Organic Acids and Polyphenols Determination in Polish Wines by Ultrasound-Assisted Solvent Extraction of Porous Membrane-Packed Liquid Samples
Abstract
:1. Introduction
2. Results and Discussion
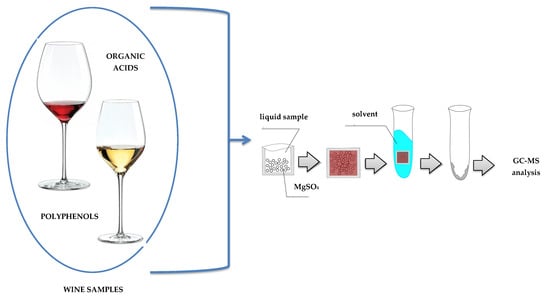
2.1. Optimization of an Ultrasound-Assisted Solvent Extraction of Porous Membrane-Packed Liquid Samples
2.2. Method Validation
2.3. Quantification of Organic Acids and Polyphenols in Polish Wines
2.4. Green Evaluation Assessment
3. Materials and Methods
3.1. Reagents
3.2. GC–MS Conditions
3.3. Wine Samples
3.4. Analytical Procedures and Statistical Analysis
3.4.1. Extraction and Derivatization Procedure
3.4.2. Quality Control (QC) Sample and Calibration Solutions
3.4.3. Method Validation
3.4.4. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Tarko, T.; Duda-Chodak, A.; Sroka, P.; Satora, P.; Jurasz, E. Polish wines: Characteristics of cool-climate wines. J. Food Compos. Anal. 2010, 23, 463–468. [Google Scholar] [CrossRef]
- Jurado-Sánchez, B.; Ballesteros, E.; Gallego, M. Gas chromatographic determination of 29 organic acids in foodstuffs after continuous solid-phase extraction. Talanta 2011, 84, 924–930. [Google Scholar] [CrossRef]
- Sumby, K.M.; Grbin, P.R.; Jiranek, V. Implications of new research and technologies for malolactic fermentation in wine. Appl. Microbiol. Biotechnol. 2014, 98, 8111–8132. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, K.; Khan, A.; Ali, M.M.; Khattak, K. Biological Significance of Ascorbic Acid (Vitamin C) in Human Health—A Review. Pak. J. Nutr. 2004, 3, 5–13. [Google Scholar]
- Bortz, J.D.; Kirschner, M.I. A1 Methods and Compositions for Enhancing Iron Absorption Patent Application Publication Pub. No. US 2016/0022631, 28 June 2016. [Google Scholar]
- Nagai, R.; Nagai, M.; Shimasaki, S.; Baynes, J.W.; Fujiwara, Y. Biochemical and Biophysical Research Communications Citric acid inhibits development of cataracts, proteinuria and ketosis in streptozotocin (type 1) diabetic rats. Biochem. Biophys. Res. Commun. 2010, 393, 118–122. [Google Scholar] [CrossRef] [PubMed]
- Marunaka, Y. The Proposal of Molecular Mechanisms of Weak Organic Acids Intake-Induced Improvement of Insulin Resistance in Diabetes Mellitus via Elevation of Interstitial Fluid pH. Int. J. Mol. Sci. 2018, 19, 3244. [Google Scholar] [CrossRef]
- Tomás- Barberán, F.A. Los polifenoles de los alimentos y la salud. Aliment. Nutr. Y Salud 2003, 10, 41–53. [Google Scholar]
- Galarce-Bustos, O.; Novoa, L.; Pavon-Perez, J.; Henriquez-Aedo, K.; Aranda, M. Chemometric Optimization of QuEChERS Extraction Method for Polyphenol Determination in Beers by Liquid Chromatography with Ultraviolet Detection. Food Anal. Methods 2019, 12, 448–457. [Google Scholar] [CrossRef]
- Caprioli, G.; Boarelli, M.C.; Ricciutelli, M.; Sagratini, G.; Fiorini, D. Micro-scaled Quantitative Method to Analyze Olive Oil Polyphenols. Food Anal. Methods 2019, 12, 1133–1139. [Google Scholar] [CrossRef]
- Perumalla, A.V.S.; Hettiarachchy, N.S. Green tea and grape seed extracts—Potential applications in food safety and quality. Food Res. Int. 2011, 44, 827–839. [Google Scholar] [CrossRef]
- Ferrandino, A.; Carlomagno, A.; Baldassarre, S.; Schubert, A. Varietal and pre-fermentative volatiles during ripening of Vitis vinifera cv Nebbiolo berries from three growing areas. Food Chem. 2012, 135, 2340–2349. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; Sang, M.; Fan, P.; Wu, B.; Wang, L.; Duan, W.; Li, S. Changes of Polyphenols, Sugars, and Organic Acid in 5 Vitis Genotypes during Berry Ripening. J. Food Sci. 2011, 76, 1231–1238. [Google Scholar] [CrossRef] [PubMed]
- Walorczyk, S.; Drozdzynski, D.; Gnusowski, B. Multiresidue determination of 160 pesticides in wines employing mixed-mode dispersive-solid phase extraction and gas chromatography—tandem mass spectrometry. Talanta 2011, 85, 1856–1870. [Google Scholar] [CrossRef] [PubMed]
- Tuzimski, T. Determination of pesticides in wines samples by HPLC-DAD and HPTLC-DAD. J. Liq. Chromatogr. Relat. Technol. 2012, 35, 1415–1428. [Google Scholar] [CrossRef]
- Raczkowska, J.; Mielcarz, G.; Howard, A.; Raczkowski, M. UPLC and Spectrophotometric Analysis of Polyphenols in Wines Available in the Polish Market. Int. J. Food Prop. 2011, 14, 514–522. [Google Scholar] [CrossRef]
- Adamski, J.; Kochana, J.; Nowak, P.; Parczewski, A. On the electrochemical biosensing of phenolic compounds in wines. J. Food Compos. Anal. 2016, 46, 1–6. [Google Scholar] [CrossRef]
- Plotka-Wasylka, J.; Rutkowska, M.; Cieslik, B.; Tyburcy, A.; Namieśnik, J. Determination of Selected Metals in Fruit Wines by Spectroscopic Techniques. J. Anal. Methods Chem. 2017, 2017, 5283917. [Google Scholar] [CrossRef]
- Plotka-Wasylka, J.; Namiesnik, J.; Klodzinska, E. Determination of Biogenic Amines in Wine using Micellar Electrokinetic Chromatography. J. Res. Anal. 2017, 3, 62–66. [Google Scholar]
- Piasta, A.M.; Jastrzebska, A.; Krzeminski, M.P.; Muziol, T.M.; Szlyk, E. New procedure of selected biogenic amines determination in wine samples by HPLC. Anal. Chim. Acta 2014, 834, 58–66. [Google Scholar] [CrossRef]
- Papageorgiou, M.; Lambropoulou, D.; Morrison, C.; Namiesnik, J.; Plotka-Wasylka, J. Direct solid phase microextraction combined with gas chromatography—Mass spectrometry for the determination of biogenic amines in wine. Talanta 2018, 183, 276–282. [Google Scholar] [CrossRef]
- Barros, P.E.; Moreira, N.; Pereira, E.G.; Gomes Ferreira Leite, S.; Moraes Rezende, C.; Guedes de Pinho, P. Development and validation of automatic HS-SPME with a gas chromatography-ion trap/mass spectrometry method for analysis of volatiles in wines. Talanta 2012, 101, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Viñas, P.; Campillo, N.; Martínez-Castillo, N.; Hernández-Córdoba, M. Solid-phase microextraction on-fiber derivatization for the analysis of some polyphenols in wine and grapes using gas chromatography-mass spectrometry. J. Chromatogr. A 2009, 1216, 1279–1284. [Google Scholar] [CrossRef] [PubMed]
- Sajid, M.; Płotka-Wasylka, J. Combined extraction and microextraction techniques: Recent trends and future perspectives. Trends Anal. Chem. 2018, 103, 74–86. [Google Scholar] [CrossRef]
- Yang, P.; Li, H.; Wang, H.; Han, F.; Jing, S.; Yuan, C.; Guo, A.; Zhang, Y.; Xu, Z. Dispersive Liquid-Liquid Microextraction Method for HPLC Determination of Phenolic Compounds in Wine. Food Anal. Methods 2017, 10, 2383–2397. [Google Scholar] [CrossRef]
- Sun, S.Y.; Jiang, W.G.; Zhao, Y.P. Comparison of aromatic and phenolic compounds in cherry wines with different cherry cultivars by HS-SPME-GC-MS and HPLC. Int. J. Food Sci. Technol. 2012, 47, 100–106. [Google Scholar] [CrossRef]
- Hashim, S.N.N.S.; Schwarz, L.J.; Boysen, R.I.; Yang, Y.; Danylec, B.; Hearn, M.T.W. Rapid solid-phase extraction and analysis of resveratrol and other polyphenols in red wine. J. Chromatogr. A 2013, 1313, 284–290. [Google Scholar] [CrossRef]
- Antalick, G.; Perello, M.; Revel, G. De Development, validation and application of a specific method for the quantitative determination of wine esters by headspace-solid-phase microextraction-gas chromatography—Mass spectrometry. Food Chem. 2010, 121, 1236–1245. [Google Scholar] [CrossRef]
- Fariña, L.; Boido, E.; Carrau, F.; Dellacassa, E. Determination of volatile phenols in red wines by dispersive liquid—Liquid microextraction and gas chromatography—Mass spectrometry detection. J. Chromatogr. A 2007, 1157, 46–50. [Google Scholar] [CrossRef]
- Li, J.; Jia, S.; Jun, S.; Ji, S.; Won, S.; Lee, J. Ion-pair dispersive liquid—Liquid microextraction solidification of floating organic droplets method for the rapid and sensitive detection of phenolic acids in wine samples using liquid chromatography combined with a core—Shell particle column. J. Food Compos. Anal. 2016, 45, 73–79. [Google Scholar] [CrossRef]
- Fontana, A.R.; Bottini, R. High-throughput method based on quick, easy, cheap, effective, rugged and safe followed by liquid chromatography-multi-wavelength detection for the quantification of multiclass polyphenols in wines. J. Chromatogr. A 2014, 1342, 44–53. [Google Scholar] [CrossRef]
- Basheer, C.; Alnedhary, A.A.; Rao, B.S.M.; Valliyaveettil, S.; Lee, H.K. Development and Application of Porous Membrane-Protected Carbon Nanotube Micro-Solid-Phase Extraction Combined with Gas Chromatography/Mass Spectrometry. Anal. Chem. 2006, 78, 2853–2858. [Google Scholar] [CrossRef] [PubMed]
- Basheer, C.; Chong, H.G.; Hii, T.M.; Lee, H.K. Application of Porous Membrane-Protected Micro-Solid-Phase Extraction Combined with HPLC for the Analysis of Acidic Drugs in Wastewater. Anal. Chem. 2007, 79, 6845–6850. [Google Scholar] [CrossRef] [PubMed]
- Basheer, C.; Wong, W.; Makahleh, A.; Tameem, A.A.; Salhin, A.; Saad, B.; Lee, H.K. Hydrazone-based ligands for micro-solid phase extraction-high performance liquid chromatographic determination of biogenic amines in orange juice. J. Chromatogr. A 2011, 1218, 4332–4339. [Google Scholar] [CrossRef] [PubMed]
- Lashgari, M.; Basheer, C.; Lee, H.K. Application of surfactant-templated ordered mesoporous material as sorbent in micro-solid phase extraction followed by liquid chromatography—Triple quadrupole mass spectrometry for determination of perfluorinated carboxylic acids in aqueous. Talanta 2015, 141, 200–206. [Google Scholar] [CrossRef]
- Sajid, M.; Basheer, C.; Narasimhan, K.; Choolani, M.; Lee, H.K. Application of microwave-assisted micro-solid-phase extraction for determination of parabens in human ovarian cancer tissues. J. Chromatogr. B 2015, 1000, 192–198. [Google Scholar] [CrossRef]
- Sajid, M.; Wozniak, M.K.; Plotka-Wasylka, J. Ultrasound-assisted solvent extraction of porous membrane packed solid samples: A new approach for extraction of target analytes from solid samples. Microchem. J. 2019, 144, 117–123. [Google Scholar] [CrossRef]
- Dobrowolska-Iwanek, J.; Gąstoł, M.; Wanat, A.; Krośniak, M.; Jancik, M.; Zagrodzki, P. Wine of Cool-climate Areas in South Poland. S. Afr. J. Enol. Vitic. 2014, 35, 1–9. [Google Scholar] [CrossRef]
- Ohira, S.; Kuhara, K.; Shigetomi, A.; Yamasaki, T.; Kodama, Y.; Dasguota, P.K.; Toda, K. On-line electrodialytic matrix isolation for chromatographic determination of organic acids in wine. J. Chromatogr. A 2014, 1372, 18–24. [Google Scholar] [CrossRef] [Green Version]
- Zeravik, J.; Fohlerova, Z.; Milovanovic, M.; Kubesa, O.; Zeisbergerova, M.; Lacina, K.; Petrovic, A.; Glatz, Z.; Skladal, P. Various instrumental approaches for determination of organic acids in wines. Food Chem. 2016, 194, 432–440. [Google Scholar] [CrossRef]
- Minuti, L.; Pellegrino, R.M.; Tesei, I. Simple extraction method and gas chromatography—Mass spectrometry in the selective ion monitoring mode for the determination of phenols in wine. J. Chromatogr. A 2006, 1114, 263–268. [Google Scholar] [CrossRef]
- Anli, R.E.; Vural, N.; Kizile, E. An alternative method for the determination of some of the antioxidant phenolics in varietal Turkish red wines. J. Inst. Brew. 2008, 114, 239–245. [Google Scholar] [CrossRef]
- Šeruga, M.; Novak, I.; Jakobek, L. Determination of polyphenols content and antioxidant activity of some red wines by differential pulse voltammetry, HPLC and spectrophotometric methods. Food Chem. 2011, 124, 1208–1216. [Google Scholar] [CrossRef]
- Mato, I.; Suarez-Luque, S.; Huidoro, J.F. Simple determination of main organic acids in grape juice and wine by using capillary zone electrophoresis with direct UV detection. Food Chem. 2007, 102, 104–112. [Google Scholar] [CrossRef]
- Cunha, S.C.; Fernandes, J.O.; Faria, M.A.; Ferreira, I.M.P.L.V.O.; Ferreira, M.A. Quantification of Organic Acids in grape musts and Port Wines. CYTA J. Food 2002, 3, 212–216. [Google Scholar]
- do Nascimento Silva, F.L.; Schmidt, E.M.; Messias, C.L.; Nogueira Eberlin, M.; Sawaya Frankland, H.A.C. Quantitation of organic acids in wine and grapes by direct infusion electrospray ionization mass spectrometry. Anal. Methods 2015, 7, 53–62. [Google Scholar] [CrossRef] [Green Version]
- Bai, S.; Cui, C.; Liu, J.; Li, P.; Li, Q.; Bi, K. Quantification of polyphenol composition and multiple statistical analyses of biological activity in Portuguese red wines. Eur. Food Res. Technol. 2018, 244, 2007–2017. [Google Scholar] [CrossRef]
- Gonçalves, J.; Mendes, B.; Silva, C.L.; Câmara, J.S. Development of a novel microextraction by packed sorbent-based approach followed by ultrahigh pressure liquid chromatography as a powerful technique for quantification phenolic constituents of biological interest in wines. J. Chromatogr. A 2012, 1229, 13–23. [Google Scholar] [CrossRef]
- Plotka-Wasylka, J. A new tool for the evaluation of the analytical procedure: Green Analytical Procedure Index. Talanta 2018, 181, 204–209. [Google Scholar] [CrossRef]
- Konieczka, P.; Namieśnik, J. Quality Assurance and Quality Control in the Analytical Chemical Laboratory. A Practical Approach, 2nd ed.; CRC Press Taylor & Francis Group: Boca Raton, FL, USA, 2018; ISBN 9781138196728. [Google Scholar]
- Sajid, M.; Basheer, C.; Alsharaa, A.; Narasimhan, K.; Buhmeida, A.; Qahtani, M.; Al-ahwal, M.S. Development of natural sorbent based micro-solid-phase extraction for determination of phthalate esters in milk samples. Anal. Chim. Acta 2016, 924, 35–44. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |





| Compounds | Linear Range (µg/mL) | r | LOD (µg/mL) | LOQ (µg/mL) |
|---|---|---|---|---|
| Lactic acid | 11–95 | 0.9927 | 3.6 | 11 |
| Fumaric acid | 0.83–30.63 | 0.9991 | 0.28 | 0.83 |
| Succinic acid | 5.6–25.4 | 0.9965 | 1.9 | 5.6 |
| L-Malic acid | 2.2–26.1 | 0.9882 | 0.75 | 2.2 |
| Tartaric acid | 25.13–150.75 | 0.9907 | 5.9 | 17 |
| Citric acid | 24–147.8 | 0.9852 | 8.2 | 24 |
| Protocatechuic acid | 1.1–32 | 0.9993 | 0.36 | 1.1 |
| p-Coumaric acid | 0.81–33.5 | 0.9981 | 0.27 | 0.81 |
| Gallic acid | 0.047–23.63 | 0.9915 | 0.016 | 0.047 |
| Ferulic acid | 1.0–32.5 | 0.9979 | 0.35 | 1.0 |
| Caffeic acid | 3.7–82.5 | 0.9992 | 1.2 | 3.7 |
| Sinapic acid | 0.95–30 | 0.9991 | 0.32 | 0.95 |
| Pterostilbene | 0.58–29 | 0.9989 | 0.17 | 0.52 |
| Resveratrol | 0.99–24 | 0.9983 | 0.33 | 0.99 |
| (+)-Catechin | 5.97–14.18 | 0.9942 | 2.0 | 5.9 |
| Compounds | Inter-day precision Accuracy (precision*) (n = 5) (c = 10 µg/mL) | Intra-day precision Accuracy (precision*) (n = 7) (c = 10 µg/mL) | Recovery | ME Accuracy (precision*) (n = 3) (c = 5 µg/mL) | |||||
|---|---|---|---|---|---|---|---|---|---|
| Day 1 | Day 2 | Day 3 | (c = µg/mL) | %R ± U%R(k = 2) (n = 5) | Red | White | Rosé | ||
| Lactic acid | 95 (17) | 101 (15) | 102.8 (3.7) | 99.7 (1.3) | 15 | 111 ± 18 | 99.7 (9.3) | 116.9 (8.0) | 100.6 (1.4) |
| 20 | 109 ± 16 | ||||||||
| Fumaric acid | 103 (12) | 98.4 (9.5) | 101 (13) | 109.1 (6.5) | 15 | 104 ± 22 | 99.5 (2.6) | 92.2 (1.2) | 102.7 (4.8) |
| 20 | 92.0 ± 8.5 | ||||||||
| Succinic acid | 108.0 (9.4) | 109.9 (2.6) | 106 (12) | 96.3 (4.9) | 15 | 105 ± 15 | 94 (13) | 94.9 (8.5) | 98.7 (2.6) |
| 20 | 103.0 ± 7.8 | ||||||||
| L- Malic acid | 87.5 (6.3) | 95 (15) | 92.3 (6.9) | 91.0 (5.5) | 15 | 99.0 ± 4.4 | 102.2 (6.0) | 103.26 (0.41) | 99 (11) |
| 20 | 101.2 ± 8.9 | ||||||||
| Tartaric acid | 110 (14) | 104 (16) | 100.4 (8.2) | 105.6 (1.2) | 15 | 102.6 ± 5.7 | 87.0 (9.6) | 102.8 (6.0) | 95.1 (6.7) |
| 20 | 113 ± 16 | ||||||||
| Citric acid | 95 (13) | 83 (15) | 94.8 (9.8) | 102.1 (7.7) | 15 | 98.6 ± 3.8 | 110.7 (7.3) | 107.4 (6.5) | 111.9 (8.8) |
| 20 | 98.5 ± 3.1 | ||||||||
| Protocatechuic acid | 111.2 (7.7) | 100.2 (6.4) | 101 (12) | 98.9 (7.2) | 5 | 108 ± 15 | 107.7 (9.1) | 112 (12) | 108.7 (7.2) |
| 10 | 98.5 ± 3.6 | ||||||||
| p-Coumaric acid | 99.2 (8.2) | 94.5 (6.1) | 95.6 (9.0) | 103.4 (7.0) | 5 | 96 ± 15 | 96.0 (5.0) | 107.9 (7.5) | 90.3 (3.4) |
| 10 | 96 ± 12 | ||||||||
| Gallic acid | 109.5 (2.9) | 102.5 (8.5) | 99 (11) | 104.2 (4.6) | 15 | 94.5 ± 7.8 | 98.77 (6.8) | 84.9 (2.2) | 103.2 (4.3) |
| 20 | 98.7 ± 4.7 | ||||||||
| Ferulic acid | 93.1 (7.8) | 91.3 (7.6) | 92 (10) | 99.4 (6.6) | 5 | 103 ± 18 | 103.4 (6.9) | 98.4 (2.6) | 99.5 (2.2) |
| 10 | 102 ± 10 | ||||||||
| Caffeic acid | 94.9 (8.1) | 100 (13) | 117 (16) | 95.0 (7.3) | 5 | 101 ± 13 | 101.4 (3.6) | 104.09 (0.58) | 99.2 (2.5) |
| 10 | 93.7 ± 7.6 | ||||||||
| Sinapic acid | 103 (14) | 99 (12) | 109 (13) | 104.9 (9.8) | 5 | 106 ± 17 | 106.5 (4.1) | 105.4 (8.5) | 103.58 (0.29) |
| 10 | 103 ± 12 | ||||||||
| Pterostilbene | 103.9 (3.9) | 110 (13) | 112.8 (9.2) | 100.7 (9.0) | 5 | 102.3 ± 2.9 | 102.2 (5.0) | 100.9 (2.0) | 96.9 (3.0) |
| 10 | 110 ± 11 | ||||||||
| Resveratrol | 102 (12) | 96 (15) | 98.9 (8.5) | 105.0 (7.5) | 5 | 99 ± 16 | 114.7 (7.9) | 99.6 (3.0) | 96 (11) |
| 10 | 106 ± 13 | ||||||||
| (+)-Catechin | 98.0 (2.8) | 107.9 (1.2) | 106.5 (7.8) | 108.3 (6.8) | 5 | 95 ± 11 | 106.5 (2.3) | 95.1 (4.8) | 103.4 (7.2) |
| 10 | 97 ± 12 | ||||||||
| Sample | Protocatechuic Acid | p-Coumaric Acid | Gallic Acid | Ferulic Acid | Caffeic Acid | Sinapic Acid | Pterostilbene | Resveratrol | (+)-Catechin |
|---|---|---|---|---|---|---|---|---|---|
| 1R | 1.830 ± 0.027 | 7.82 ± 0.10 | 2.360 ± 0.077 | <LOQ | 25.18 ± 0.20 | <LOQ | <LOD | 2.880 ± 0.036 | 454 ± 112 |
| 2R | 2.50 ± 0.10 | 10.62 ± 0.33 | 2.210 ± 0.081 | <LOQ | 15.41 ± 0.33 | <LOQ | <LOD | 2.460 ± 0.019 | 336 ± 61 |
| 3R | 5.22 ± 0.45 | 2.990 ± 0.070 | 2.49 ± 0.51 | <LOQ | 9.14 ± 0.41 | <LOQ | <LOD | 2.960 ± 0.074 | 383 ± 36 |
| 4R | 6.75 ± 0.72 | 11.65 ± 0.81 | 4.97 ± 0.91 | <LOQ | 21.03 ± 2.11 | 0.960 ± 0.035 | <LOD | 2.980 ± 0.060 | 965 ± 349 |
| 5R | 2.48 ± 0.33 | 4.39 ± 0.61 | 2.51 ± 0.20 | <LOQ | 12.1 ± 1.4 | <LOQ | <LOD | 2.330 ± 0.052 | 66.9 ± 7.3 |
| 6R | <LOQ | 12.33 ± 0.67 | 2.72 ± 0.16 | <LOQ | 25.3 ± 2.3 | <LOQ | <LOD | 2.540 ± 0.045 | 140 ± 19 |
| 7R | <LOQ | 7.15 ± 0.79 | 6.59 ± 0.97 | <LOQ | 30.7 ± 3.0 | <LOQ | <LOD | 5.09 ± 0.23 | 6226 ± 243 |
| 8R | 5.26 ± 0.12 | 10.87 ± 0.53 | 6.11 ± 0.41 | <LOQ | 8.36 ± 0.41 | 0.96 ± 0.41 | <LOD | 4.0 ± 0.14 | 2860 ± 116 |
| 9R | 2.24 ± 0.17 | 7.12 ± 0.31 | 1.02000 ± 0.00051 | <LOQ | 14.3 ± 2.6 | <LOQ | <LOD | 2.280 ± 0.061 | 10.4 ± 2.9 |
| 10R | <LOQ | 12.4 ±1.1 | 0.86 ± 0.51 | <LOQ | 10.2 ± 1.3 | 0.950 ± 0.041 | <LOD | 4.70 ± 0.54 | 5525 ± 994 |
| 1Ro | <LOQ | <LOQ | 0.100 ± 0.012 | <LOQ | 6.52 ± 0.37 | <LOQ | <LOD | 2.320 ± 0.099 | <LOQ |
| 2Ro | 2.37 ± 0.85 | 22.3 ± 3.8 | 1.18 ± 0.42 | <LOQ | 13.6 ± 3.2 | 1.03 ± 0.13 | <LOD | 2.290 ± 0.056 | 38 ± 13 |
| 3Ro | <LOQ | <LOQ | 0.1200 ± 0.0006 | <LOQ | 5.49 ± 0.77 | <LOQ | <LOD | 2.210 ± 0.013 | <LOQ |
| 1W | <LOQ | 1.110 ± 0.059 | 0.240 ± 0.014 | <LOQ | 8.88 ± 0.22 | <LOQ | <LOD | 2.2700 ± 0.0046 | 119 ± 12 |
| 2W | <LOQ | 0.800 ± 0.052 | 0.190 ± 0.016 | <LOQ | 8.31 ± 0.15 | <LOQ | <LOD | 2.250 ± 0.021 | 41.9 ± 1.4 |
| 3W | <LOQ | 0.94 ± 0.25 | 0.320 ± 0.051 | <LOQ | 6.17 ± 0.26 | <LOQ | <LOD | 2.270 ± 0.025 | 16.8 ± 4.7 |
| 4W | <LOQ | 2.23 ± 0.33 | 0.470 ± 0.040 | <LOQ | 7.570 ± 0.040 | <LOQ | <LOD | 2.380 ± 0.029 | 34.9 ± 2.7 |
| 5W | <LOQ | 0.9100 ± 0.0091 | 0.610 ± 0.093 | <LOQ | 7.69 ± 0.60 | <LOQ | <LOD | 2.360 ± 0.021 | 29.6 ± 1.5 |
| 6W | <LOQ | <LOQ | 0.1200 ± 0.0072 | <LOQ | 5.78 ± 0.33 | <LOQ | <LOD | 2.2400 ± 0.0042 | 34.2 ± 6.4 |
| 7W | 1.34 ± 0.21 | 1.23 ± 0.23 | 0.210 ± 0.013 | <LOQ | 6.33 ± 0.26 | <LOQ | <LOD | 2.510 ± 0.025 | 110 ± 28 |
| 8W | <LOQ | <LOQ | 0.09 ± 0.10 | <LOQ | 4.4500 ± 0.0048 | <LOQ | <LOD | 2.2000 ± 0.0096 | <LOQ |
| 9W | <LOQ | 1.1200 ± 0.0054 | 0.250 ± 0.020 | <LOQ | 5.27 ± 0.17 | <LOQ | <LOD | 2.250 ± 0.020 | 15 ± 11 |
| 10W | <LOQ | 2.220 ± 0.042 | 0.300 ± 0.051 | <LOQ | 6.22 ± 0.12 | <LOQ | <LOD | 2.3100 ± 0.0043 | 29.9 ± 1.6 |
| Sample | Lactic Acid | Succinic Acid | Fumaric Acid | L-Malic Acid | Tartaric Acid | Citric Acid |
|---|---|---|---|---|---|---|
| 1R | 260.7 ± 4.5 | 259.3 ± 4.8 | <LOQ | 23.5 ± 1.8 | 58.9 ± 1.3 | <LOQ |
| 2R | 340 ± 13 | 457 ± 11 | <LOQ | 117 ± 10 | 46.8 ± 3.5 | <LOQ |
| 3R | 299 ± 18 | 456 ± 28 | <LOQ | 54.2 ± 3.6 | 44.8 ± 3.4 | 26.8 ± 3.4 |
| 4R | 316 ± 37 | 465 ± 51 | <LOQ | 571 ± 62 | 48.5 ± 2.0 | 54 ± 17 |
| 5R | 306 ± 23 | 466 ± 52 | <LOQ | 37.9 ± 4.8 | 65.0 ± 4.9 | <LOQ |
| 6R | 316.0 ± 8.9 | 388 ± 28 | <LOQ | 31.1 ± 6.2 | 39.3 ± 2.8 | <LOQ |
| 7R | 356.2 ± 1.9 | 351 ± 19 | <LOQ | 115 ± 29 | 39.9 ± 2.9 | <LOQ |
| 8R | 333.3 ± 5.1 | 355.3 ± 2.4 | <LOQ | 217.7 ± 7.2 | 41.5 ± 1.6 | 32.9 ± 5.0 |
| 9R | 310 ± 16 | 401.7 ± 1.4 | <LOQ | 20.9 ± 4.0 | 46.8 ± 4.5 | <LOQ |
| 10R | 439 ± 60 | 370.7 ± 4.4 | <LOQ | 23.3 ± 3.2 | 43.3 ± 6.3 | <LOQ |
| 1Ro | 125.6 ± 7.8 | 338 ± 34 | <LOQ | 867 ± 104 | 44.2 ± 4.1 | 163.33 ± 0.72 |
| 2Ro | 221 ± 11 | 950 ± 287 | 1.38 ± 0.51 | 2185 ± 593 | 78.9 ± 7.9 | 371 ± 51 |
| 3Ro | 320 ± 34 | 248 ± 13 | <LOQ | 205.7 ± 5.5 | 35.5 ± 1.5 | 187.6 ± 1.0 |
| 1W | 45.2 ± 1.0 | 256 ± 12 | <LOQ | 870 ± 65 | 76 ± 11 | 163 ± 12 |
| 2W | 328 ± 12 | 316 ± 31 | <LOQ | 175.4 ± 4.9 | 33.2 ± 2.3 | <LOQ |
| 3W | 117.0 ± 3.9 | 310.9 ± 4.7 | 2.060 ± 0.087 | 901.7 ± 4.4 | 34.2 ± 2.9 | 206.2 ± 1.9 |
| 4W | 53.9 ± 7.8 | 272 ± 29 | 1.55 ± 0.13 | 1081 ± 175 | 40.47 ± 0.80 | 222 ± 34 |
| 5W | 79.6 ± 4.5 | 474 ± 16 | 3.10 ± 0.11 | 1421 ± 34 | 35.2 ± 1.3 | 329 ± 11 |
| 6W | 60.5 ± 2.9 | 253 ± 18 | <LOQ | 921 ± 62 | 42.6 ± 2.4 | 172.6 ± 3.9 |
| 7W | 99 ± 10 | 576 ± 34 | 1.47 ± 0.19 | 1158 ± 85 | 47.86 ± 0.51 | 312 ± 19 |
| 8W | 75.9 ± 1.4 | 226 ± 19 | <LOQ | 1027 ± 61 | 33.1 ± 1.8 | 172 ± 22 |
| 9W | 226 ± 16 | 338 ± 45 | 2.77 ± 0.36 | 1661 ± 247 | 37.5 ± 2.6 | 297 ± 33 |
| 10W | 315 ± 19 | 385 ± 13 | 1.67 ± 0.11 | 1640 ± 87 | 34.5 ± 1.1 | 322 ± 35 |
| Procedure 1 (This Work) | Procedure 2 [44] | Procedure 3 [45] | Procedure 4 [46] | Procedure 5 [47] | Procedure 6 [48] | Procedure 7 [41] | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Reagents | PPs | Reagents | PPs | Reagents | PPs | Reagents | PPs | Reagents | PPs | Reagents | PPs | Reagents | PPs |
| MgSO4 | 0 | NaH2PO4 | 0 | benzylmalonic acid (IS) | 1 | Methanol | 3 | Formic acid | 2 | MeOH | 3 | Phenanthrene | 1 |
| EtAc | 1 | Na2HPO4 | 0 | O-(4-nitrobenzyl)-N,N’-diisopropylisourea | 2 | Water | 0 | Acetonitrile | 6 | Formic acid | 2 | 2,3-benzophenanthrene | 3 |
| DCM | 1 | Tetradecyltrimethyl ammonium hydroxide | 2 | Dioxane | 6 | Ammonium hydroxide | 6 | MeOH | 3 | Acetic acid | 2 | BSTFA + 1% TMCS | 2 |
| MeOH | 3 | CaCl2 | 0 | Acetonitrile | 6 | Water | 0 | Ethanol absolute | 3 | Pyridine | 3 | ||
| BSTFA + 1% TMCS | 2 | Water | 0 | Water | 0 | NaOH | 2 | EtAc | 1 | ||||
| 3-methylbenzoic acid (IS) | 0 | Water | 0 | ||||||||||
| Ʃ 7 | Ʃ 2 | Ʃ 15 | Ʃ 9 | Ʃ 11 | Ʃ 12 | Ʃ 10 | |||||||
| Instruments | PPs | Instruments | PPs | Instruments | PPs | Instruments | PPs | Instruments | PPs | Instruments | PPs | Instruments | PPs |
| Transport | 1 | Transport | 1 | Transport | 1 | Transport | 1 | Transport | 1 | Transport | 1 | Transport | 1 |
| GC–MS | 2 | Water Capillary Ion Analyzer | 2 | Heater | 2 | ESI-MS | 2 | Filtration | 0 | MEPS | 2 | LLE | 2 |
| Ultrasound bath | 1 | Occupational hazard | 0 | HPLC-UV | 2 | Occupational hazard | 0 | UFLC-MS/MS | 2 | UHPLC-PDA | 0 | GC–MS | 2 |
| Occupational hazard | 0 | Waste | 1 | Occupational hazard | 0 | Waste | 1 | Occupational hazard | 0 | Occupational hazard | 0 | Occupational hazard | 1 |
| Waste | 1 | Waste | 3 | Waste | 3 | Waste | 1 | Waste | 5 | ||||
| Ʃ 5 | Ʃ 4 | Ʃ 8 | Ʃ 4 | Ʃ 6 | Ʃ 4 | Ʃ 11 | |||||||
| Total PPs: 12 | Total PPs: 6 | Total PPs: 23 | Total PPs: 13 | Total PPs: 17 | Total PPs: 16 | Total PPs: 21 | |||||||
| Score: 88 | Score: 94 | Score: 77 | Score: 87 | Score: 83 | Score: 84 | Score: 79 | |||||||
| Labeled Peaks | Compound | Rt [min] | Quantitative Ion | Qualitative Ions |
|---|---|---|---|---|
| 1 | Lactic acid | 5.50 | 147 | 117, 191 |
| 2 | Succinic acid | 9.01 | 147 | 75, 148 |
| 3 | Fumaric acid | 9.50 | 245 | 147, 246 |
| 4 | L-Malic acid | 11.24 | 73 | 147, 233 |
| 5 | Tartaric acid | 11.98 | 73 | 147, 292 |
| 6 | Citric acid | 15.04 | 273 | 147, 347 |
| 7 | Protocatechuic acid | 15.08 | 193 | 355, 370 |
| 8 | p-Coumaric acid | 16.35 | 293 | 219, 308 |
| 9 | Gallic acid | 16.45 | 281 | 282, 458 |
| 10 | Ferulic acid | 17.80 | 338 | 308, 323 |
| 11 | Caffeic acid | 18.20 | 219 | 396, 397 |
| 12 | Sinapic acid | 19.16 | 368 | 353, 338 |
| 13 | Pterostilbene | 22.42 | 328 | 327, 329 |
| 14 | Resveratrol | 23.05 | 444 | 445, 446 |
| 15 | (+)-Catechin | 24.63 | 368 | 355, 369 |
| 16 | IS | 9.55 | 193 | 119, 149 |
| Label | Vineyard | Year | Type of Wine | Origin | % Alcohol | Grape Type | Sugar Content |
|---|---|---|---|---|---|---|---|
| 1R | HOPLE Winnica Poraj Paczkow | 2015 | Red | Paczkow | 11.0 | Regent | dry |
| 2R | Winnica Chodorowa | 2017 | Red | Grybów | 12.0 | Regent | dry |
| 3R | Dom Bliskowice | 2014 | Red | Bliskowice | 12.1 | Rondo | dry |
| 4R | Winnica Witanowice | 2013 | Red | Witanowice | 12.5 | Regent | dry |
| 5R | Adoria Vineyards | 2017 | Red | Zachowice | 13.5 | Dornfelder | dry |
| 6R | Winnica Chodorowa | 2017 | Red | Grybów | 11.0 | Rondo | dry |
| 7R | Adoria Vineyards | 2017 | Red | Zachowice | 13.5 | Pinot Noir | dry |
| 8R | Winnica Turnau | 2016 | Red | Banie | 13.0 | Rondo/Regent | dry |
| 9R | HOPLE Winnica Poraj Paczkow | 2015 | Red | Paczków | 11.5 | Rondo | dry |
| 10R | Winnica Golesz | 2016 | Red | Jasło | 12.5 | Mix of three grapes | dry |
| 1W | Winnica Solaris | 2016 | White | Opole Lubelskie | 12.0 | Johanniter | dry |
| 2W | Adoria Vineyards | 2017 | White | Zachowice | 12.0 | Riesling | semi-dry |
| 3W | Winnica Saint Vincent | 2016 | White | Borów Wielki | 12.0 | Pinot Gris, Riesling, Muscat Ottonel, Gewurztraminer | semi-dry |
| 4W | Winnica Srebrna Gora | 2017 | White | Kraków | 12.0 | Seyval Blanc, Hibernal, Johanniter, Solaris | semi-dry |
| 5W | Winnica Saint Vincent | 2016 | White | Borów Wielki | 13.0 | Pinot Gris | semi-dry |
| 6W | Winnica Solaris | 2016 | White | Opole Lubelskie | 12.5 | Solaris | sweet |
| 7W | Winnica Witanowice | 2014 | White | Witanowice | 12.0 | Bianca | dry |
| 8W | Winnica Turnau | 2017 | White | Banie | 12.5 | Solaris | dry |
| 9W | Winnica Golesz | 2017 | White | Jasło | 12.0 | Mix of grapes | semi-sweet |
| 10W | Winnica Golesz | 2015 | White | Jaslo | 11.5 | Mix of eight grapes | dry |
| 1Ro | Winnica Srebrna Gora | 2014 | Rosé | Krakow | 10.5 | Zweiglet | semi-dry |
| 2Ro | Winnica De Sas | 2015 | Rosé | Krosnice | 10.5 | Regent | dry |
| 3Ro | Winnica Golesz | 2016 | Rosé | Jaslo | 11.5 | Mix of three grapes | dry |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Robles, A.D.; Fabjanowicz, M.; Płotka-Wasylka, J.; Konieczka, P. Organic Acids and Polyphenols Determination in Polish Wines by Ultrasound-Assisted Solvent Extraction of Porous Membrane-Packed Liquid Samples. Molecules 2019, 24, 4376. https://doi.org/10.3390/molecules24234376
Robles AD, Fabjanowicz M, Płotka-Wasylka J, Konieczka P. Organic Acids and Polyphenols Determination in Polish Wines by Ultrasound-Assisted Solvent Extraction of Porous Membrane-Packed Liquid Samples. Molecules. 2019; 24(23):4376. https://doi.org/10.3390/molecules24234376
Chicago/Turabian StyleRobles, Alicia D., Magdalena Fabjanowicz, Justyna Płotka-Wasylka, and Piotr Konieczka. 2019. "Organic Acids and Polyphenols Determination in Polish Wines by Ultrasound-Assisted Solvent Extraction of Porous Membrane-Packed Liquid Samples" Molecules 24, no. 23: 4376. https://doi.org/10.3390/molecules24234376