Regulation of Noise-Induced Loss of Serotonin Transporters with Resveratrol in a Rat Model Using 4-[18F]-ADAM/Small-Animal Positron Emission Tomography
Abstract
:1. Introduction
2. Results
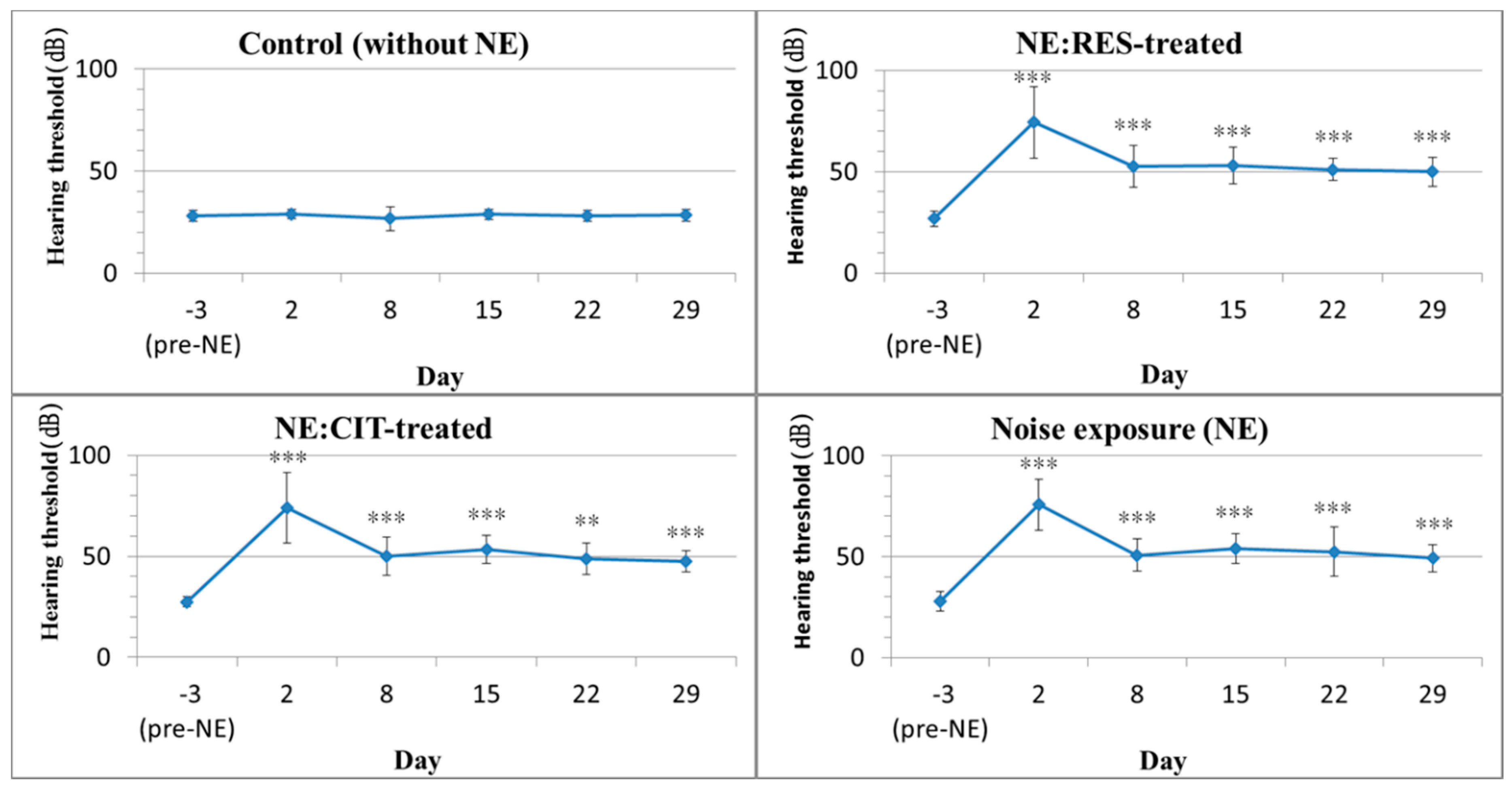
2.1. High Intensity Noise Induced Permanent Hearing Loss
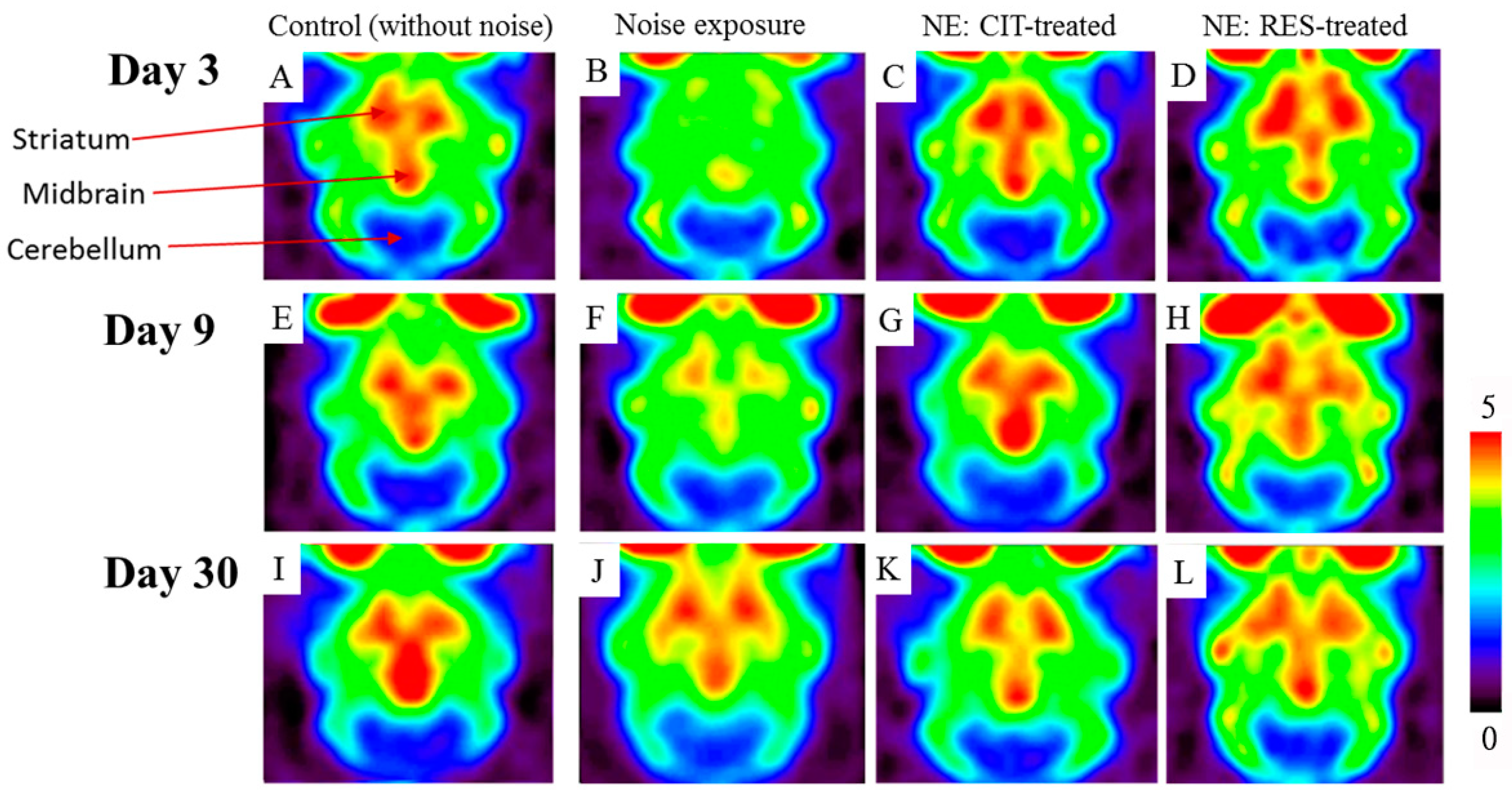
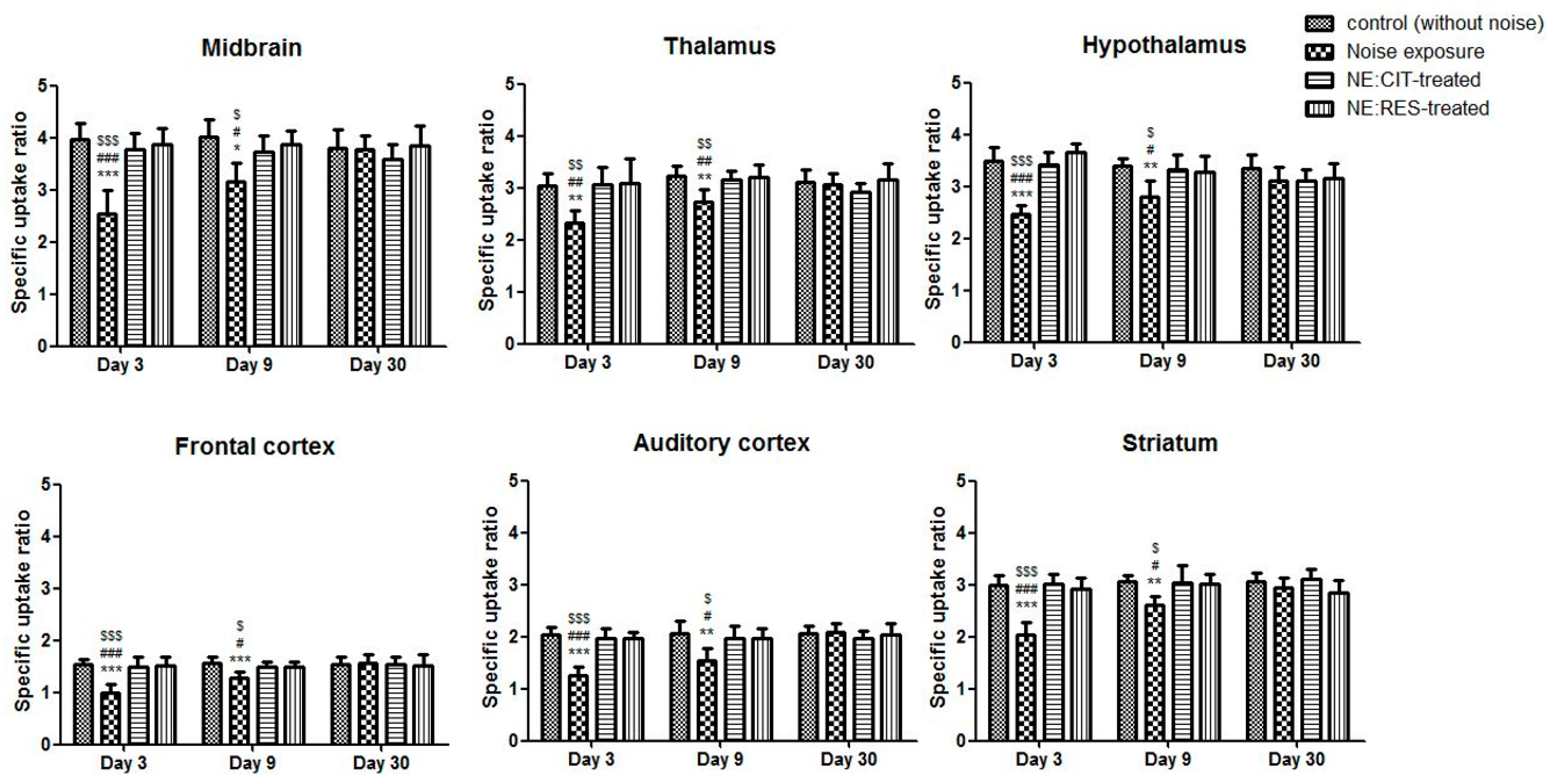
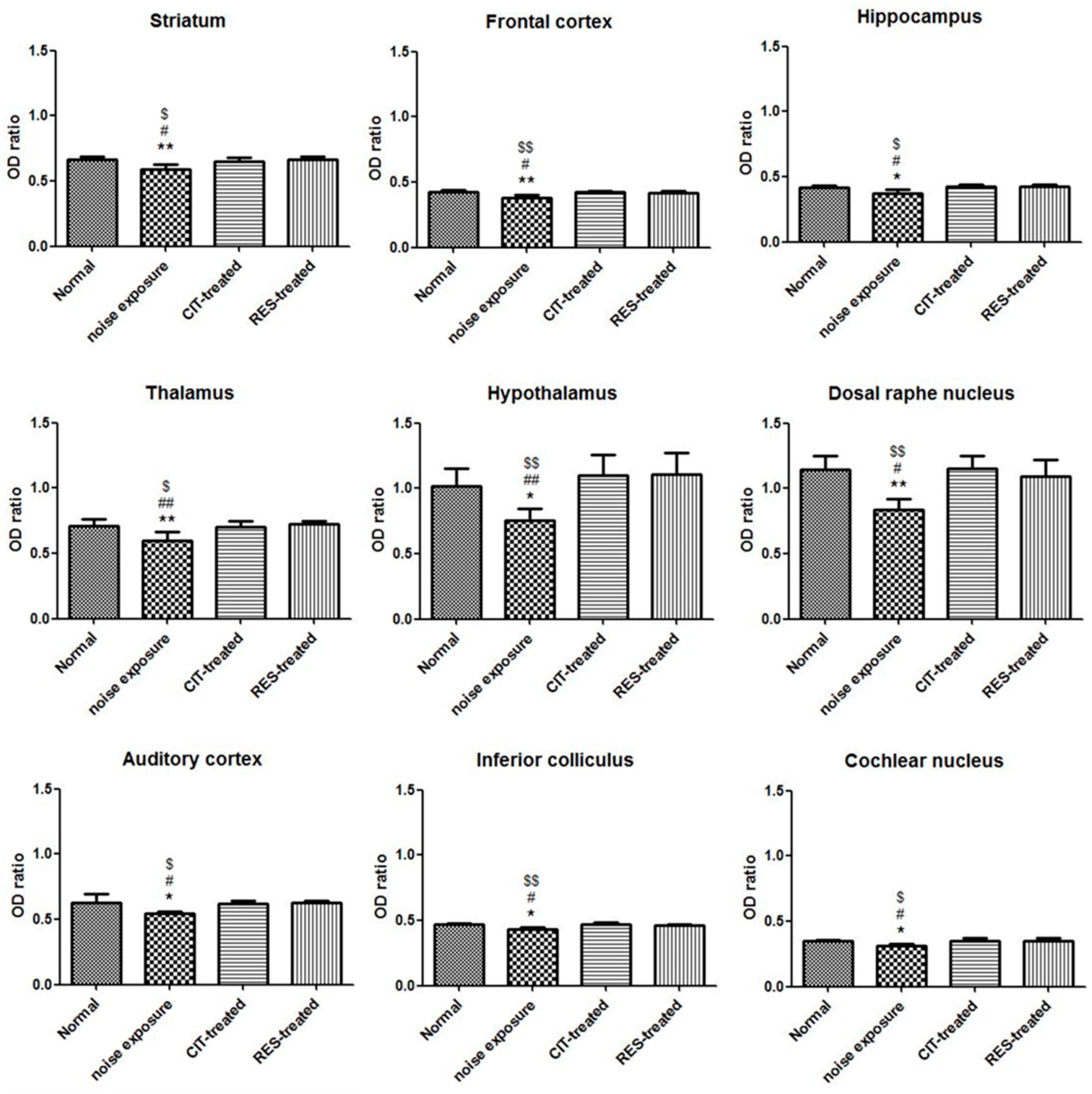
2.2. Noise Exposure Decreased SERT Levels in Multiple Brain Regions
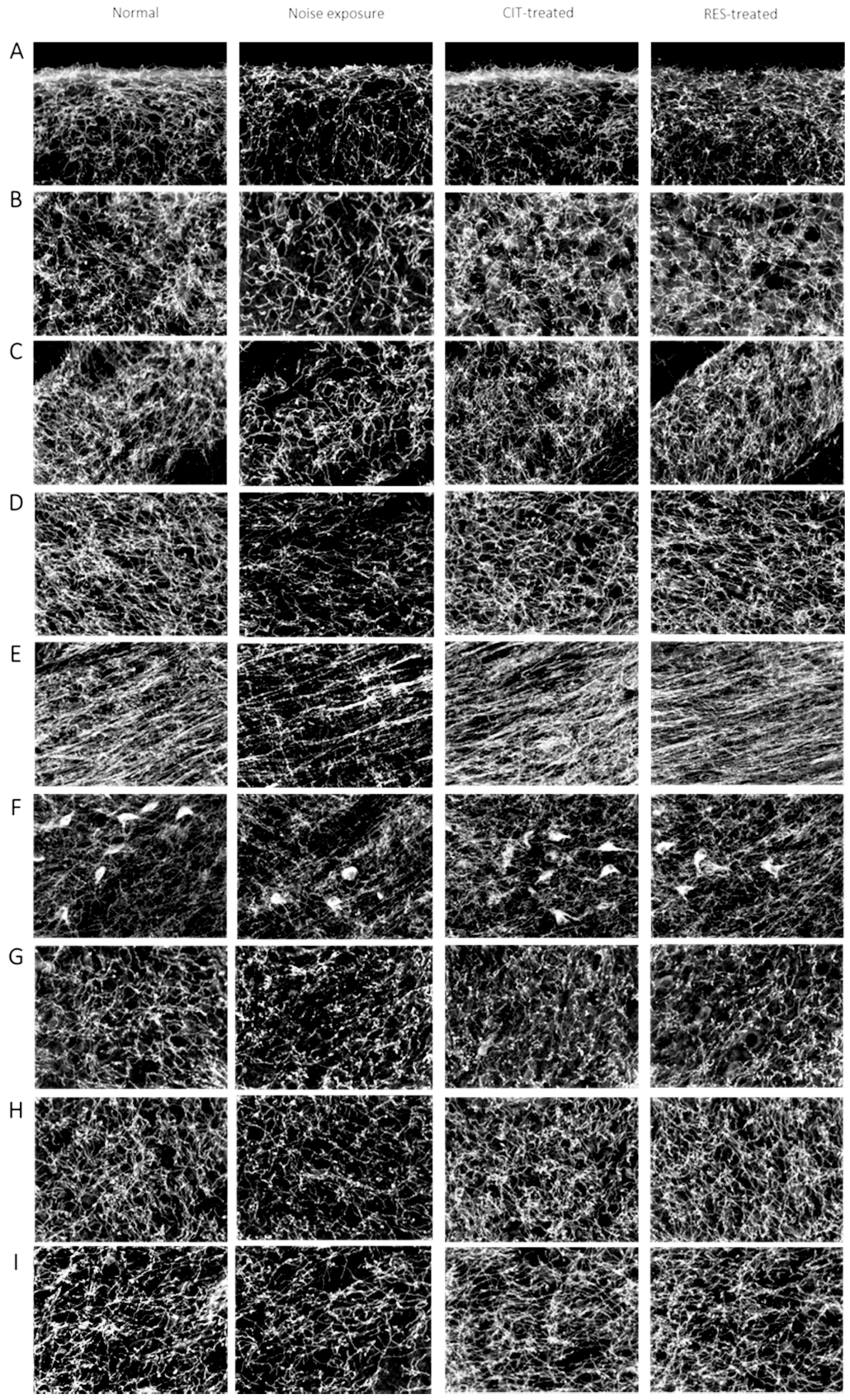
2.3. Resveratrol Conferred Neuroprotection against Noise-Induced SERT Loss
3. Discussion
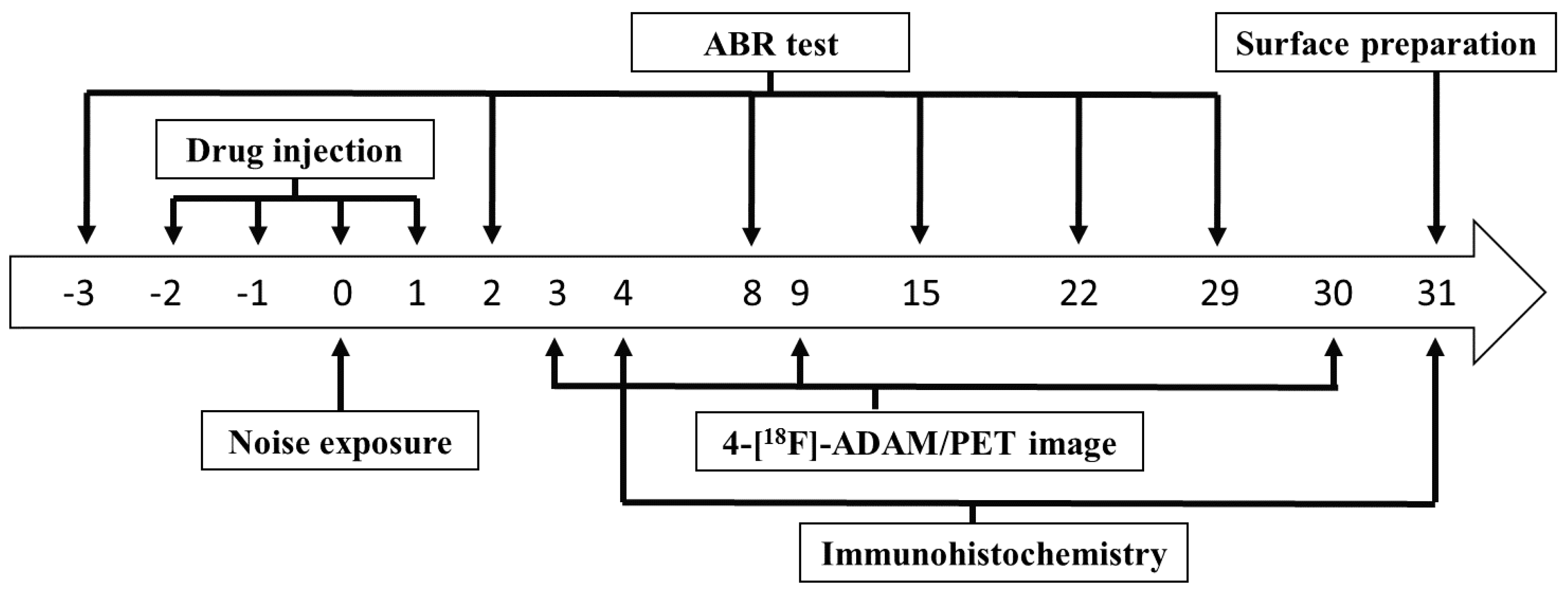
4. Materials and Methods
4.1. Experimental Animals
4.2. Hearing Threshold Detection
4.3. Cochlear Surface Preparation and Actin-Staining
4.4. Small Animal-PET Imaging
4.5. Immunohistochemistry
4.6. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Pelegrin, A.C.; Canuet, L.; Rodriguez, A.A.; Morales, M.P. Predictive factors of occupational noise-induced hearing loss in Spanish workers: A prospective study. Noise Health 2015, 17, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Saunders, J.C.; Dear, S.P.; Schneider, M.E. The anatomical consequences of acoustic injury: A review and tutorial. J. Acoust. Soc. Am. 1985, 78, 833–860. [Google Scholar] [CrossRef] [PubMed]
- Ottersen, O.P.; Takumi, Y.; Matsubara, A.; Landsend, A.S.; Laake, J.H.; Usami, S. Molecular organization of a type of peripheral glutamate synapse: The afferent synapses of hair cells in the inner ear. Prog. Neurobiol. 1998, 54, 127–148. [Google Scholar] [CrossRef]
- Dong, X.X.; Wang, Y.; Qin, Z.H. Molecular mechanisms of excitotoxicity and their relevance to pathogenesis of neurodegenerative diseases. Acta Pharmacol. Sin. 2009, 30, 379–387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, H.H.; Wang, C.H.; Chen, H.C.; Li, I.H.; Cheng, C.Y.; Liu, R.S.; Huang, W.S.; Shiue, C.Y.; Ma, K.H. Investigating the effects of noise-induced hearing loss on serotonin transporters in rat brain using 4-[18F]-ADAM/small animal PET. NeuroImage 2013, 75, 262–269. [Google Scholar] [CrossRef]
- Gil-Loyzaga, P.; Bartolome, V.; Vicente-Torres, A.; Carricondo, F. Serotonergic innervation of the organ of Corti. Acta Oto-Laryngol. 2000, 120, 128–132. [Google Scholar]
- Gil Loyzaga, P.E. Innervation of the auditory receptor and cochlear nuclei. An. R. Acad. Nac. Med. 1997, 114, 1063–1086, Discussion 1086–1087. [Google Scholar]
- Cransac, H.; Cottet-Emard, J.M.; Hellstrom, S.; Peyrin, L. Specific sound-induced noradrenergic and serotonergic activation in central auditory structures. Hear. Res. 1998, 118, 151–156. [Google Scholar] [CrossRef]
- Hurley, L.M.; Thompson, A.M.; Pollak, G.D. Serotonin in the inferior colliculus. Hear. Res. 2002, 168, 1–11. [Google Scholar] [CrossRef]
- Kotak, V.C.; Fujisawa, S.; Lee, F.A.; Karthikeyan, O.; Aoki, C.; Sanes, D.H. Hearing loss raises excitability in the auditory cortex. J. Neurosci. 2005, 25, 3908–3918. [Google Scholar] [CrossRef]
- Li, L.; Yue, Q. Auditory gating processes and binaural inhibition in the inferior colliculus. Hear. Res. 2002, 168, 98–109. [Google Scholar] [CrossRef]
- Rao, D.; Basura, G.J.; Roche, J.; Daniels, S.; Mancilla, J.G.; Manis, P.B. Hearing loss alters serotonergic modulation of intrinsic excitability in auditory cortex. J. Neurophysiol. 2010, 104, 2693–2703. [Google Scholar] [CrossRef] [PubMed]
- Wutzler, A.; Winter, C.; Kitzrow, W.; Uhl, I.; Wolf, R.J.; Heinz, A.; Juckel, G. Loudness dependence of auditory evoked potentials as indicator of central serotonergic neurotransmission: Simultaneous electrophysiological recordings and in vivo microdialysis in the rat primary auditory cortex. Neuropsychopharmacology 2008, 33, 3176–3181. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Kotak, V.C.; Sanes, D.H. Conductive hearing loss disrupts synaptic and spike adaptation in developing auditory cortex. J. Neurosci. 2007, 27, 9417–9426. [Google Scholar] [CrossRef] [PubMed]
- Pehrson, A.L.; Sanchez, C. Serotonergic modulation of glutamate neurotransmission as a strategy for treating depression and cognitive dysfunction. CNS Spectr. 2014, 19, 121–133. [Google Scholar] [CrossRef] [PubMed]
- Cruz, O.L.; Kasse, C.A.; Sanchez, M.; Barbosa, F.; Barros, F.A. Serotonin reuptake inhibitors in auditory processing disorders in elderly patients: Preliminary results. Laryngoscope 2004, 114, 1656–1659. [Google Scholar] [CrossRef]
- Ignatowicz, E.; Baer-Dubowska, W. Resveratrol, a natural chemopreventive agent against degenerative diseases. Pol. J. Pharmacol. 2001, 53, 557–569. [Google Scholar]
- Hao, H.D.; He, L.R. Mechanisms of cardiovascular protection by resveratrol. J. Med. Food 2004, 7, 290–298. [Google Scholar] [CrossRef]
- Soleas, G.J.; Diamandis, E.P.; Goldberg, D.M. Resveratrol: A molecule whose time has come? And gone? Clin. Biochem. 1997, 30, 91–113. [Google Scholar] [CrossRef]
- Sonmez, U.; Sonmez, A.; Erbil, G.; Tekmen, I.; Baykara, B. Neuroprotective effects of resveratrol against traumatic brain injury in immature rats. Neurosci. Lett. 2007, 420, 133–137. [Google Scholar] [CrossRef] [PubMed]
- Yanez, M.; Fraiz, N.; Cano, E.; Orallo, F. Inhibitory effects of cis- and trans-resveratrol on noradrenaline and 5-hydroxytryptamine uptake and on monoamine oxidase activity. Biochem. Biophys. Res. Commun. 2006, 344, 688–695. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wang, Z.; You, W.; Zhang, X.; Li, S.; Barish, P.A.; Vernon, M.M.; Du, X.; Li, G.; Pan, J.; et al. Antidepressant-like effect of trans-resveratrol: Involvement of serotonin and noradrenaline system. Eur. Neuropsychopharmacol. 2010, 20, 405–413. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Wang, R.; Chen, C.; Du, X.; Ruan, L.; Sun, J.; Li, J.; Zhang, L.; O’Donnell, J.M.; Pan, J.; et al. Antidepressant-like effect of trans-resveratrol in chronic stress model: Behavioral and neurochemical evidences. J. Psychiatr. Res. 2013, 47, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Shih, J.H.; Ma, K.H.; Chen, C.F.; Cheng, C.Y.; Pao, L.H.; Weng, S.J.; Huang, Y.S.; Shiue, C.Y.; Yeh, M.K.; Li, I.H. Evaluation of brain SERT occupancy by resveratrol against MDMA-induced neurobiological and behavioral changes in rats: A 4-[(1)(8)F]-ADAM/small-animal PET study. Eur. Neuropsychopharmacol. 2016, 26, 92–104. [Google Scholar] [CrossRef] [PubMed]
- Vicente-Torres, M.A.; Davila, D.; Bartolome, M.V.; Carricondo, F.; Gil-Loyzaga, P. Biochemical evidence for the presence of serotonin transporters in the rat cochlea. Hear. Res. 2003, 182, 43–47. [Google Scholar] [CrossRef]
- Gil-Loyzaga, P.; Vicente-Torres, M.A.; Garcia-Bonacho, M.; Esquifino, A. Presence of catecholamines and serotonin in the rat vestibule. Brain Res. 1997, 746, 265–268. [Google Scholar] [CrossRef]
- Mroz, E.A.; Sewell, W.F. Pharmacological alterations of the activity of afferent fibers innervating hair cells. Hear. Res. 1989, 38, 141–162. [Google Scholar] [CrossRef]
- Hurley, L.M.; Pollak, G.D. Serotonin differentially modulates responses to tones and frequency-modulated sweeps in the inferior colliculus. J. Neurosci. 1999, 19, 8071–8082. [Google Scholar] [CrossRef]
- Ahveninen, J.; Jaaskelainen, I.P.; Pennanen, S.; Liesivuori, J.; Ilmoniemi, R.J.; Kahkonen, S. Auditory selective attention modulated by tryptophan depletion in humans. Neurosci. Lett. 2003, 340, 181–184. [Google Scholar] [CrossRef] [Green Version]
- Kahkonen, S.; Ahveninen, J.; Pennanen, S.; Liesivuori, J.; Ilmoniemi, R.J.; Jaaskelainen, I.P. Serotonin modulates early cortical auditory processing in healthy subjects: Evidence from MEG with acute tryptophan depletion. Neuropsychopharmacology 2002, 27, 862–888. [Google Scholar] [CrossRef]
- Thompson, A.M.; Thompson, G.C. Serotonin projection patterns to the cochlear nucleus. Brain Res. 2001, 907, 195–207. [Google Scholar] [CrossRef]
- Weisz, C.; Glowatzki, E.; Fuchs, P. The postsynaptic function of type II cochlear afferents. Nature 2009, 461, 1126–1129. [Google Scholar] [CrossRef] [PubMed]
- Liberman, M.C.; Kujawa, S.G. Cochlear synaptopathy in acquired sensorineural hearing loss: Manifestations and mechanisms. Hear. Res. 2017, 349, 138–147. [Google Scholar] [CrossRef]
- Wu, J.S.; Vyas, P.; Glowatzki, E.; Fuchs, P.A. Opposing expression gradients of calcitonin-related polypeptide alpha (Calca/Cgrpalpha) and tyrosine hydroxylase (Th) in type II afferent neurons of the mouse cochlea. J. Comp. Neurol. 2018, 526, 425–438. [Google Scholar] [CrossRef] [PubMed]
- Vyas, P.; Wu, J.S.; Zimmerman, A.; Fuchs, P.; Glowatzki, E. Tyrosine Hydroxylase Expression in Type II Cochlear Afferents in Mice. J. Assoc. Res. Otolaryngol. 2017, 18, 139–151. [Google Scholar] [CrossRef] [PubMed]
- Kujawa, S.G.; Liberman, M.C. Synaptopathy in the noise-exposed and aging cochlea: Primary neural degeneration in acquired sensorineural hearing loss. Hear. Res. 2015, 330, 191–199. [Google Scholar] [CrossRef] [Green Version]
- Pujol, R.; Puel, J.L. Excitotoxicity, synaptic repair, and functional recovery in the mammalian cochlea: A review of recent findings. Ann. N. Y. Acad. Sci. 1999, 884, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Zhang, C.; Chen, A.; Liu, W.; Li, J.; Sun, Q.; Wang, H. Protective Effect of Edaravone on Glutamate-Induced Neurotoxicity in Spiral Ganglion Neurons. Neural Plast. 2016, 2016, 4034218. [Google Scholar] [CrossRef] [PubMed]
- Lafon-Cazal, M.; Pietri, S.; Culcasi, M.; Bockaert, J. NMDA-dependent superoxide production and neurotoxicity. Nature 1993, 364, 535–537. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, K.; Brask, D.; Knudsen, G.M.; Aznar, S. Immunodetection of the serotonin transporter protein is a more valid marker for serotonergic fibers than serotonin. Synapse 2006, 59, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Invernizzi, R.; Bramante, M.; Samanin, R. Extracellular concentrations of serotonin in the dorsal hippocampus after acute and chronic treatment with citalopram. Brain Res. 1995, 696, 62–66. [Google Scholar] [CrossRef]
- Quincozes-Santos, A.; Bobermin, L.D.; Tramontina, A.C.; Wartchow, K.M.; Tagliari, B.; Souza, D.O.; Wyse, A.T.; Goncalves, C.A. Oxidative stress mediated by NMDA, AMPA/KA channels in acute hippocampal slices: Neuroprotective effect of resveratrol. Toxicol. In Vitro 2014, 28, 544–551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de la Lastra, C.A.; Villegas, I. Resveratrol as an antioxidant and pro-oxidant agent: Mechanisms and clinical implications. Biochem. Soc. Trans. 2007, 35, 1156–1160. [Google Scholar] [CrossRef] [PubMed]
- Gülçin, İ. Antioxidant properties of resveratrol: A structure–activity insight. Innov. Food Sci. Emerg. Technol. 2010, 11, 210–218. [Google Scholar] [CrossRef]
- Rauschecker, J.P.; Leaver, A.M.; Muhlau, M. Tuning out the noise: Limbic-auditory interactions in tinnitus. Neuron 2010, 66, 819–826. [Google Scholar] [CrossRef] [Green Version]
- Ramamoorthy, S.; Shippenberg, T.S.; Jayanthi, L.D. Regulation of monoamine transporters: Role of transporter phosphorylation. Pharmacol. Ther. 2011, 129, 220–238. [Google Scholar] [CrossRef]
- Marriage, J.; Barnes, N.M. Is central hyperacusis a symptom of 5-hydroxytryptamine (5-HT) dysfunction? J. Laryngol. Otol. 1995, 109, 915–921. [Google Scholar] [CrossRef] [PubMed]
- Papesh, M.A.; Hurley, L.M. Plasticity of serotonergic innervation of the inferior colliculus in mice following acoustic trauma. Hear. Res. 2012, 283, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Deniz, M.; Bayazit, Y.A.; Celenk, F.; Karabulut, H.; Yilmaz, A.; Gunduz, B.; Saridogan, C.; Dagli, M.; Erdal, E.; Menevse, A. Significance of serotonin transporter gene polymorphism in tinnitus. Otol. Neurotol. 2010, 31, 19–24. [Google Scholar] [CrossRef]
- Wu, W.L.; Wang, C.H.; Huang, E.Y.; Chen, C.C. Asic3(-/-) female mice with hearing deficit affects social development of pups. PLoS ONE 2009, 4, e6508. [Google Scholar] [CrossRef] [PubMed]
- Viberg, A.; Canlon, B. The guide to plotting a cochleogram. Hear. Res. 2004, 197, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Li, I.H.; Huang, W.S.; Shiue, C.Y.; Huang, Y.Y.; Liu, R.S.; Chyueh, S.C.; Hu, S.H.; Liao, M.H.; Shen, L.H.; Liu, J.C.; et al. Study on the neuroprotective effect of fluoxetine against MDMA-induced neurotoxicity on the serotonin transporter in rat brain using micro-PET. NeuroImage 2010, 49, 1259–1270. [Google Scholar] [CrossRef]
- Ma, K.H.; Huang, W.S.; Kuo, Y.Y.; Peng, C.J.; Liou, N.H.; Liu, R.S.; Hwang, J.J.; Liu, J.C.; Chen, H.J.; Shiue, C.Y. Validation of 4-[18F]-ADAM as a SERT imaging agent using micro-PET and autoradiography. NeuroImage 2009, 45, 687–693. [Google Scholar] [CrossRef] [PubMed]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates, 6th ed.; Academic Press/Elsevier: Amsterdam, The Netherlands; Boston, MA, USA, 2007. [Google Scholar]
- Glavaski-Joksimovic, A.; Virag, T.; Chang, Q.A.; West, N.C.; Mangatu, T.A.; McGrogan, M.P.; Dugich-Djordjevic, M.; Bohn, M.C. Reversal of dopaminergic degeneration in a parkinsonian rat following micrografting of human bone marrow-derived neural progenitors. Cell Transplant. 2009, 18, 801–814. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, Y.; Sawada, T.; Blunt, S.; Jenner, P.; Marsden, C.D. Effects of 6-hydroxydopamine lesions of the nigrostriatal pathway on striatal serotonin innervation in adult rats. Brain Res. 1991, 562, 301–305. [Google Scholar] [CrossRef]
- Zhou, F.C.; Chiang, Y.H.; Wang, Y. Constructing a new nigrostriatal pathway in the Parkinsonian model with bridged neural transplantation in substantia nigra. J. Neurosci. 1996, 16, 6965–6974. [Google Scholar] [CrossRef] [PubMed]
- Weng, S.J.; Shiue, C.Y.; Huang, W.S.; Cheng, C.Y.; Huang, S.Y.; Li, I.H.; Tao, C.C.; Chou, T.K.; Liao, M.H.; Chang, Y.P.; et al. PET imaging of serotonin transporters with 4-[18F]-ADAM in a Parkinsonian rat model. Cell Transplant. 2013, 22, 1295–1305. [Google Scholar] [CrossRef] [PubMed]
- Noristani, H.N.; Olabarria, M.; Verkhratsky, A.; Rodriguez, J.J. Serotonin fibre sprouting and increase in serotonin transporter immunoreactivity in the CA1 area of hippocampus in a triple transgenic mouse model of Alzheimer’s disease. Eur. J. Neurosci. 2010, 32, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Joyce, J.N.; Woolsey, C.; Ryoo, H.; Borwege, S.; Hagner, D. Low dose pramipexole is neuroprotective in the MPTP mouse model of Parkinson’s disease, and downregulates the dopamine transporter via the D3 receptor. BMC Biol. 2004, 2, 22. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, I.-H.; Shih, J.-H.; Jhao, Y.-T.; Chen, H.-C.; Chiu, C.-H.; Chen, C.-F.F.; Huang, Y.-S.; Shiue, C.-Y.; Ma, K.-H. Regulation of Noise-Induced Loss of Serotonin Transporters with Resveratrol in a Rat Model Using 4-[18F]-ADAM/Small-Animal Positron Emission Tomography. Molecules 2019, 24, 1344. https://doi.org/10.3390/molecules24071344
Li I-H, Shih J-H, Jhao Y-T, Chen H-C, Chiu C-H, Chen C-FF, Huang Y-S, Shiue C-Y, Ma K-H. Regulation of Noise-Induced Loss of Serotonin Transporters with Resveratrol in a Rat Model Using 4-[18F]-ADAM/Small-Animal Positron Emission Tomography. Molecules. 2019; 24(7):1344. https://doi.org/10.3390/molecules24071344
Chicago/Turabian StyleLi, I-Hsun, Jui-Hu Shih, Yun-Tin Jhao, Hsin-Chien Chen, Chuang-Hsin Chiu, Chien-Fu F. Chen, Yuahn-Sieh Huang, Chyng-Yann Shiue, and Kuo-Hsing Ma. 2019. "Regulation of Noise-Induced Loss of Serotonin Transporters with Resveratrol in a Rat Model Using 4-[18F]-ADAM/Small-Animal Positron Emission Tomography" Molecules 24, no. 7: 1344. https://doi.org/10.3390/molecules24071344





