Isotonic Beverage Pigmented with Water-Dispersible Emulsion from Astaxanthin Oleoresin
Abstract
:1. Introduction
2. Results and Discussion
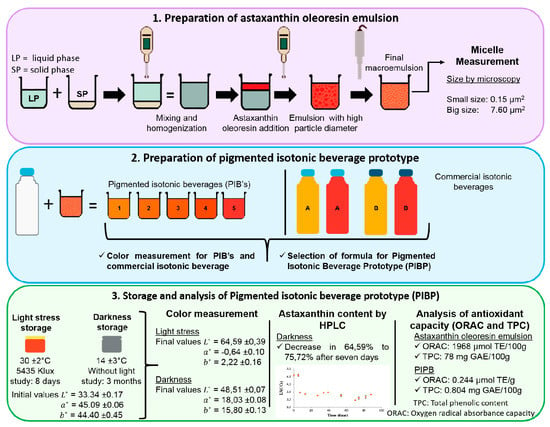
2.1. Determination of the Size of the Micelles of Astaxanthin Oleoresin Emulsion at 10%
2.2. Chromatic Characteristic in Commercial and Pigmented AOE with Isotonic Beverages
2.3. Stability of Astaxanthin Concentration and Variation of Chromatic Coordinates in PIBP for a Period of Three Months
2.4. Color Variation (ΔE) of Prototype Isotonic Beverage Pigmented (PIBP) over a Period of Time
2.5. Stability of Astaxanthin Developed in the Isotonic Beverage
2.6. Analysis of Some Control Variables of the Prototype Isotonic Beverage Pigmented (PIBP)
2.7. Analysis of Capacity Antioxidant Assay in Oleoresin Astaxanthin Emulsion (AOE)
3. Materials and Methods
3.1. Materials
3.2. Preparation of Astaxanthin Oleoresin Emulsion (AOE)
3.3. Chromatic Coordinates Measurement
- (luminosity difference),
- (red-green difference) and
- (blue-yellow difference).
3.4. Preparation of Pigmented Isotonic Beverage Prototype (PIBP)
3.5. Storage of Pigmented Isotonic Beverage Prototype (PIBP)
3.6. Sample Preparation for Alkaline Hydrolysis
3.7. Alkaline Hydrolysis
3.8. HPLC Analysis
3.9. Control Variables
3.10. Oxygen Radical Absorbance Capacity (ORAC) Assay
3.11. Determination of Total Polyphenols Content
3.12. Statistical Analysis of the Results
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gateau, E.; Solymosi, K.; Marchand, J.; Scoefs, B. Carotenoids of microalgae used in food industry and medicine. Mini Rev. Med. Chem. 2017, 13, 1140–1172. [Google Scholar] [CrossRef] [PubMed]
- Solymosi, K.; Latruffe, N.; Morant-Manceaut, A.; Schoefs, B. Food colour additives of natural origin. In Colour Additives for Foods and Beverages: Development, Safety and Applications; Scotter, M.J., Ed.; Woodhead Publishing: Cambridge, UK, 2015; Volume 1, pp. 1–34. [Google Scholar] [CrossRef]
- Christaki, E.; Bonos, E.; Giannenas, I.; Florou-Paneri, P. Functional properties of carotenoids originating from algae. J. Sci. Food Agric. 2012, 93, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Rao, R.; Sarada, A.R.; Baskaran, V.; Ravishankar, G.A. Identification of carotenoids from Green alga Haematococcus pluvialis by HPLC and LC-MS (APCI) and their antioxidant properties. J. Microbiol. Biotechnol. 2009, 19, 1333–1341. [Google Scholar] [CrossRef]
- Hussein, G.; Sankawa, U.; Goto, H.; Matsumoto, K.; Watanabe, H. Astaxanthin, a carotenoid with potential in human health and nutrition. J. Nat. Prod. 2006, 69, 443–449. [Google Scholar] [CrossRef]
- Guerin, M.; Huntley, M.; Olaizola, M. Haematococcus astaxanthin applications for human health and nutrition. Trends Biotechnol. 2003, 21, 210–216. [Google Scholar] [CrossRef]
- Fassett, R.G.; Coombes, J.S. Astaxanthin: A potential therapeutic agent in cardiovascular disease. Mar. Drugs 2011, 9, 447–465. [Google Scholar] [CrossRef] [Green Version]
- Odeberg, J.M.; Lignell, Å.; Pettersson, A.; Höglund, P. Oral bioavailability of the antioxidant astaxanthin in humans is enhanced by incorporation of lipid based formulations. Eur. J. Pharm. Sci. 2003, 19, 299–304. [Google Scholar] [CrossRef]
- Anarjan, N.; Mirhosseini, H.; Baharin, B.S.; Tan, C.P. Effect of processing conditions on physicochemical properties of astaxanthin nanodispersions. Food Chem. 2010, 123, 477–483. [Google Scholar] [CrossRef]
- Hou, Z.; Gao, Y.; Yuan, F.; Liu, Y.; Li, C.; Xu, D. Investigation into the physicochemical stability and rheological properties of β-carotene emulsion stabilized by soybean soluble polysaccharides and chitosan. J. Agric. Food Chem. 2010, 58, 8604–8611. [Google Scholar] [CrossRef]
- McClements, D.J. Critical review of techniques and methodologies for characterization of emulsion stability. Crit. Rev. Food Sci. Nutr. 2007, 47, 611–649. [Google Scholar] [CrossRef]
- Guzey, D.; McClements, D.J. Formation, stability and properties of multilayer emulsion for application in the food industry. Adv. Colloid Interface Sci. 2006, 128–130, 227–248. [Google Scholar] [CrossRef] [PubMed]
- Rosseau, D. Fat crystals and emulsion stability—A review. Food Res. Int. 2000, 33, 3–14. [Google Scholar] [CrossRef]
- Wang, Y.C.; Bleich, S.N.; Gortmaker, S.L. Increasing caloric contribution from sugar-sweetened beverages and 100% fruit juices among US children and adolescents, 1988–2004. Pediatrics 2014, 121, e1604–e1614. [Google Scholar] [CrossRef] [PubMed]
- Fresno, M.C.; Angel, P.; Cisternas, P.; Muñoz, A. Grado de acidez y potencial erosiva de las bebidas deportivas isotónicas disponibles en Chile. Rev. Dent. Chile 2011, 102, 13–16. [Google Scholar] [CrossRef] [Green Version]
- Coombes, J.S. Sport drinks and dental. Am. J. Dent. 2005, 18, 101–104. [Google Scholar]
- Coombes, J.S.; Hamilton, K.L. The effectiveness of commercially available sports drinks. Sport Med. 2000, 29, 181–209. [Google Scholar] [CrossRef]
- Villalobos-Castillejos, F.; Cerezal-Mezquita, P.; Hernández-De Jesús, M.L.; Barragán-Huerta, B.E. Production and stability of water-dispersible astaxanthin oleoresin from Phaffia rhodozyma. Int. J. Food Sci. Technol. 2013, 48, 1243–1251. [Google Scholar] [CrossRef]
- Mesnier, X.; Gregory, C.; Fança-Berthon, P.; Boukobza, F.; Bily, A. Heat and light colour stability of beverages coloured with a natural carotene emulsion: Effect of synthetic versus natural water soluble antioxidants. Food Res. Int. 2014, 65, 149–155. [Google Scholar] [CrossRef]
- Gironés-Vilaplana, A.; Mena, P.; Moreno, D.A.; García-Viguera, C. Evaluation of sensorial, phytochemical and biological properties of new isotonic beverages enriched with lemon and berries during shelf life. J. Sci. Food Agric. 2014, 94, 1090–1100. [Google Scholar] [CrossRef]
- Gironés-Vilaplana, A.; Huertas, J.P.; Moreno, D.A.; Periago, P.M.; García-Viguera, C. Quality and microbial safety evaluation of new isotonic beverages upon thermal treatments. Food Chem. 2016, 194, 455–462. [Google Scholar] [CrossRef]
- Restrepo, M.; Acosta, E.V.; Ocampo, J.C.; Morales, C. Sustitución de tartrazina por betacaroteno en la elaboración de bebidas no alcohólicas. Rev. Lasallista Investig. 2007, 3, 7–12. [Google Scholar]
- Serdaroğlu, M.; Öztürk, B.; Kara, A. An overview of food emulsions: Description, classification and recent potential applications. Turk. J. Agric. Food Sci. Technol. 2015, 3, 430–438. [Google Scholar] [CrossRef] [Green Version]
- McClements, D. (Ed.) Food emulsions in practice. In Food Emulsions: Principles, Practices and Techniques, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2015; Volume 12, pp. 547–575. ISBN 9781498726689. [Google Scholar]
- Espinosa-Álvarez, C.; Jaime-Matus, C.; Cerezal-Mezquita, P. Some physical characteristics of the O/W macroemulsion of oleoresin of astaxanthin obtained from biomass of Haematococcus pluvialis. DYNA 2019, 86, 136–142. [Google Scholar] [CrossRef]
- Mason, T.G.; Wilking, J.N.; Meleson, K.; Chang, C.B.; Graves, S.M. Nanoemulsions: Formation, structure, and physical properties. J. Phys. Condens. Matter 2006, 18, R635–R666. [Google Scholar] [CrossRef] [Green Version]
- Waraho, T.; McClements, D.J.; Decker, E.A. Mechanism of lipid oxidation in food dispersions. Trends Food Sci. Technol. 2011, 22, 3–13. [Google Scholar] [CrossRef]
- Cerezal, P.; Acosta, E.; Rojas, G.; Romero, N.; Arcos, R. Desarrollo de una bebida de alto contenido proteico a partir de algarrobo, lupino y quinoa para la dieta de preescolares. Nutr. Hosp. 2012, 27, 232–243. [Google Scholar]
- Obón, J.M.; Castellar, M.R.; Alacid, M.; Fernández-López, J.A. Production of a red-purple food colorant from Opuntia stricta fruits by spray drying and its application in food model system. J. Food Eng. 2009, 90, 471–479. [Google Scholar] [CrossRef]
- Hong, S.; Han, J.H.; Krochta, J.M. Optical and surface properties of whey protein isolate coatings on plastic films as influenced by substrate, protein concentration, and plasticizer type. J. Appl. Polym. Sci. 2004, 92, 335–343. [Google Scholar] [CrossRef]
- Lui, Y.; Hou, Z.; Yang, J.; Gao, Y. Effects of antioxidants on the stability of β-carotene in O/W emulsion stabilized by Gum Arabic. J. Food Sci. Technol. 2015, 52, 3300–3311. [Google Scholar] [CrossRef] [Green Version]
- Sherestha, S.; Sadiq, M.B.; Anal, A.K. Culled banana resistant starch-soy protein isolate conjugate based emulsion enriched with astaxanthin to enhance its stability. Int. J. Biol. Macromol. 2018, 120, 449–459. [Google Scholar] [CrossRef]
- Qian, C.; Decker, E.A.; Xiao, H.; McClements, D.J. Physical and chemical stability of β-carotene-enriched nanoemulsions: Influence of pH, ionic strength, temperature, and emulsifier type. Food Chem. 2012, 132, 1221–1229. [Google Scholar] [CrossRef] [PubMed]
- McClements, D.J. Theoretical prediction of emulsion color. Adv. Colloid Interface 2002, 97, 63–89. [Google Scholar] [CrossRef]
- Zepka, L.Q.; Borsarelli, C.D.; da Silva, M.A.; Mercadante, A.Z. Thermal degradation kinetics of carotenoids in a casher Apple juice model and its impact on the system color. J. Agric. Food Chem. 2009, 57, 7841–7845. [Google Scholar] [CrossRef] [PubMed]
- Boon, C.S.; McClements, D.J.; Weiss, J.; Decker, E.A. Role of iron and hydroperoxides in the degradation of lycopene in oil-in-water emulsions. J. Agric. Food Chem. 2009, 57, 2993–2998. [Google Scholar] [CrossRef] [PubMed]
- Meléndez-Martínez, A.J.; Vicario, I.M.; Heredia, F.J. Estabilidad de los pigmentos carotenoides en los alimentos. In Archivos Latinoamericanos de Nutrición; Publisher: Caracas, Venezuela, 2004; Volume 54, pp. 209–215. ISSN 0004-0622. [Google Scholar]
- Villa, T.; Calo, P.; Blanco, P.; Sieiro, C. Phaffia rhodozyma: Primer microorganismo explotado para la producción de astaxantina. Proceedings of Biotecnología y aplicaciones de microorganismos pigmentados: IV simposio científico en biología celular y molecular, La Coruña, Spain. 1998. Available online: https://ruc.udc.es/dspace/handle/2183/10771 (accessed on 11 December 2019).
- Kundu, P.; Agrawal, A.; Mateen, H.; Mishra, I.M. Stability of oil-in-water macro-emulsion with anionic surfactant: Effect of electrolytes and temperature. Chem. Eng. Sci. 2013, 102, 176–185. [Google Scholar] [CrossRef]
- Raposo, M.F.; Morais, A.M.; Morais, R.M. Effects of spray-drying and storage on astaxanthin content of Haematococcus pluvialis biomass. World J. Microbiol. Biotechnol. 2012, 20, 1253–1257. [Google Scholar] [CrossRef] [Green Version]
- Arrazola, G.; Herazo, I.; Alvis, A. Obtención y evaluación de la estabilidad de antocianinas de berenjena (Solanum melongena L.) en bebidas. Inf. Tecnol. 2014, 25, 43–52. [Google Scholar] [CrossRef] [Green Version]
- Rosso, V.V.; Mercadante, A.Z. Evaluation of colour and stability of anthocyanins from tropical fruits in an isotonic soft drink system. Innov. Food Sci. Emerg. Technol. 2007, 8, 347–352. [Google Scholar] [CrossRef]
- Ambati, R.R.; Phang, S.M.; Ravi, S.; Aswathanarayana, R.G. Astaxanthin: Sources, extraction, stability, biological activities and its commercial applications—A review. Mar. Drugs 2014, 12, 128–152. [Google Scholar] [CrossRef]
- Mortensen, A.; Skibsted, L.H. Kinetics and Mechanism of the primary steps of degradation of carotenoids by acid in homogeneous solution. J. Agric. Food Chem. 2000, 48, 279–286. [Google Scholar] [CrossRef]
- Demetriades, K.; Coupland, J.N.; McClements, D.J. Physical properties of whey protein stabilized emulsions as related to pH and NaCl. J. Food Sci. 1997, 62, 342–347. [Google Scholar] [CrossRef]
- Mt. Carpa. Available online: https://mtcapra.com/product/solar-synergy/ (accessed on 9 December 2019).
- Blasa, M.; Gennari, L.; Angelino, D.; Ninfali, P. Fruit and vegetable antioxidant in health. In Bioactive Foods in Promoting Health (Fruits and Vegetables), 1st ed.; Watson, R.R., Preedy, V.R., Eds.; Academic Press: San Diego, CA, USA, 2010; Volume 3, pp. 37–58. [Google Scholar] [CrossRef]
- Bioflavia. Available online: https://www.southbrook.com/Bioflavia (accessed on 11 December 2019).
- Gironés-Vilaplana, A.; Villaño, D.; Moreno, D.A.; García-Viguera, C. New isotonic drinks with antioxidant and biological capacities from berries (maqui, aҫai and blackthorn) and lemon juice. Int. J. Food Sci. Nutr. 2013, 64, 897–906. [Google Scholar] [CrossRef] [PubMed]
- Seeram, N.P.; Aviram, M.; Zhang, Y.; Henning, S.M.; Feng, L.; Dreher, M.; Herber, D. Comparison of antioxidant potency of commonly consumed polyphenol-rich beverages in the United States. J. Agric. Food. Chem. 2008, 56, 1415–1422. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, R. Process for Solubilization of Flavor Oils. U.S. Patent 20100323066 A1, 19 June 2010. [Google Scholar]
- Cerezal, P.; Barragán, B.E.; Palma, J.C.; Ortíz, C.P. Milks pigmentation with astaxanthin and determination of colour stability during short period cold storage. J. Food Sci. Technol. 2013, 52, 1634–1641. [Google Scholar] [CrossRef] [Green Version]
- Prior, R.L.; Hoang, H.; Gu, L.; Wu, X.; Bacchiocca, M.; Howard, L.; Hampsch-Woodill, M.; Huang, D.; Ou, B.; Jacob, R. Assays for hydrophilic and lipophilic antioxidant capacity (oxygen radical absorbance capacity (ORACFL))) of plasma and other biological and food samples. J. Agric. Food Chem. 2003, 51, 3273–3279. [Google Scholar] [CrossRef] [PubMed]
- Zuleta, A.; Esteve, M.J.; Frígola, A. ORAC and TEAC assays comparison to measure the antioxidant capacity of food products. Food Chem. 2009, 114, 313–316. [Google Scholar] [CrossRef]
- Wu, X.; Beecher, G.R.; Holden, J.M.; Haytowitz, D.B.; Gebhardt, S.E.; Prior, R.L. Lipophilic and hydrophilic antioxidant capacities of common foods in the United States. J. Agric. Food Chem. 2004, 52, 4026–4037. [Google Scholar] [CrossRef]
- Fu, R.; Zhang, Y.; Guo, Y.; Liu, F.; Cheng, F. Determination of phenolic contents and antioxidant activities of extracts of Jatropha curcas L. seed shell, a by-product, a new source of natural antioxidant. Ind. Crop Prod. 2014, 58, 265–270. [Google Scholar] [CrossRef]
- Añalón, M.E.; Castro-Vásquez, L.; Díaz-Maroto, M.C.; Gordon, M.H.; Pérez-Coello, M.S. A study of the antioxidant capacity of oak Wood used in wine ageing and the correlation with polyphenol composition. Food Chem. 2011, 128, 997–1002. [Google Scholar] [CrossRef] [Green Version]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventos, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. In Methods in Enzymology (Oxidants and Antioxidants, Part A); Lester Packer, L., Ed.; Academic Press: San Diego, CA, USA, 1999; Volume 299, pp. 152–178. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |








| Commercial Isotonic Beverage | Brand of Beverage | Chromatic Coordinates | |||
|---|---|---|---|---|---|
| Colorless | A | 61.72 ± 0.56 | −0.98 ± 0.13 | 1.74 ± 0.17 | −0.56 |
| Orange | A | 47.43 ± 3.16 a | 24.00 ± 0.86 a | 78.21 ± 2.21 a | 0.31 |
| B | 46.57 ± 0.34 a | 22.58 ± 0.17 b | 75.41 ± 0.67 a | 0.30 | |
| Red | A | 33.34 ± 0.17 b | 45.09 ± 0.06 b | 44.40 ± 0.45 b | 1.02 |
| B | 35.74 ± 1.32 a | 41.96 ± 0.34 a | 29.69 ± 2.17 a | 1.41 | |
| Pigmented Isotonic Beverage | Concentration of AOE (ppm) | Chromatic Coordinates | ||
|---|---|---|---|---|
| 1 | 530 | 42.36 ± 0.06 a | 28.59 ± 0.04 a | 39.54 ± 0.21 a |
| 2 | 992 | 35.07 ± 0.03 b | 35.49 ± 0.04 b | 48.56 ± 0.20 e |
| 3 | 1548 | 30.74 ± 0.05 c | 38.00 ± 0.06 c | 47.19 ± 0.16 d |
| 4 | 2076 | 28.18 ± 0.04 d | 38.80 ± 0.05 d | 44.31 ± 0.20 c |
| 5 | 2520 | 25.80 ± 0.03 e | 39.20 ± 0.06 e | 41.09 ± 0.17 b |
| Formulas | Concentration of | ∆E Between Commercial IB and Developed IB | |||
|---|---|---|---|---|---|
| Brand (Orange-Colored) | Brand (Red-Colored) | ||||
| Pigmented IB | AOE (ppm) | A | B | A | B |
| 1 | 530 | 39.27 ± 0.18 c,a | 36.61 ± 0.20 c,b | 19.42 ± 0.11 a,a | 17.88 ± 0.14 c,b |
| 2 | 992 | 34.11 ± 0.17 e,a | 31.94 ± 0.22 e,b | 10.60 ± 0.08 b,a | 19.96 ± 0.19 a,b |
| 3 | 1548 | 37.90 ± 0.18 d,a | 35.84 ± 0.17 d,b | 8.05 ± 0.09 d,a | 18.63 ± 0.15 b,b |
| 4 | 2076 | 41.70 ± 0.22 b,a | 39.61 ± 0.15 b,b | 8.14 ± 0.03 d,a | 16.76 ± 0.19 d,b |
| 5 | 2520 | 45.57 ± 0.15 a,a | 43.43 ± 0.18 a,b | 10.13 ± 0.07 c,a | 15.38 ± 0.15 e,b |
| Control Variables | Days | PIBP | |
|---|---|---|---|
| Darkness | Under Light Stress | ||
| Soluble solids (°Bx) | 0–30 | 1.3 ± 0.20 a | 1.3 ± 0.20 |
| 31–90 | 1.4 ± 0.20 a | ------ | |
| pH | 0–14 | 4.07 ± 0.02 a | 4.15 ± 0.01 |
| 15–35 | 4.09 ± 0.02 ab | ------ | |
| 36–90 | 4.13 ± 0.02 b | ------ | |
| Products | TPC (mg GAE/g) | ORAC (µmol TE/g) | Ratio ORAC/TPC |
|---|---|---|---|
| Sweetener | 0.02 ± 0.003 * | 0.08 ± 0.02 * | 4.00 ** |
| Sweetener with Green tea extract | 1.43 ± 0.11 * | 25.56 ± 1.9 * | 17.87 ** |
| Yogurt drink | 0.16 ± 0.02 * | 1.76 ± 0.25 * | 11.00 ** |
| Yogurt drink with catechins | 0.41 ± 0.05 * | 8.99 ± 1.06 * | 21.93 ** |
| Isotonic beverage | 0.18 ± 0.05 * | 1.13 ± 0.04 * | 6.28 ** |
| Isotonic beverage with green tea extract | 0.63 ± 0.08 * | 5.07 ± 0.20 * | 8.05 ** |
| Isotonic beverage with AOE* | 0.008 ** | 0.24 ** | 30 ** |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cerezal Mezquita, P.; Espinosa Álvarez, C.; Palma Ramírez, J.; Bugueño Muñoz, W.; Salinas Fuentes, F.; Ruiz-Domínguez, M.d.C. Isotonic Beverage Pigmented with Water-Dispersible Emulsion from Astaxanthin Oleoresin. Molecules 2020, 25, 841. https://doi.org/10.3390/molecules25040841
Cerezal Mezquita P, Espinosa Álvarez C, Palma Ramírez J, Bugueño Muñoz W, Salinas Fuentes F, Ruiz-Domínguez MdC. Isotonic Beverage Pigmented with Water-Dispersible Emulsion from Astaxanthin Oleoresin. Molecules. 2020; 25(4):841. https://doi.org/10.3390/molecules25040841
Chicago/Turabian StyleCerezal Mezquita, Pedro, Carolina Espinosa Álvarez, Jenifer Palma Ramírez, Waldo Bugueño Muñoz, Francisca Salinas Fuentes, and María del Carmen Ruiz-Domínguez. 2020. "Isotonic Beverage Pigmented with Water-Dispersible Emulsion from Astaxanthin Oleoresin" Molecules 25, no. 4: 841. https://doi.org/10.3390/molecules25040841