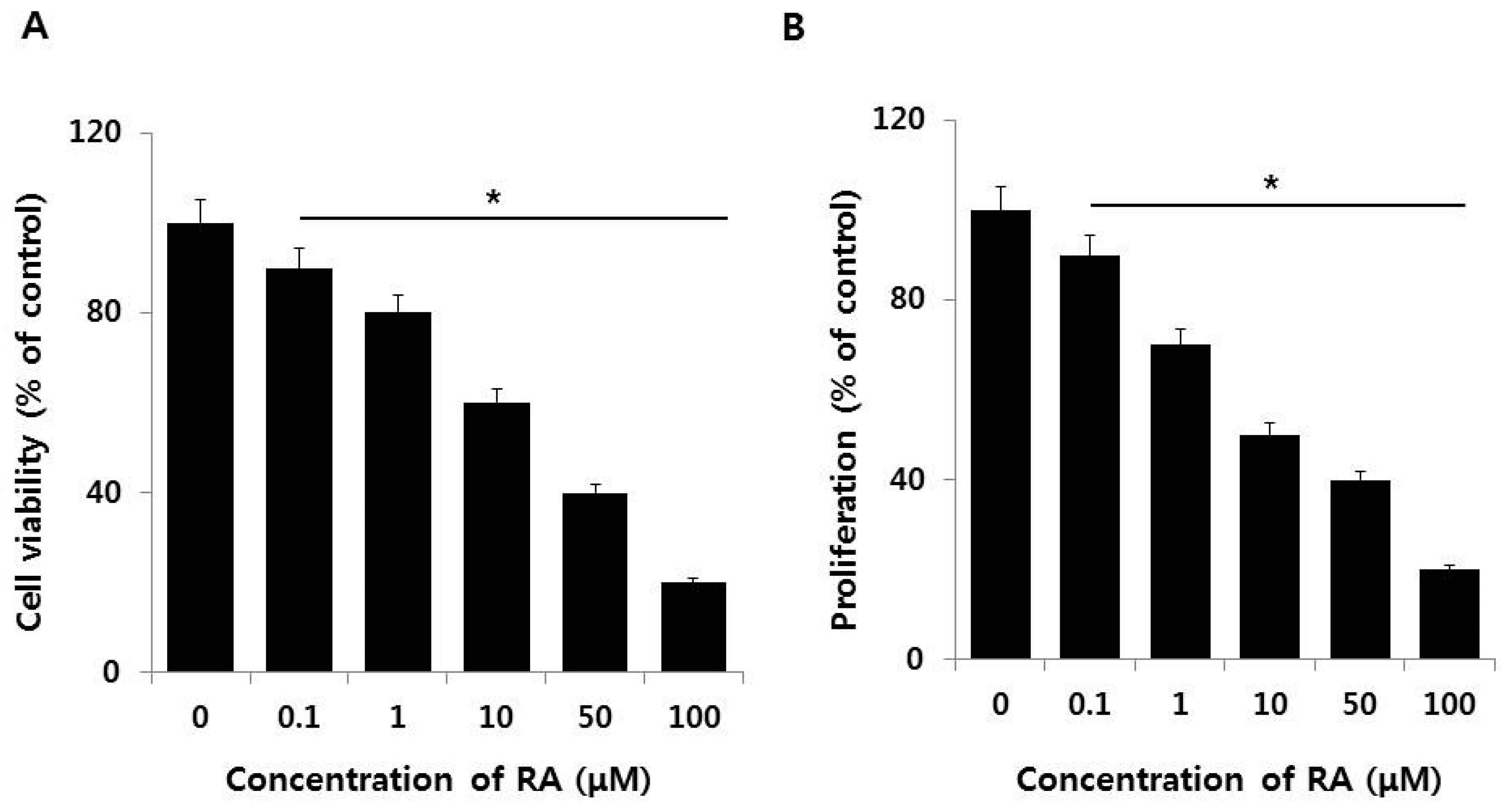
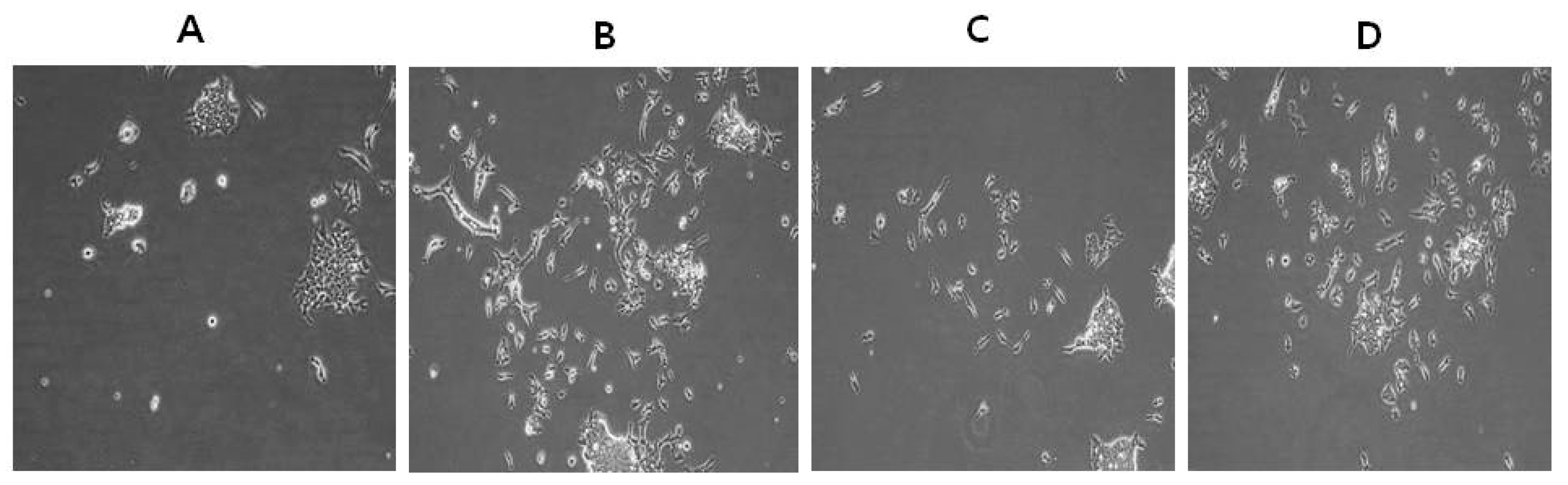
2.3. Effect of AuNPs on RA-Induced Cell Death and Proliferation
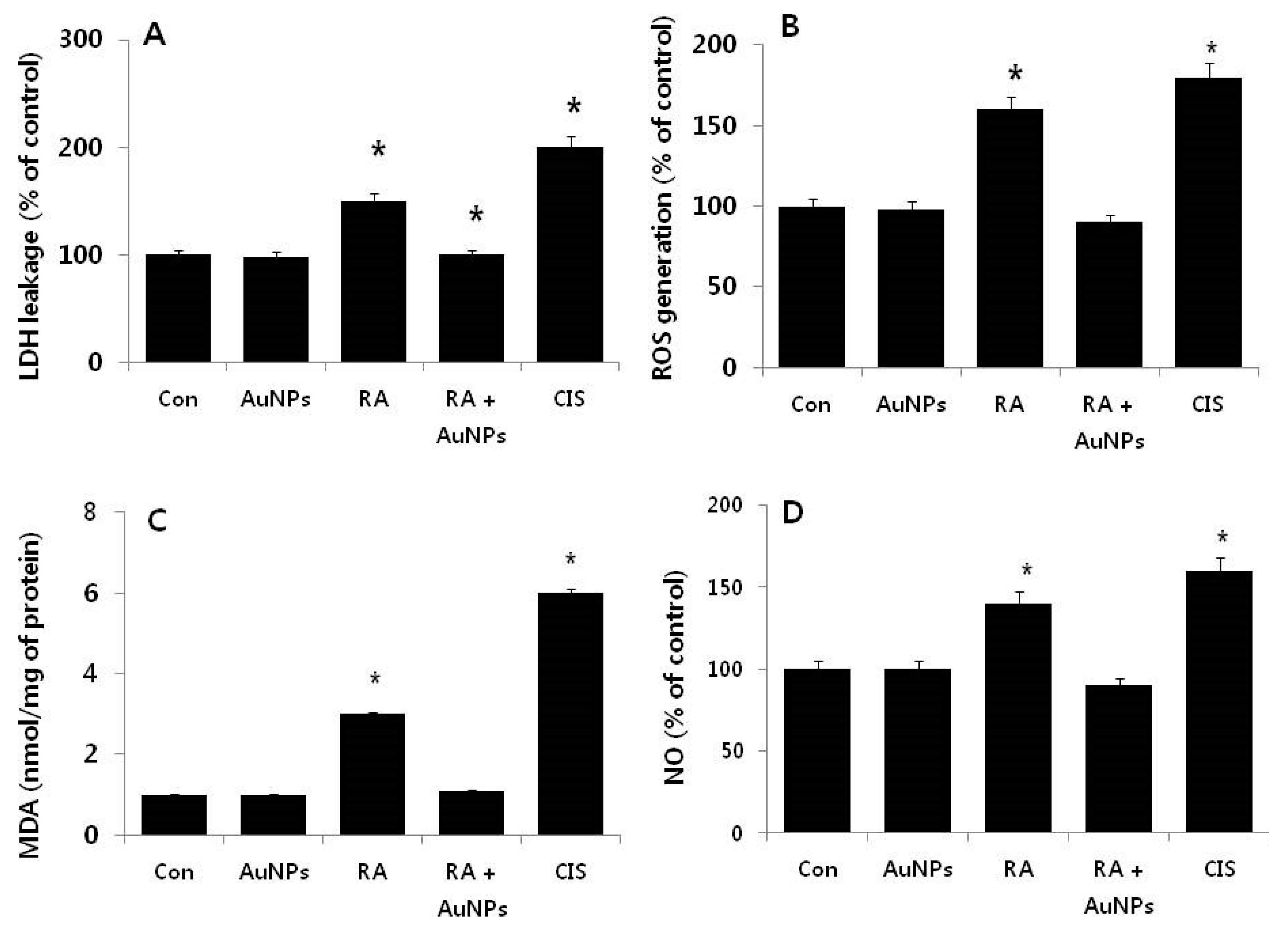
2.4. Effect of AuNPs on RA-Induced Cytotoxicity
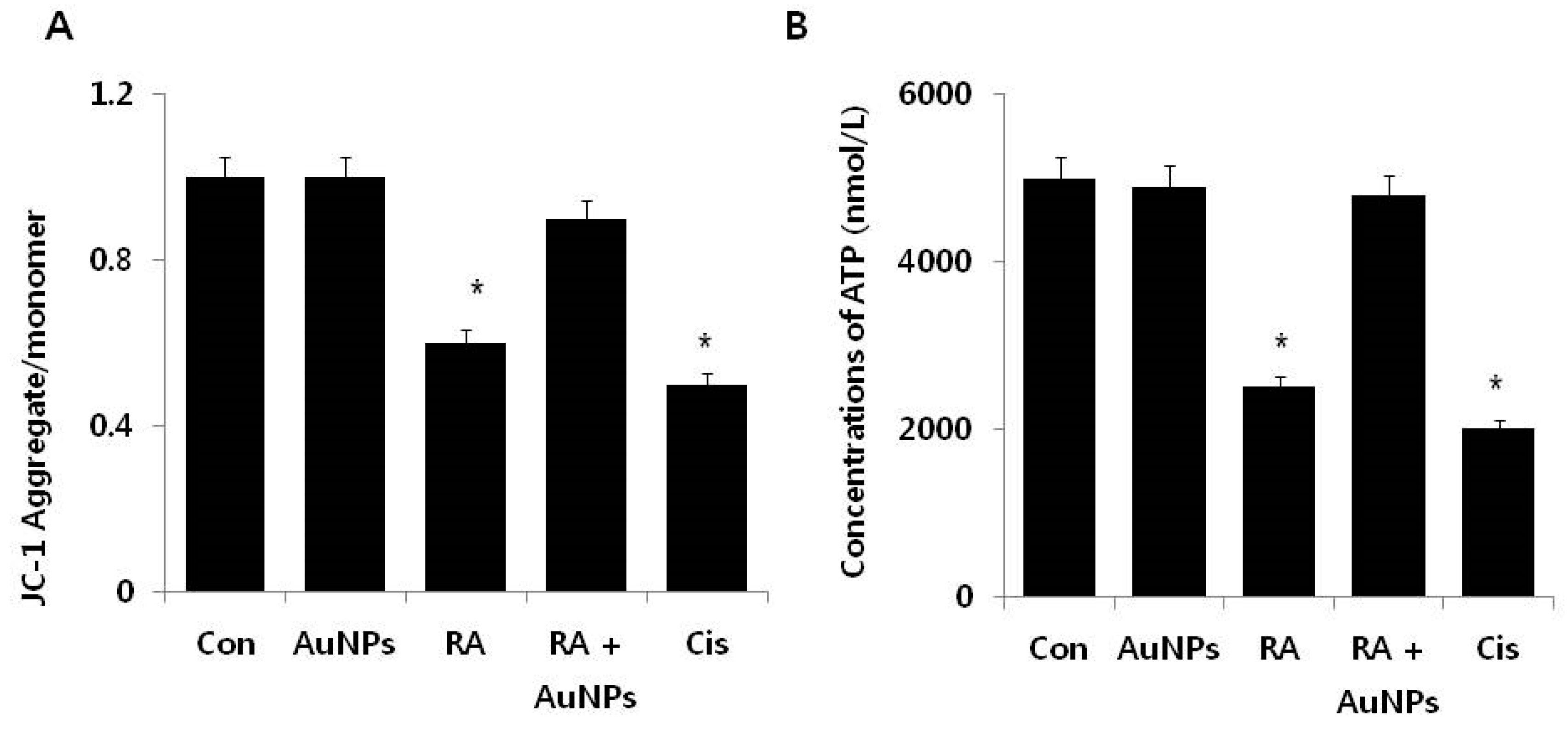
2.5. Effect of AuNPs on RA-Induced Mitochondrial Dysfunction
2.6. Effect of AuNPs on RA-Induced Expression of Anti-Oxidative Stress Markers
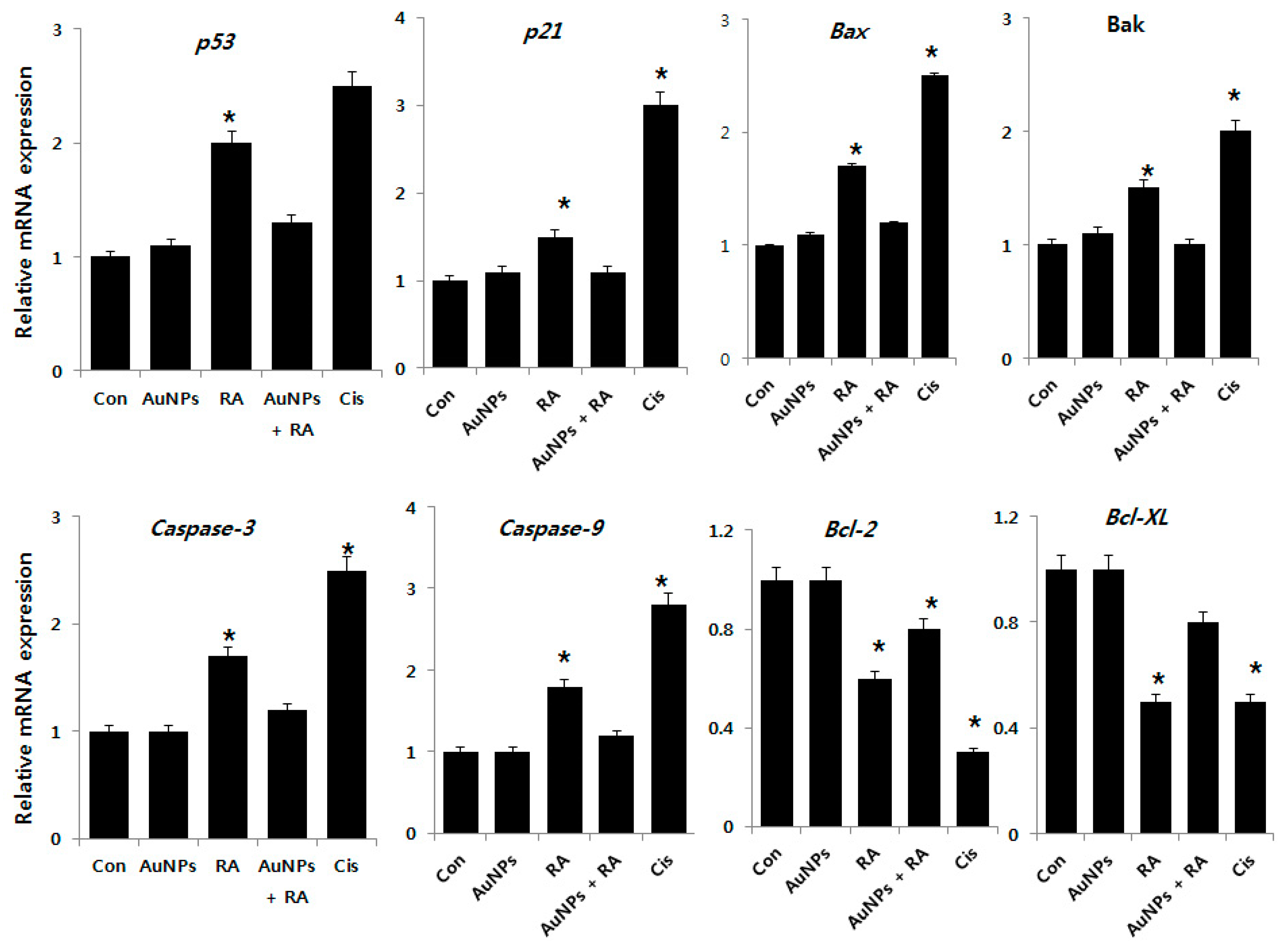
2.7. Effect of AuNPs and RA on Expression of Pro- and Anti-Apoptotic Genes in F9 Cells
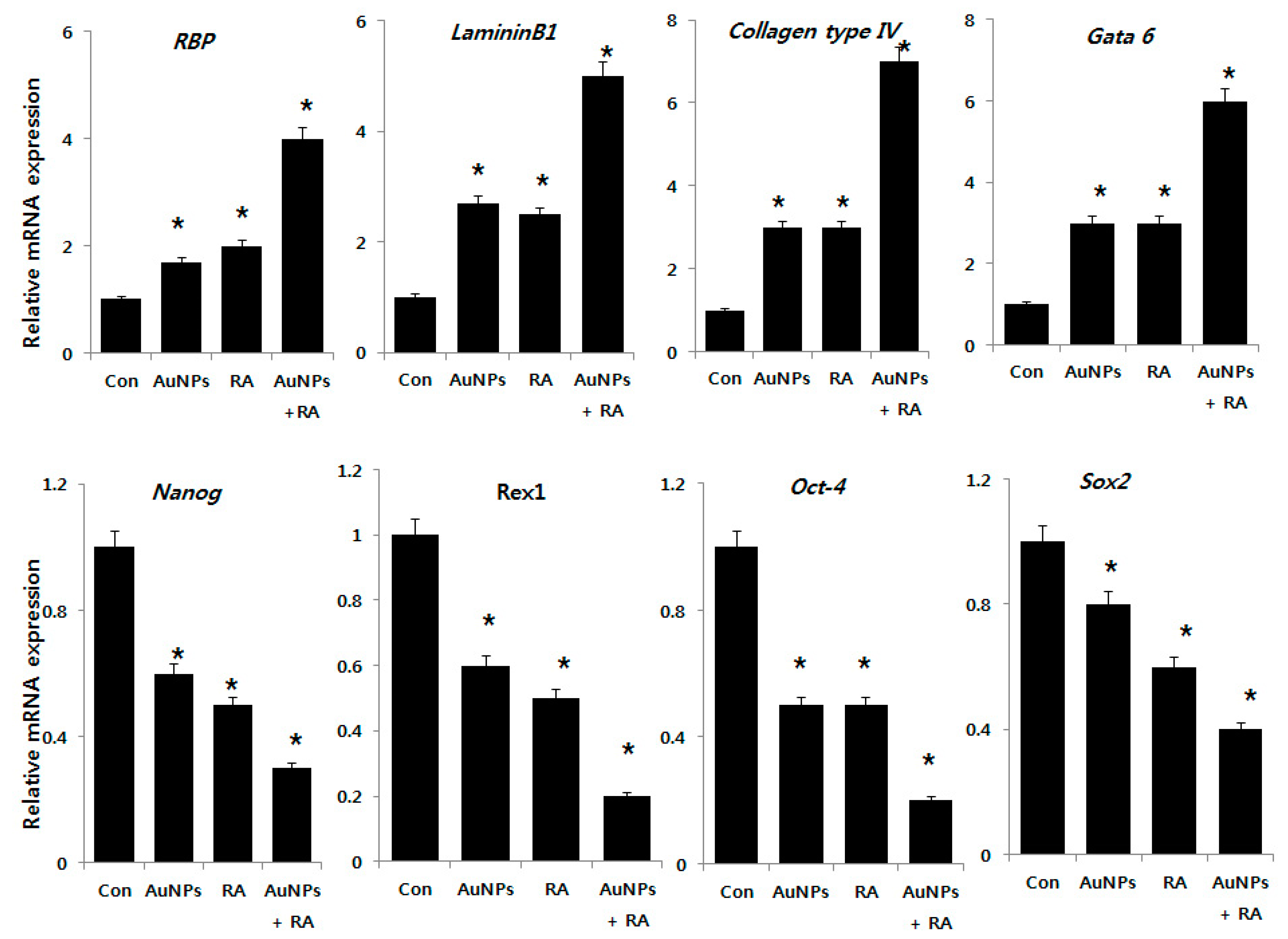
2.8. Differentiation Effect of AuNPs and RA in F9 Cells
2.9. Effect of AuNPs and RA on Expression of Differentiation and Stem Cell Markers in F9 Cells
3. Materials and Methods
3.1. Synthesis and Characterization of AuNPs
3.2. NO Measurement
3.3. Mitochondrial Transmembrane Potential (MTP) Assay
3.4. Measurement of ATP
3.5. Measurement of Anti-Oxidative Stress Markers
3.6. Reverse Transcription-Quantitative Polymerase Chain Reaction (RT-qPCR)
3.7. Statistical Analyses
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Barathmanikanth, S.; Kalishwaralal, K.; Sriram, M.; Pandian, S.R.; Youn, H.S.; Eom, S.; Gurunathan, S. Anti-oxidant effect of gold nanoparticles restrains hyperglycemic conditions in diabetic mice. J. Nanobiotechnol. 2010, 8, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dykman, L.A.; Khlebtsov, N.G. Gold nanoparticles in biology and medicine: Recent advances and prospects. Acta Nat. 2011, 3, 34–55. [Google Scholar]
- Jeon, K.I.; Byun, M.S.; Jue, D.M. Gold compound auranofin inhibits ikappab kinase (ikk) by modifying cys-179 of ikkbeta subunit. Exp. Mol. Med. 2003, 35, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Shah Zahoor, A.; Vohora Shashi, B. Antioxidant/restorative effects of calcined gold preparations used in indian systems of medicine against global and focal models of Ischaemia. Pharmacol. Toxicol. 2002, 90, 254–259. [Google Scholar] [CrossRef]
- Mukherjee, P.; Bhattacharya, R.; Wang, P.; Wang, L.; Basu, S.; Nagy, J.A.; Atala, A.; Mukhopadhyay, D.; Soker, S. Antiangiogenic properties of gold nanoparticles. Clin. Cancer Res. 2005, 11, 3530–3534. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.F.; Shen, W.; Gurunathan, S. Biologically synthesized gold nanoparticles ameliorate cold and heat stress-induced oxidative stress in Escherichia coli. Molecules 2016, 21, 731. [Google Scholar] [CrossRef] [PubMed]
- Sobhana, S.S.L.; Sundaraseelan, J.; Sekar, S.; Sastry, T.P.; Mandal, A.B. Gelatin–chitosan composite capped gold nanoparticles: A matrix for the growth of hydroxyapatite. J. Nanopart. Res. 2009, 11, 333–340. [Google Scholar] [CrossRef]
- Yi, C.; Liu, D.; Fong, C.C.; Zhang, J.; Yang, M. Gold nanoparticles promote osteogenic differentiation of mesenchymal stem cells through p38 MAPK pathway. ACS Nano 2010, 4, 6439–6448. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.Y.; Song, M.S.; Ryu, P.D.; Lam, A.T.; Joo, S.W.; Lee, S.Y. Gold nanoparticles promote osteogenic differentiation in human adipose-derived mesenchymal stem cells through the wnt/beta-catenin signaling pathway. Int. J. Nanomed. 2015, 10, 4383–4392. [Google Scholar]
- Zhang, D.; Liu, D.; Zhang, J.; Fong, C.; Yang, M. Gold nanoparticles stimulate differentiation and mineralization of primary osteoblasts through the ERK/MAPK signaling pathway. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 42, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Shi, X.; Chen, F. The effect of gold nanoparticles on the proliferation and differentiation of murine osteoblast: A study of mc3t3-e1 cells in vitro. J. Nanosci. Nanotechnol. 2014, 14, 4851–4857. [Google Scholar] [CrossRef] [PubMed]
- Dvir, T.; Timko, B.P.; Brigham, M.D.; Naik, S.R.; Karajanagi, S.S.; Levy, O.; Jin, H.; Parker, K.K.; Langer, R.; Kohane, D.S. Nanowired three-dimensional cardiac patches. Nat. Nanotechnol. 2011, 6, 720–725. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tian, B.; Liu, J.; Dvir, T.; Jin, L.; Tsui, J.H.; Qing, Q.; Suo, Z.; Langer, R.; Kohane, D.S.; Lieber, C.M. Macroporous nanowire nanoelectronic scaffolds for synthetic tissues. Nat. Mater. 2012, 11, 986–994. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, K.; Chen, X.; Chen, W.; Zhang, L.; Li, J.; Ye, J.; Zhang, Y.; Zhang, L.; Li, C.H.; Yin, L.; et al. Neuroprotective effect of gold nanoparticles composites in parkinson’s disease model. Nanomedicine 2018, 14, 1123–1136. [Google Scholar] [CrossRef] [PubMed]
- Wightman, J.; Roberson, M.S.; Lamkin, T.J.; Varvayanis, S.; Yen, A. Retinoic acid-induced growth arrest and differentiation: Retinoic acid up-regulates cd32 expression, the ectopic expression of which retards the cell cycle. Mol. Cancer Ther. 2002, 1, 493–506. [Google Scholar] [PubMed]
- Castro-Obregon, S.; Covarrubias, L. Role of retinoic acid and oxidative stress in embryonic stem cell death and neuronal differentiation. FEBS Lett. 1996, 381, 93–97. [Google Scholar] [CrossRef] [Green Version]
- Cunningham, T.J.; Duester, G. Mechanisms of retinoic acid signalling and its roles in organ and limb development. Nat. Rev. Mol. Cell Biol. 2015, 16, 110–123. [Google Scholar] [PubMed] [Green Version]
- Ammer, H.; Schulz, R. Retinoic acid-induced differentiation of human neuroblastoma sh-sy5y cells is associated with changes in the abundance of g proteins. J. Neurochem. 2008, 62, 1310–1318. [Google Scholar] [CrossRef]
- Mandili, G.; Marini, C.; Carta, F.; Zanini, C.; Prato, M.; Khadjavi, A.; Turrini, F.; Giribaldi, G. Identification of phosphoproteins as possible differentiation markers in all-trans-retinoic acid-treated neuroblastoma cells. PLoS ONE 2011, 6, e18254. [Google Scholar] [CrossRef] [PubMed]
- Kalemkerian Gregory, P.; Jiroutek, M.; Ettinger David, S.; Dorighi John, A.; Johnson David, H.; Mabry, M. A phase ii study of all-trans-retinoic acid plus cisplatin and etoposide in patients with extensive stage small cell lung carcinoma. Cancer 2000, 83, 1102–1108. [Google Scholar] [CrossRef]
- Syed, Z.; Cheepala, S.B.; Gill, J.N.; Stein, J.; Nathan, C.A.; DiGiovanni, J.; Batra, V.; Adegboyega, P.; Kleiner, H.E.; Clifford, J.L. All-trans retinoic acid suppresses STAT-3 signaling during skin carcinogenesis. Cancer Prev. Res. 2009, 2, 903–911. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Duvic, M. Treatment of cutaneous t-cell lymphoma with retinoids. Dermatol. Ther. 2006, 19, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Sanz, M.A.; Montesinos, P.; Rayón, C.; Holowiecka, A.; de la Serna, J.; Milone, G.; de Lisa, E.; Brunet, S.; Rubio, V.; Ribera, J.M.; et al. Risk-adapted treatment of acute promyelocytic leukemia based on retinoic acid and anthracycline with addition of cytarabine in consolidation therapy for high-risk patients: Further improvements in treatment outcome. Blood 2010, 115, 5137–5146. [Google Scholar] [CrossRef] [PubMed]
- Gudas Lorraine, J.; Wagner John, A. Retinoids regulate stem cell differentiation. J. Cell. Physiol. 2010, 226, 322–330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toma, S.; Isnardi, L.; Raffo, P.; Dastoli, G.; De Francisci, E.; Riccardi, L.; Palumbo, R.; Bollag, W. Effects of all-trans-retinoic acid and 13-cis-retinoic acid on breast-cancer cell lines: Growth inhibition and apoptosis induction. Int. J. Cancer 1998, 70, 619–627. [Google Scholar] [CrossRef]
- Nagy, L.; Thomazy, V.A.; Shipley, G.L.; Fesus, L.; Lamph, W.; Heyman, R.A.; Chandraratna, R.A.; Davies, P.J. Activation of retinoid x receptors induces apoptosis in hl-60 cell lines. Mol. Cell Biol. 1995, 15, 3540–3551. [Google Scholar] [CrossRef] [PubMed]
- Mrass, P.; Rendl, M.; Mildner, M.; Gruber, F.; Lengauer, B.; Ballaun, C.; Eckhart, L.; Tschachler, E. Retinoic acid increases the expression of p53 and proapoptotic caspases and sensitizes keratinocytes to apoptosis: A possible explanation for tumor preventive action of retinoids. Cancer Res. 2004, 64, 6542–6548. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, N.D.; Sun, Y.; Price, B.D. Activation of the kinase activity of atm by retinoic acid is required for creb-dependent differentiation of neuroblastoma cells. J. Biol. Chem. 2007, 282, 16577–16584. [Google Scholar] [CrossRef] [PubMed]
- Tokarz, P.; Piastowska-Ciesielska, A.W.; Kaarniranta, K.; Blasiak, J. All-trans retinoic acid modulates DNA damage response and the expression of the vegf-a and mki67 genes in ARPE-19 cells subjected to oxidative stress. Int. J. Mol. Sci. 2016, 17, 898. [Google Scholar] [CrossRef] [PubMed]
- Gramzinski, R.A.; Parchment, R.E.; Pierce, G.B. Evidence linking programmed cell death in the blastocyst to polyamine oxidation. Differentiation 1990, 43, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Haiss, W.; Thanh, N.T.K.; Aveyard, J.; Fernig, D.G. Determination of size and concentration of gold nanoparticles from uv−vis spectra. Anal. Chem. 2007, 79, 4215–4221. [Google Scholar] [CrossRef] [PubMed]
- Gurunathan, S.; Han, J.; Park, J.H.; Kim, J.H. A green chemistry approach for synthesizing biocompatible gold nanoparticles. Nanoscale Res. Lett. 2014, 9, 248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levchenko, L.A.; Golovanova, S.A.; Lariontseva, N.V.; Sadkov, A.P.; Voilov, D.N.; Shul’ga, Y.M.; Nikitenko, N.G.; Shestakov, A.F. Synthesis and study of gold nanoparticles stabilized by bioflavonoids. Russ. Chem. Bull. 2011, 6, 426. [Google Scholar] [CrossRef]
- Kalishwaralal, K.; Sheikpranbabu, S.; BarathManiKanth, S.; Haribalaganesh, R.; Ramkumarpandian, S.; Gurunathan, S. Retracted article: Gold nanoparticles inhibit vascular endothelial growth factor-induced angiogenesis and vascular permeability via src dependent pathway in retinal endothelial cells. Angiogenesis 2011, 14, 29–45. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Wang, Y.; Ma, J.; Yang, G. A biocompatible synthesis of gold nanoparticles by tris(hydroxymethyl)aminomethane. Nanoscale Res. Lett. 2014, 9, 220. [Google Scholar] [CrossRef] [PubMed]
- Kalishwaralal, K.; Deepak, V.; Ram Kumar Pandian, S.; Gurunathan, S. Biological synthesis of gold nanocubes from bacillus licheniformis. Bioresour. Technol. 2009, 100, 5356–5358. [Google Scholar] [CrossRef] [PubMed]
- Gurunathan, S.; Han, J.; Park, J.H.; Kim, J.H. An in vitro evaluation of graphene oxide reduced by ganoderma spp. In human breast cancer cells (MDA-MB-231). Int. J. Nanomed. 2014, 9, 1783–1797. [Google Scholar] [CrossRef] [PubMed]
- Murugan, M.; Anthony, K.J.P.; Jeyaraj, M.; Rathinam, N.K.; Gurunathan, S. Biofabrication of gold nanoparticles and its biocompatibility in human breast adenocarcinoma cells (MCF-7). J. Ind. Eng. Chem. 2014, 20, 1713–1719. [Google Scholar] [CrossRef]
- Pan, Y.; Neuss, S.; Leifert, A.; Fischler, M.; Wen, F.; Simon, U.; Schmid, G.; Brandau, W.; Jahnen-Dechent, W. Size-dependent cytotoxicity of gold nanoparticles. Small 2007, 3, 1941–1949. [Google Scholar] [CrossRef] [PubMed]
- Hau, H.; Khanal, D.; Rogers, L.; Suchowerska, N.; Kumar, R.; Sridhar, S.; McKenzie, D.; Chrzanowski, W. Dose enhancement and cytotoxicity of gold nanoparticles in colon cancer cells when irradiated with kilo- and mega-voltage radiation. Bioeng. Transl. Med. 2016, 1, 94–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Connor Ellen , E.; Mwamuka, J.; Gole, A.; Murphy Catherine , J.; Wyatt Michael, D. Gold nanoparticles are taken up by human cells but do not cause acute cytotoxicity. Small 2005, 1, 325–327. [Google Scholar] [CrossRef] [PubMed]
- Chueh, P.J.; Liang, R.-Y.; Lee, Y.-H.; Zeng, Z.-M.; Chuang, S.-M. Differential cytotoxic effects of gold nanoparticles in different mammalian cell lines. J. Hazard. Mater. 2014, 264, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Koch, F.; Möller, A.-M.; Frenz, M.; Pieles, U.; Kuehni-Boghenbor, K.; Mevissen, M. An in vitro toxicity evaluation of gold-, plla- and pcl-coated silica nanoparticles in neuronal cells for nanoparticle-assisted laser-tissue soldering. Toxicol. In Vitro 2014, 28, 990–998. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Zhang, J.; Yi, C.; Yang, M. The effects of gold nanoparticles on the proliferation, differentiation, and mineralization function of MC3T3-E1 cells in vitro. Chin. Sci. Bull. 2010, 55, 1013–1019. [Google Scholar] [CrossRef]
- Ponzoni, M.; Bocca, P.; Chiesa, V.; Decensi, A.; Pistoia, V.; Raffaghello, L.; Rozzo, C.; Montaldo, P.G. Differential effects of N-(4-hydroxyphenyl) retinamide and retinoic acid on neuroblastoma cells: Apoptosis versus differentiation. Cancer Res. 1995, 55, 853–861. [Google Scholar] [PubMed]
- Lovat, P.E.; Irving, H.; Pearson, A.D.J.; Redfern, C.P.F.; Malcolm, A.J.; Annicchiarico-Petruzzelli, M.; Bernassola, F.; Melino, G. Apoptosis of n-type neuroblastoma cells after differentiation with 9-cis-retinoic acid and subsequent washout. JNCI J. Natl. Cancer Inst. 1997, 89, 446–452. [Google Scholar] [CrossRef] [PubMed]
- Voigt, A.; Hartmann, P.; Zintl, F. Differentiation, proliferation and adhesion of human neuroblastoma cells after treatment with retinoic acid. Cell Adhes. Commun. 2000, 7, 423–440. [Google Scholar] [CrossRef] [PubMed]
- Voigt, A.; Zintl, F. Effects of retinoic acid on proliferation, apoptosis, cytotoxicity, migration, and invasion of neuroblastoma cells. Med. Pediatr. Oncol. 2003, 40, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Zhang, E.; Fu, A. Promotion of sh-sy5y cell growth by gold nanoparticles modified with 6-mercaptopurine and a neuron-penetrating peptide. Nanoscale Res. Lett. 2017, 12, 641. [Google Scholar] [CrossRef] [PubMed]
- Gunduz, N.; Ceylan, H.; Guler, M.O.; Tekinay, A.B. Intracellular accumulation of gold nanoparticles leads to inhibition of macropinocytosis to reduce the endoplasmic reticulum stress. Sci. Rep. UK 2017, 7, 40493. [Google Scholar] [CrossRef] [PubMed]
- Leeman, W.R.; van de Gevel, I.A.; Rutten, A.A.J.J.L. Cytotoxicity of retinoic acid, menadione and aflatoxin b1 in rat liver slices using netwell inserts as a new culture system. Toxicol. In Vitro 1995, 9, 291–298. [Google Scholar] [CrossRef]
- Schieber, M.; Chandel, N.S. Ros function in redox signaling and oxidative stress. Curr. Biol. CB 2014, 24, R453–R462. [Google Scholar] [CrossRef] [PubMed]
- Dayem, A.A.; Kim, B.; Gurunathan, S.; Choi, H.Y.; Yang, G.; Saha, S.K.; Han, D.; Han, J.; Kim, K.; Kim, J.H.; et al. Biologically synthesized silver nanoparticles induce neuronal differentiation of SH-SY5Y cells via modulation of reactive oxygen species, phosphatases, and kinase signaling pathwayss. Biotechnol. J. 2014, 9, 934–943. [Google Scholar] [CrossRef] [PubMed]
- Hodges, D.M.; DeLong, J.M.; Forney, C.F.; Prange, R.K. Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 1999, 207, 604–611. [Google Scholar] [CrossRef]
- Nagl, F.; Schönhofer, K.; Seidler, B.; Mages, J.; Allescher, H.-D.; Schmid, R.M.; Schneider, G.; Saur, D. Retinoic acid-induced nnos expression depends on a novel pi3k/akt/dax1 pathway in human TGW-NU-I neuroblastoma cells. Am. J. Physiol. Cell Physiol. 2009, 297, C1146–C1156. [Google Scholar] [CrossRef] [PubMed]
- Tamplenizza, M.; Lenardi, C.; Maffioli, E.; Nonnis, S.; Negri, A.; Forti, S.; Sogne, E.; De Astis, S.; Matteoli, M.; Schulte, C.; et al. Nitric oxide synthase mediates PC12 differentiation induced by the surface topography of nanostructured TiO2. J. Nanobiotechnol. 2013, 11, 35. [Google Scholar] [CrossRef] [PubMed]
- Personett, D.; Fass, U.; Panickar, K.; McKinney, M. Retinoic acid-mediated enhancement of the cholinergic/neuronal nitric oxide synthase phenotype of the medial septal sn56 clone. J. Neurochem. 2002, 74, 2412–2424. [Google Scholar] [CrossRef]
- de Oliveira, M.R.; Oliveira, M.W.S.; Behr, G.A.; Hoff, M.L.M.; da Rocha, R.F.; Moreira, J.C.F. Evaluation of the effects of vitamin a supplementation on adult rat substantia nigra and striatum redox and bioenergetic states: Mitochondrial impairment, increased 3-nitrotyrosine and α-synuclein, but decreased d2 receptor contents. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2009, 33, 353–362. [Google Scholar] [CrossRef] [PubMed]
- Bernardi, P.; Broekemeier, K.M.; Pfeiffer, D.R. Recent progress on regulation of the mitochondrial permeability transition pore; a cyclosporin-sensitive pore in the inner mitochondrial membrane. J. Bioenerg. Biomembr. 1994, 26, 509–517. [Google Scholar] [CrossRef] [PubMed]
- Nieminen, A.L.; Saylor, A.K.; Tesfai, S.A.; Herman, B.; Lemasters, J.J. Contribution of the mitochondrial permeability transition to lethal injury after exposure of hepatocytes to butylhydroperoxide. Biochem. J. 1995, 307, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, M.P.C.; Santos, A.E.; Santos, M.S.; Custódio, J.B.A. Effects of all-trans-retinoic acid on the permeability transition and bioenergetic functions of rat liver mitochondria in combination with endoxifen. Life Sci. 2013, 93, 96–107. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B. Reactive species and antioxidants. Redox biology is a fundamental theme of aerobic life. Plant Physiol. 2006, 141, 312–322. [Google Scholar] [CrossRef] [PubMed]
- Klamt, F.; Roberto de Oliveira, M.; Moreira, J.C. Retinol induces permeability transition and cytochrome c release from rat liver mitochondria. Biochim. Biophys. Acta 2005, 1726, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Silva, F.S.G.; Ribeiro, M.P.C.; Santos, M.S.; Rocha-Pereira, P.; Santos-Silva, A.; Custódio, J.B.A. Acitretin affects bioenergetics of liver mitochondria and promotes mitochondrial permeability transition: Potential mechanisms of hepatotoxicity. Toxicology 2013, 306, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Sawada, O.; Perusek, L.; Kohno, H.; Howell, S.J.; Maeda, A.; Matsuyama, S.; Maeda, T. All-trans-retinal induces bax activation via DNA damage to mediate retinal cell apoptosis. Exp. Eye Res. 2014, 123, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B. Role of free radicals in the neurodegenerative diseases: Therapeutic implications for antioxidant treatment. Drugs Aging 2001, 18, 685–716. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Garcia, A.; Hevia, D.; Mayo, J.C.; Gonzalez-Menendez, P.; Coppo, L.; Lu, J.; Holmgren, A.; Sainz, R.M. Thioredoxin 1 modulates apoptosis induced by bioactive compounds in prostate cancer cells. Redox Biol. 2017, 12, 634–647. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.-S. The signaling mechanism of ros in tumor progression. Cancer Metastasis Rev. 2006, 25, 695–705. [Google Scholar] [CrossRef] [PubMed]
- Benhar, M.; Engelberg, D.; Levitzki, A. Ros, stress-activated kinases and stress signaling in cancer. EMBO Rep. 2002, 3, 420–425. [Google Scholar] [CrossRef] [PubMed]
- Pelicano, H.; Carney, D.; Huang, P. Ros stress in cancer cells and therapeutic implications. Drug Resist. Updates Rev. Comment. Antimicrob. Anticancer Chemother. 2004, 7, 97–110. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.-W.; Zhang, J.; Townsend, D.M.; Tew, K.D. Oxidative stress, redox regulation and diseases of cellular differentiation. Biochim. Biophys. Acta 2015, 1850, 1607–1621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Filomeni, G.; De Zio, D.; Cecconi, F. Oxidative stress and autophagy: The clash between damage and metabolic needs. Cell Death Differ. 2015, 22, 377–388. [Google Scholar] [CrossRef] [PubMed]
- Opferman, J.T.; Korsmeyer, S.J. Apoptosis in the development and maintenance of the immune system. Nat. Immunol. 2003, 4, 410–415. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Parant, J.M.; Lang, G.; Chau, P.; Chavez-Reyes, A.; El-Naggar, A.K.; Multani, A.; Chang, S.; Lozano, G. Chromosome stability, in the absence of apoptosis, is critical for suppression of tumorigenesis in TRP53 mutant mice. Nat. Genet. 2003, 36, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Yang, L.; Guo, S. All-trans retinoic acid inhibits migration, invasion and proliferation, and promotes apoptosis in glioma cells in vitro. Oncol. Lett. 2015, 9, 2833–2838. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bao George, C.; Wang, J.-G.; Jong, A. Increased p21 expression and complex formation with cyclin E/CDK2 in retinoid-induced pre-b lymphoma cell apoptosis. FEBS Lett. 2006, 580, 3687–3693. [Google Scholar]
- Assefa, Z.; Vantieghem, A.; Garmyn, M.; Declercq, W.; Vandenabeele, P.; Vandenheede, J.R.; Bouillon, R.; Merlevede, W.; Agostinis, P. P38 mitogen-activated protein kinase regulates a novel, caspase-independent pathway for the mitochondrial cytochromec release in ultraviolet b radiation-induced apoptosis. J. Biol. Chem. 2000, 275, 21416–21421. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.W.; Wang, J.Y.; Chen, S.J.; Chen, Z. Mechanisms of all-trans retinoic acid-induced differentiation of acute promyelocytic leukemia cells. J. Biosci. 2000, 25, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Holmes, W.F.; Soprano, D.R.; Soprano, K.J. Elucidation of molecular events mediating induction of apoptosis by synthetic retinoids using a CD437-resistant ovarian carcinoma cell line. J. Biol. Chem. 2002, 277, 45408–45419. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Lara, A.M.; Aranda, A.; Gronemeyer, H. Retinoic acid protects human breast cancer cells against etoposide-induced apoptosis by NF-KAPPAB-dependent but CIAP2-independent mechanisms. Mol. Cancer 2010, 9, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kitabayashi, I.; Chiu, R.; Umesono, K.; Evans, R.M.; Gachelin, G.; Yokoyama, K. A novel pathway for retinoic acid-induced differentiation of f9 cells that is distinct from receptor-mediatedtrans-activation. In Vitro Cell. Dev. Biol. Anim. 1994, 30, 761–768. [Google Scholar]
- Cheung, Y.-T.; Lau, W.K.-W.; Yu, M.-S.; Lai, C.S.-W.; Yeung, S.-C.; So, K.-F.; Chang, R.C.-C. Effects of all-trans-retinoic acid on human SH-SY5Y neuroblastoma as in vitro model in neurotoxicity research. NeuroToxicology 2009, 30, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Wendling, O.; Ghyselinck, N.B.; Chambon, P.; Mark, M. Roles of retinoic acid receptors in early embryonic morphogenesis and hindbrain patterning. Development 2001, 128, 2031–2038. [Google Scholar] [PubMed]
- Wei, M.; Li, S.; Yang, Z.; Zheng, W.; Le, W. Gold nanoparticles enhance the differentiation of embryonic stem cells into dopaminergic neurons via mtor/p70s6k pathway. Nanomedicine 2017, 12, 1305–1317. [Google Scholar] [PubMed]
- Han, J.W.; Gurunathan, S.; Choi, Y.-J.; Kim, J.-H. Dual functions of silver nanoparticles in f9 teratocarcinoma stem cells, a suitable model for evaluating cytotoxicity- and differentiation-mediated cancer therapy. Int. J. Nanomed. 2017, 12, 7529–7549. [Google Scholar]
- Kohl, Y.; Gorjup, E.; Katsen-Globa, A.; Büchel, C.; von Briesen, H.; Thielecke, H. Effect of gold nanoparticles on adipogenic differentiation of human mesenchymal stem cells. J. Nanopart. Res. 2011, 13, 6789–6803. [Google Scholar]
- Fan, J.H.; Hung, W.I.; Li, W.T.; Yeh, J.M. Biocompatibility study of gold nanoparticles to human cells. In Proceedings of the 13th International Conference on Biomedical Engineering, Singapore, 3–6 December 2008; Lim, C.T., Goh, J.C.H., Eds.; Springer: Berlin/Heidelberg, Germany, 2009; pp. 870–873. [Google Scholar]
- Senut, M.C.; Zhang, Y.; Liu, F.; Sen, A.; Ruden Douglas, M.; Mao, G. Size-dependent toxicity of gold nanoparticles on human embryonic stem cells and their neural derivatives. Small 2015, 12, 631–646. [Google Scholar] [PubMed]
- Rogers, M.B.; Hosler, B.A.; Gudas, L.J. Specific expression of a retinoic acid-regulated, zinc-finger gene, rex-1, in preimplantation embryos, trophoblast and spermatocytes. Development 1991, 113, 815–824. [Google Scholar] [PubMed]
- Woo, D.G.; Shim, M.-S.; Park, J.S.; Yang, H.N.; Lee, D.-R.; Park, K.-H. The effect of electrical stimulation on the differentiation of hescs adhered onto fibronectin-coated gold nanoparticles. Biomaterials 2009, 30, 5631–5638. [Google Scholar] [CrossRef] [PubMed]
- Gordeeva, O.; Khaydukov, S. Tumorigenic and differentiation potentials of embryonic stem cells depend on tgfβfamily signaling: Lessons from teratocarcinoma cells stimulated to differentiate with retinoic acid. Stem Cells Int. 2017, 2017, 7284872. [Google Scholar] [PubMed]
- Gurunathan, S.; Kim, J.-H. Graphene oxide–silver nanoparticles nanocomposite stimulates differentiation in human neuroblastoma cancer cells (SH-SY5Y). Int. J. Mol. Sci. 2017, 18. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-B.; Han, A.-R.; Park, E.-Y.; Kim, J.-Y.; Cho, W.; Lee, J.; Seo, E.-K.; Lee, K.-T. Inhibition of lps-induced inos, cox-2 and cytokines expression by poncirin through the NF-κB inactivation in raw 264.7 macrophage cells. Biol. Pharm. Bull. 2007, 30, 2345–2351. [Google Scholar] [CrossRef] [PubMed]



© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gurunathan, S.; Kim, J.-H. Biocompatible Gold Nanoparticles Ameliorate Retinoic Acid-Induced Cell Death and Induce Differentiation in F9 Teratocarcinoma Stem Cells. Nanomaterials 2018, 8, 396. https://doi.org/10.3390/nano8060396
Gurunathan S, Kim J-H. Biocompatible Gold Nanoparticles Ameliorate Retinoic Acid-Induced Cell Death and Induce Differentiation in F9 Teratocarcinoma Stem Cells. Nanomaterials. 2018; 8(6):396. https://doi.org/10.3390/nano8060396
Chicago/Turabian StyleGurunathan, Sangiliyandi, and Jin-Hoi Kim. 2018. "Biocompatible Gold Nanoparticles Ameliorate Retinoic Acid-Induced Cell Death and Induce Differentiation in F9 Teratocarcinoma Stem Cells" Nanomaterials 8, no. 6: 396. https://doi.org/10.3390/nano8060396




