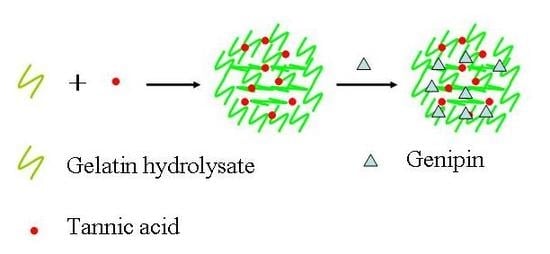
Gelatin Hydrolysate Hybrid Nanoparticles as Soft Edible Pickering Stabilizers for Oil-In-Water Emulsions
Abstract
:1. Introduction
2. Results
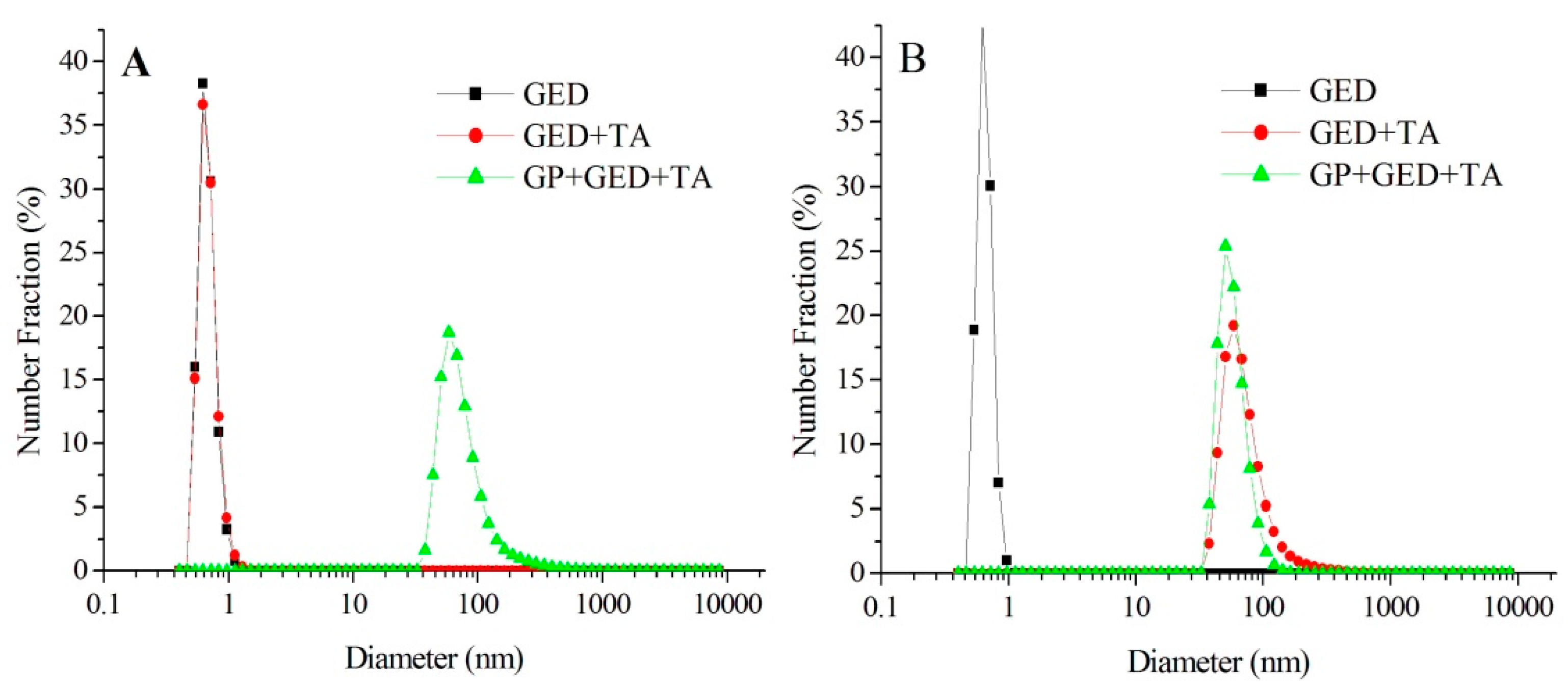
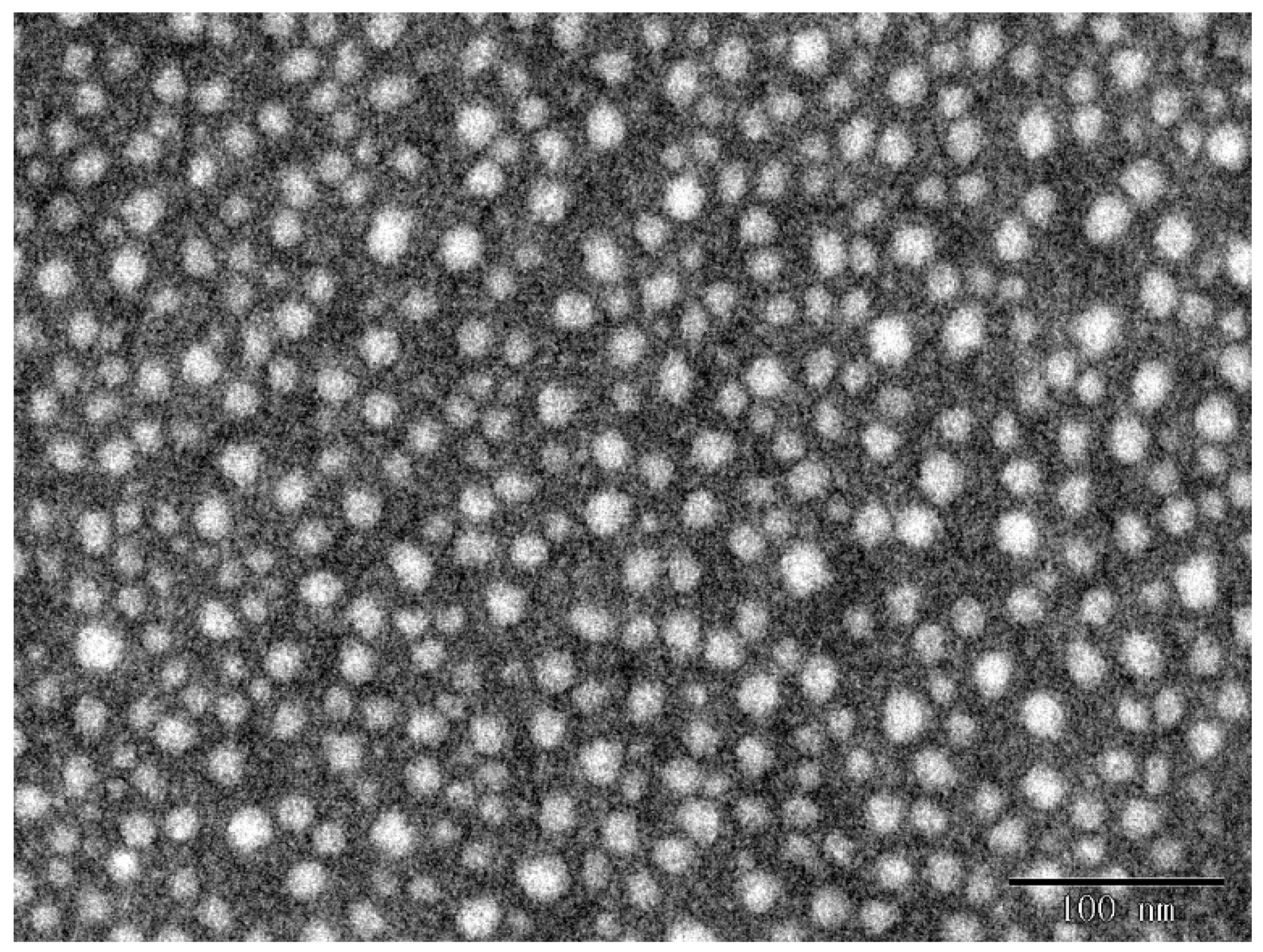
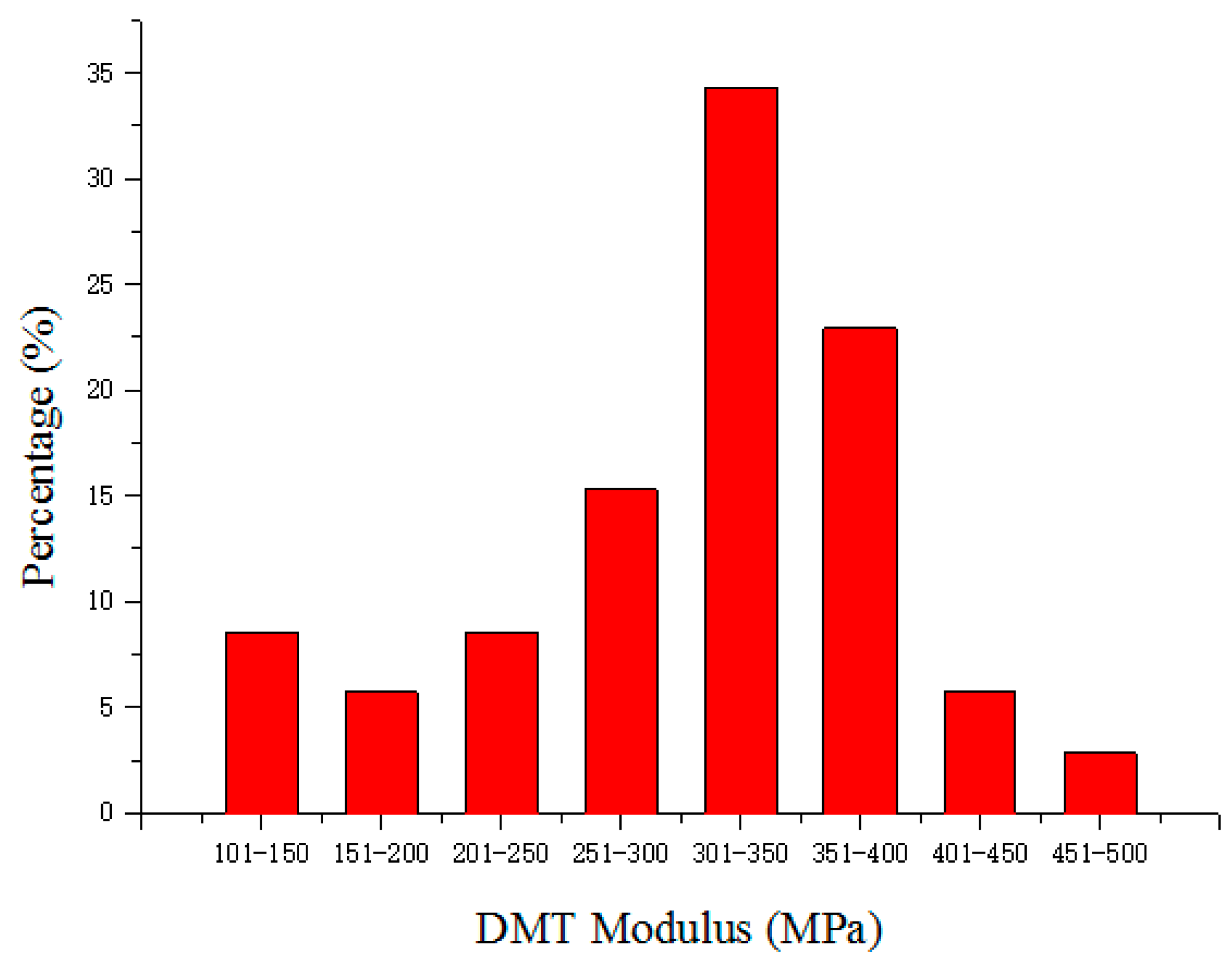
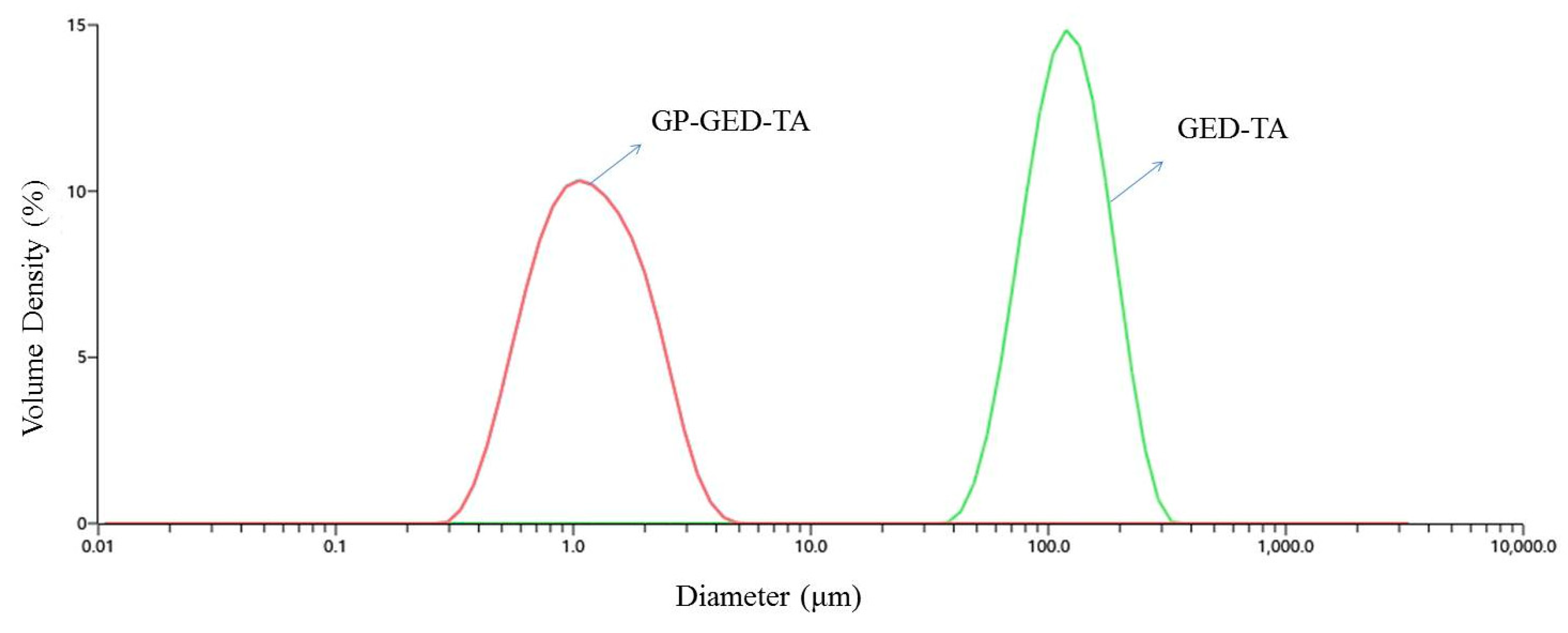
2.1. Characterization of Gel Particles
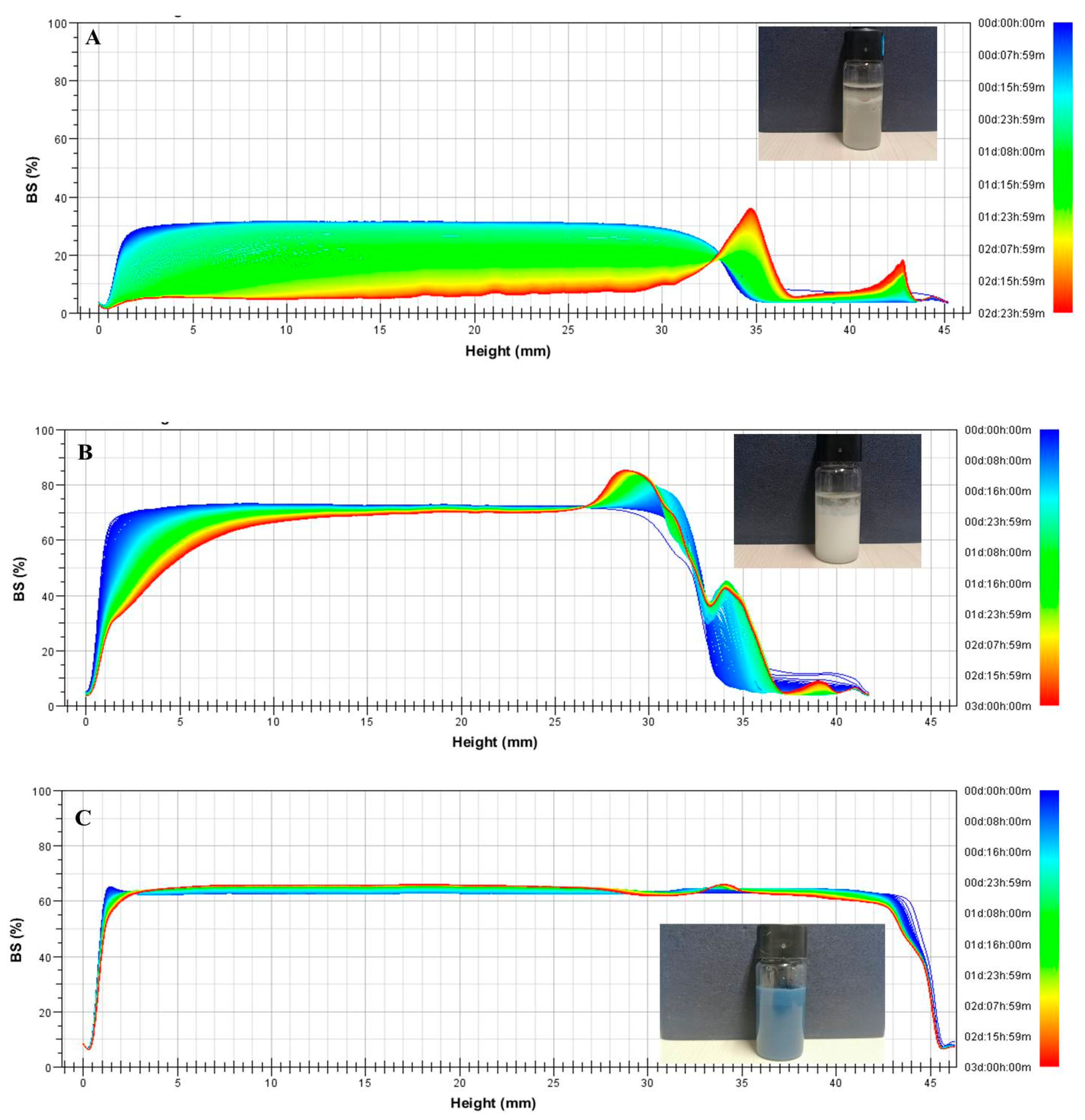
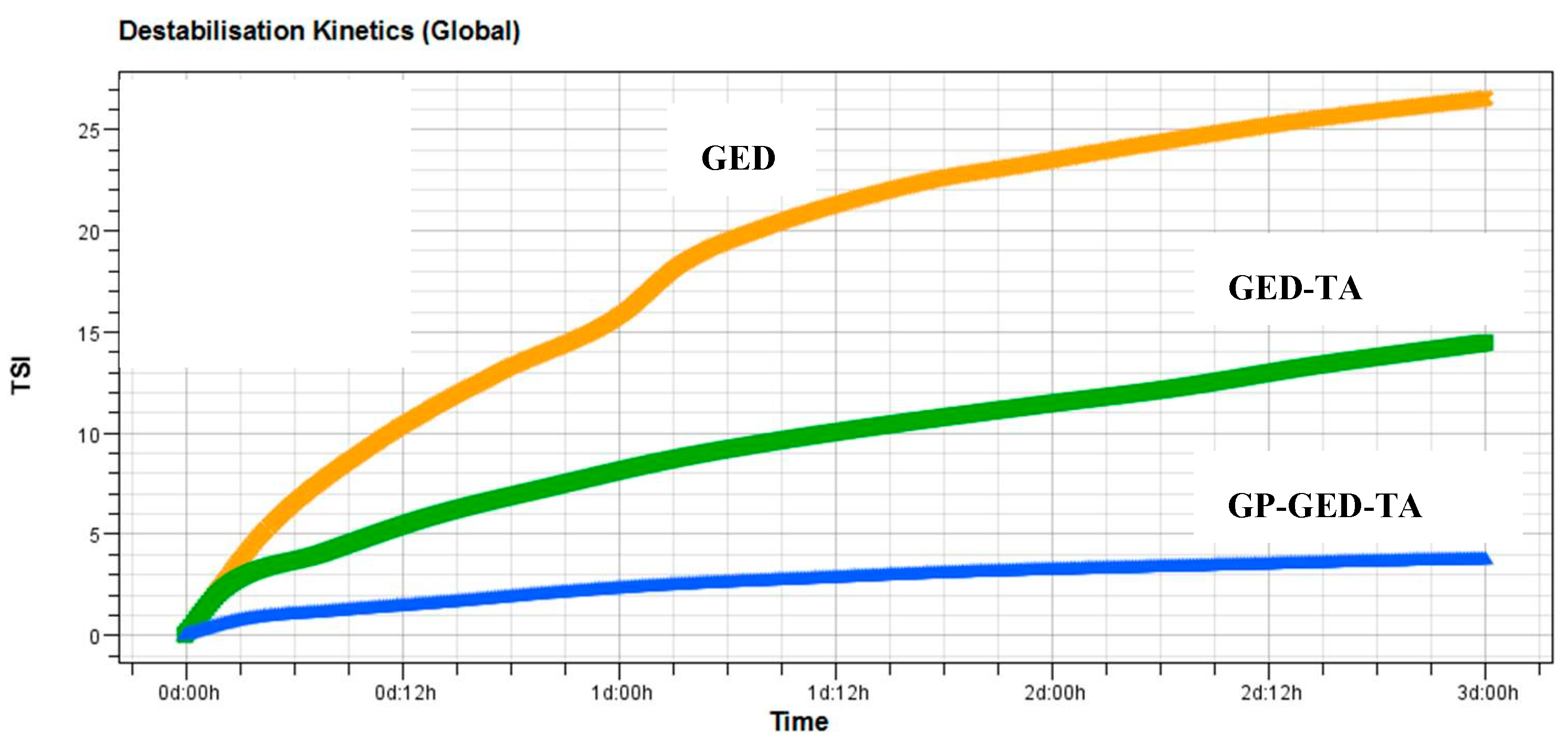
2.2. Stability of Pickering Emulsions
2.3. Viscosity and Size Distribution of the Emulsions
3. Materials and Methods
3.1. Materials
3.2. Preparation and Characterization of Gel Particles
3.3. Preparation and Characterization of O/W Emulsions
3.4. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wei, Z.; Huang, Q. Edible Pickering emulsions stabilized by ovotransferrin–gum arabic particles. Food Hydrocoll. 2019, 89, 590–601. [Google Scholar] [CrossRef]
- Sanchez-Salvador, J.L.; Balea, A.; Monte, M.C.; Blanco, A.; Negro, C. Pickering Emulsions Containing Cellulose Microfibers Produced by Mechanical Treatments as Stabilizer in the Food Industry. Appl. Sci. 2019, 9, 359. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Guo, J.; Wan, Z.-L.; Liu, Y.-Y.; Ruan, Q.-J.; Yang, X.-Q. Wheat gluten-stabilized high internal phase emulsions as mayonnaise replacers. Food Hydrocoll. 2018, 77, 168–175. [Google Scholar] [CrossRef]
- Murray, B.S. Pickering emulsions for food and drinks. Curr. Opin. Food Sci. 2019, 27, 57–63. [Google Scholar] [CrossRef]
- Dai, L.; Sun, C.; Wei, Y.; Mao, L.; Gao, Y. Characterization of Pickering emulsion gels stabilized by zein/gum arabic complex colloidal nanoparticles. Food Hydrocoll. 2018, 74, 239–248. [Google Scholar] [CrossRef]
- Nallamilli, T.; Basavaraj, M.G. Synergistic stabilization of Pickering emulsions by in situ modification of kaolinite with non ionic surfactant. Appl. Clay Sci. 2017, 148, 68–76. [Google Scholar] [CrossRef]
- Ma, L.; Zou, L.; McClements, D.J.; Liu, W. One-step preparation of high internal phase emulsions using natural edible Pickering stabilizers: Gliadin nanoparticles/gum Arabic. Food Hydrocoll. 2020, 100, 105381. [Google Scholar] [CrossRef]
- Ye, F.; Miao, M.; Cui, S.W.; Jiang, B.; Jin, Z.; Li, X. Characterisations of oil-in-water Pickering emulsion stabilized hydrophobic phytoglycogen nanoparticles. Food Hydrocoll. 2018, 76, 78–87. [Google Scholar] [CrossRef]
- Winuprasith, T.; Khomein, P.; Mitbumrung, W.; Suphantharika, M.; Nitithamyong, A.; McClements, D.J. Encapsulation of vitamin D3 in pickering emulsions stabilized by nanofibrillated mangosteen cellulose: Impact on in vitro digestion and bioaccessibility. Food Hydrocoll. 2018, 83, 153–164. [Google Scholar] [CrossRef]
- Zhu, Y.; McClements, D.J.; Zhou, W.; Peng, S.; Zhou, L.; Zou, L.; Liu, W. Influence of ionic strength and thermal pretreatment on the freeze-thaw stability of Pickering emulsion gels. Food Chem. 2020, 303, 125401. [Google Scholar] [CrossRef]
- Destribats, M.; Wolfs, M.; Pinaud, F.; Lapeyre, V.; Sellier, E.; Schmitt, V.; Ravaine, V. Pickering Emulsions Stabilized by Soft Microgels: Influence of the Emulsification Process on Particle Interfacial Organization and Emulsion Properties. Langmuir 2013, 29, 12367–12374. [Google Scholar] [CrossRef] [PubMed]
- Deshmukh, O.S.; van den Ende, D.; Stuart, M.C.; Mugele, F.; Duits, M.H.G. Hard and soft colloids at fluid interfaces: Adsorption, interactions, assembly & rheology. Adv. Colloid Interface Sci. 2015, 222, 215–227. [Google Scholar] [PubMed]
- Berton-Carabin, C.C.; Schroën, K. Pickering emulsions for food applications: Background, trends, and challenges. Annu. Rev. Food Sci. Technol. 2015, 6, 263–297. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhou, W.; Cao, J.; Liu, W.; Zhu, S. Preparation of core–shell CaCO3 capsules via Pickering emulsion templates. J. Colloid Interface Sci. 2012, 372, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Pei, Y.; Qiao, M.; Ma, F.; Ren, H.; Zhao, Q. Preparation and characterizations of Pickering emulsions stabilized by hydrophobic starch particles. Food Hydrocoll. 2015, 45, 256–263. [Google Scholar] [CrossRef]
- Lu, X.; Zhang, H.; Li, Y.; Huang, Q. Fabrication of milled cellulose particles-stabilized Pickering emulsions. Food Hydrocoll. 2018, 77, 427–435. [Google Scholar] [CrossRef]
- Noguchi, S.; Sato, K.; Yamamoto, K.; Kadokawa, J.-I. Preparation of composite and hollow particles from self-assembled chitin nanofibers by Pickering emulsion polymerization. Int. J. Biol. Macromol. 2019, 126, 187–192. [Google Scholar] [CrossRef]
- Zou, Y.; Guo, J.; Yin, S.-W.; Wang, J.-M.; Yang, X.-Q. Pickering Emulsion Gels Prepared by Hydrogen-Bonded Zein/Tannic Acid Complex Colloidal Particles. J. Agric. Food Chem. 2015, 63, 7405–7414. [Google Scholar] [CrossRef]
- Sarkar, A.; Murray, B.; Holmes, M.; Ettelaie, R.; Abdalla, A.; Yang, X. In vitro digestion of Pickering emulsions stabilized by soft whey protein microgel particles: Influence of thermal treatment. Soft Matter 2016, 12, 3558–3569. [Google Scholar] [CrossRef]
- Xu, Y.; Tang, C.; Binks, B.P. High internal phase emulsions stabilized solely by a globular protein glycated to form soft particles. Food Hydrocoll. 2020, 98, 105254. [Google Scholar] [CrossRef]
- Wang, P.; Chen, C.; Guo, H.; Zhang, H.; Yang, Z.; Ren, F. Casein gel particles as novel soft Pickering stabilizers: The emulsifying property and packing behaviour at the oil-water interface. Food Hydrocoll. 2018, 77, 689–698. [Google Scholar] [CrossRef]
- Soltani, S.; Madadlou, A. Two-step sequential cross-linking of sugar beet pectin for transforming zein nanoparticle-based Pickering emulsions to emulgels. Carbohydr. Polym. 2016, 136, 738–743. [Google Scholar] [CrossRef] [PubMed]
- Ning, F.; Wang, X.; Zheng, H.; Zhang, K.; Bai, C.; Peng, H.; Huang, Q.; Xiong, H. Improving the bioaccessibility and in vitro absorption of 5-demethylnobiletin from chenpi by se-enriched peanut protein nanoparticles-stabilized pickering emulsion. J. Funct. Foods 2019, 55, 76–85. [Google Scholar] [CrossRef]
- Ju, M.; Zhu, G.; Huang, G.; Shen, X.; Zhang, Y.; Jiang, L.; Sui, X. A novel pickering emulsion produced using soy protein-anthocyanin complex nanoparticles. Food Hydrocoll. 2020, 99, 105329. [Google Scholar] [CrossRef]
- Zhu, X.; Zheng, J.; Liu, F.; Qiu, C.-Y.; Lin, W.-F.; Tang, C.-H. Freeze-thaw stability of Pickering emulsions stabilized by soy protein nanoparticles. Influence of ionic strength before or after emulsification. Food Hydrocoll. 2018, 74, 37–45. [Google Scholar] [CrossRef]
- Tan, H.; Zhao, L.; Tian, S.; Wen, H.; Gou, X.; Ngai, T. Gelatin particle-stabilized high-internal phase emulsions for use in oral delivery systems: Protection effect and in vitro digestion study. J. Agric. Food Chem. 2017, 65, 900–907. [Google Scholar] [CrossRef]
- Feng, X.; Dai, H.; Ma, L.; Yu, Y.; Tang, M.; Li, Y.; Hu, W.; Liu, T.; Zhang, Y. Food-Grade Gelatin Nanoparticles: Preparation, Characterization, and Preliminary Application for Stabilizing Pickering Emulsions. Foods 2019, 8, 479. [Google Scholar] [CrossRef] [Green Version]
- Xiao, J.; Li, Y.; Huang, Q. Recent advances on food-grade particles stabilized Pickering emulsions: Fabrication, characterization and research trends. Trends Food Sci. Technol. 2016, 55, 48–60. [Google Scholar] [CrossRef] [Green Version]
- Wu, S.-J.; Ho, Y.-C.; Jiang, S.-Z.; Mi, F.-L. Effect of tannic acid–fish scale gelatin hydrolysate hybrid nanoparticles on intestinal barrier function and α-amylase activity. Food Funct. 2015, 6, 2283–2292. [Google Scholar] [CrossRef]
- Gerrard, J.A. Protein–protein crosslinking in food: Methods, consequences, applications. Trends Food Sci. Technol. 2002, 13, 391–399. [Google Scholar] [CrossRef]
- Wang, L.; Cao, Y.; Zhang, K.; Fang, Y.; Nishinari, K.; Phillips, G.O. Hydrogen bonding enhances the electrostatic complex coacervation between κ-carrageenan and gelatin. Colloids Surf. A Physicochem. Eng. Asp. 2015, 482, 604–610. [Google Scholar] [CrossRef]
- Huppertz, T.; Smiddy, M.A.; de Kruif, C.G. Biocompatible Micro-Gel Particles from Cross-Linked Casein Micelles. Biomacromolecules 2007, 8, 1300–1305. [Google Scholar] [CrossRef] [PubMed]
- Silva, N.F.N.; Saint-Jalmes, A.; de Carvalho, A.F.; Gaucheron, F. Development of Casein Microgels from Cross-Linking of Casein Micelles by Genipin. Langmuir 2014, 30, 10167–10175. [Google Scholar] [CrossRef] [PubMed]
- Xing, R.; Jiao, T.; Yan, L.; Ma, G.; Liu, L.; Dai, L.; Li, J.; Möhwald, H.; Yan, X. Colloidal gold–collagen protein core–shell nanoconjugate: One-step biomimetic synthesis, layer-by-layer assembled film, and controlled cell growth. ACS Appl. Mater. Interfaces 2015, 7, 24733–24740. [Google Scholar] [CrossRef]
- Krivosheeva, O.; Sababi, M.; Dedinaite, A.; Claesson, P.M. Nanostructured Composite Layers of Mussel Adhesive Protein and Ceria Nanoparticles. Langmuir 2013, 29, 9551–9561. [Google Scholar] [CrossRef]
- Suk, J.W.; Na, S.R.; Stromberg, R.J.; Stauffer, D.; Lee, J.; Ruoff, R.S.; Liechti, K.M. Probing the adhesion interactions of graphene on silicon oxide by nanoindentation. Carbon 2016, 103, 63–72. [Google Scholar] [CrossRef] [Green Version]
- Zheng, L.; Jerrams, S.; Xu, Z.; Zhang, L.; Liu, L.; Wen, S. Enhanced gas barrier properties of graphene oxide/rubber composites with strong interfaces constructed by graphene oxide and sulfur. Chem. Eng. J. 2020, 383, 123100. [Google Scholar] [CrossRef]
- Leal-Castañeda, E.J.; García-Tejeda, Y.; Hernández-Sánchez, H.; Alamilla-Beltrán, L.; Téllez-Medina, D.I.; Calderón-Domínguez, G.; García, H.S.; Gutiérrez-López, G.F. Pickering emulsions stabilized with native and lauroylated amaranth starch. Food Hydrocoll. 2018, 80, 177–185. [Google Scholar] [CrossRef]
- Cabezas, D.M.; Pascual, G.N.; Wagner, J.R.; Palazolo, G.G. Nanoparticles assembled from mixtures of whey protein isolate and soluble soybean polysaccharides. Structure, interfacial behavior and application on emulsions subjected to freeze-thawing. Food Hydrocoll. 2019, 95, 445–453. [Google Scholar] [CrossRef]
- Silva, E.K.; Zabot, G.L.; Meireles, M.A.A. Ultrasound-assisted encapsulation of annatto seed oil: Retention and release of a bioactive compound with functional activities. Food Res. Int. 2015, 78, 159–168. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |
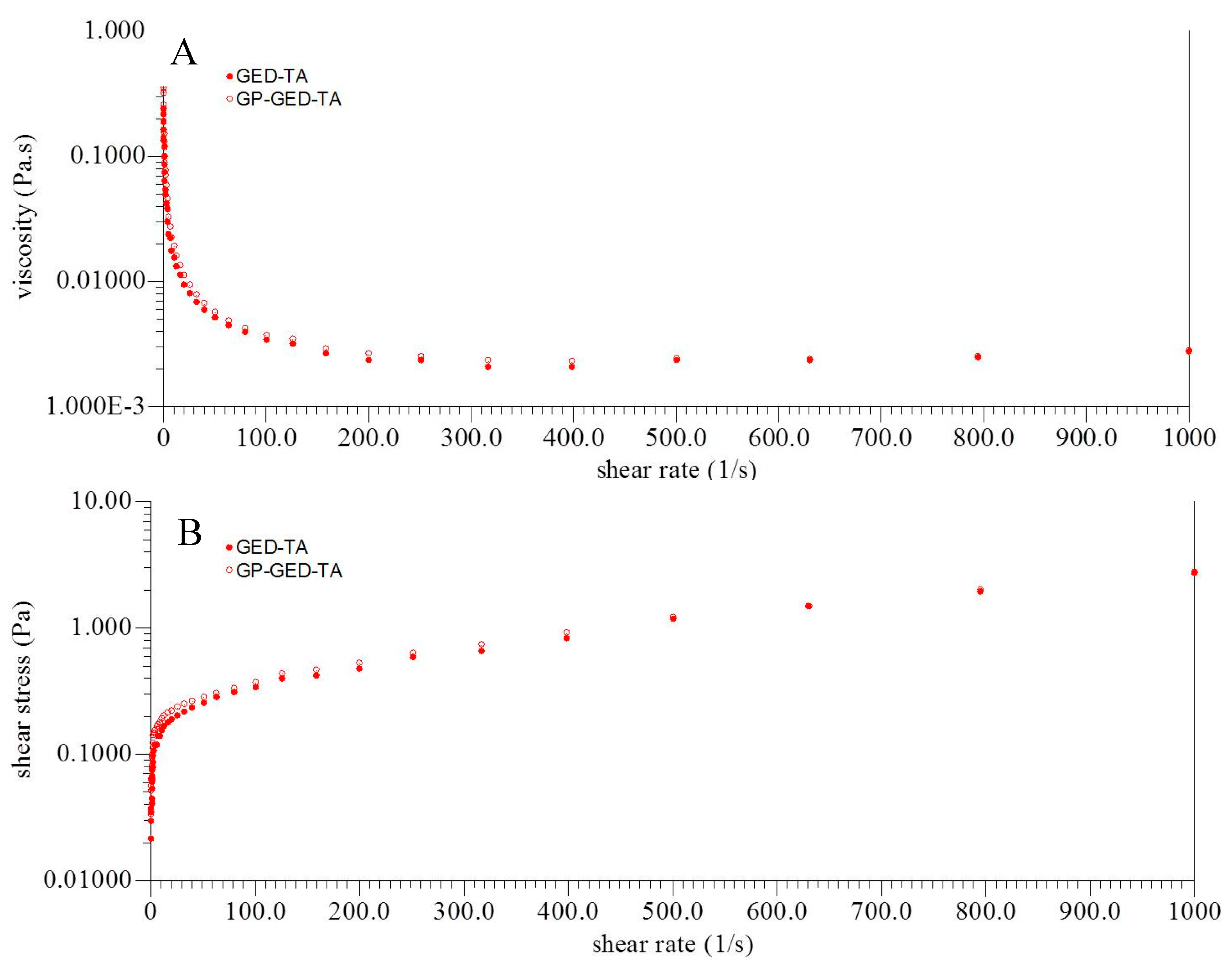
| Group | σ0 (Pa) | K (Pa·sn, 10−4) | n |
|---|---|---|---|
| GED-TA | 0.09 ± 0.06 | 8.55 ± 2.24 | 1.17 ± 0.02 |
| GP-GED-TA | 0.11 ± 0.00 | 6.26 ± 1.69 | 1.19 ± 0.04 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, Z.; Wang, P. Gelatin Hydrolysate Hybrid Nanoparticles as Soft Edible Pickering Stabilizers for Oil-In-Water Emulsions. Molecules 2020, 25, 393. https://doi.org/10.3390/molecules25020393
Du Z, Wang P. Gelatin Hydrolysate Hybrid Nanoparticles as Soft Edible Pickering Stabilizers for Oil-In-Water Emulsions. Molecules. 2020; 25(2):393. https://doi.org/10.3390/molecules25020393
Chicago/Turabian StyleDu, Zhongyao, and Pengjie Wang. 2020. "Gelatin Hydrolysate Hybrid Nanoparticles as Soft Edible Pickering Stabilizers for Oil-In-Water Emulsions" Molecules 25, no. 2: 393. https://doi.org/10.3390/molecules25020393