Molecular Simulation of the Adsorption and Diffusion in Cylindrical Nanopores: Effect of Shape and Fluid–Solid Interactions
Abstract
:1. Introduction
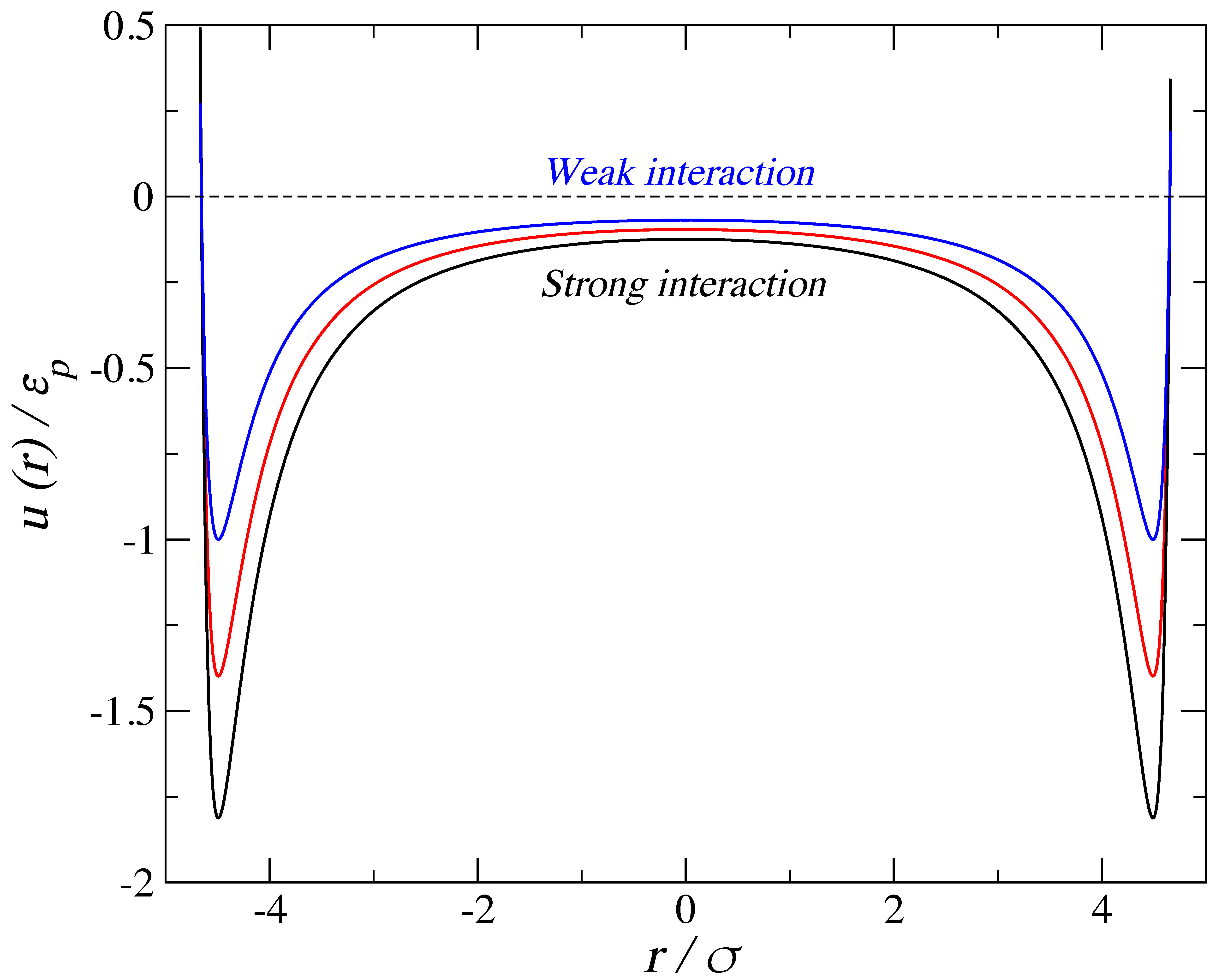
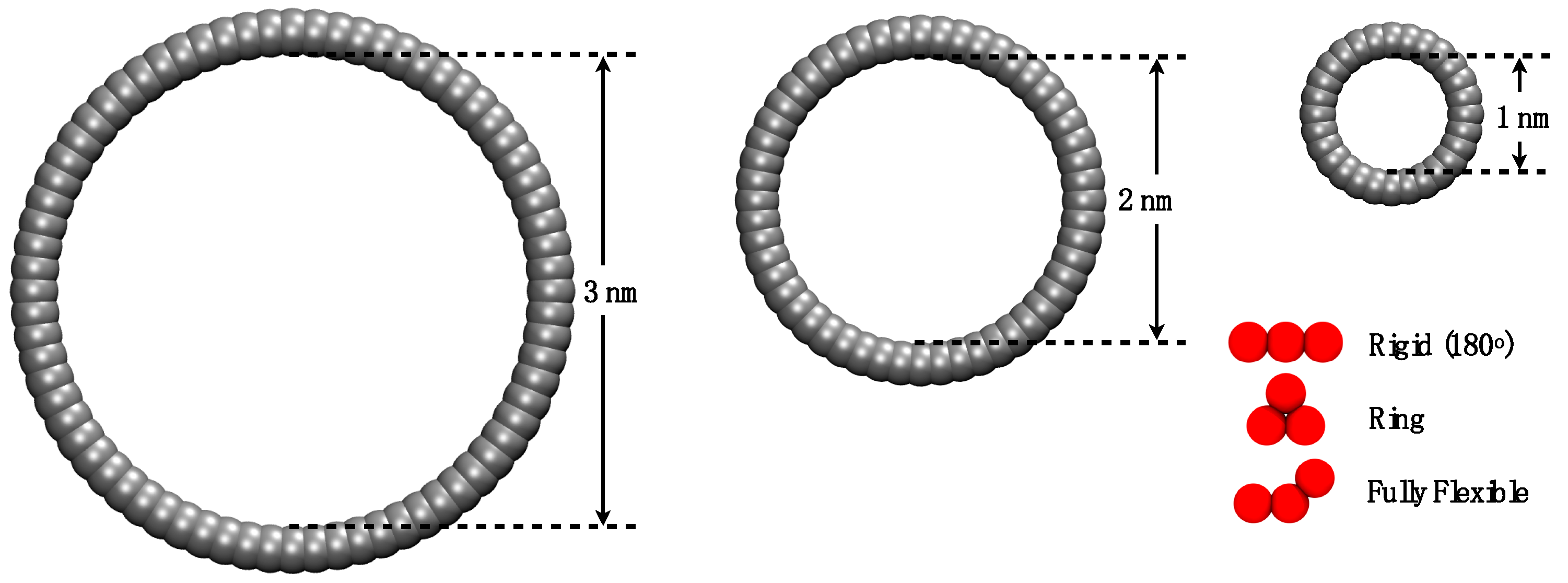
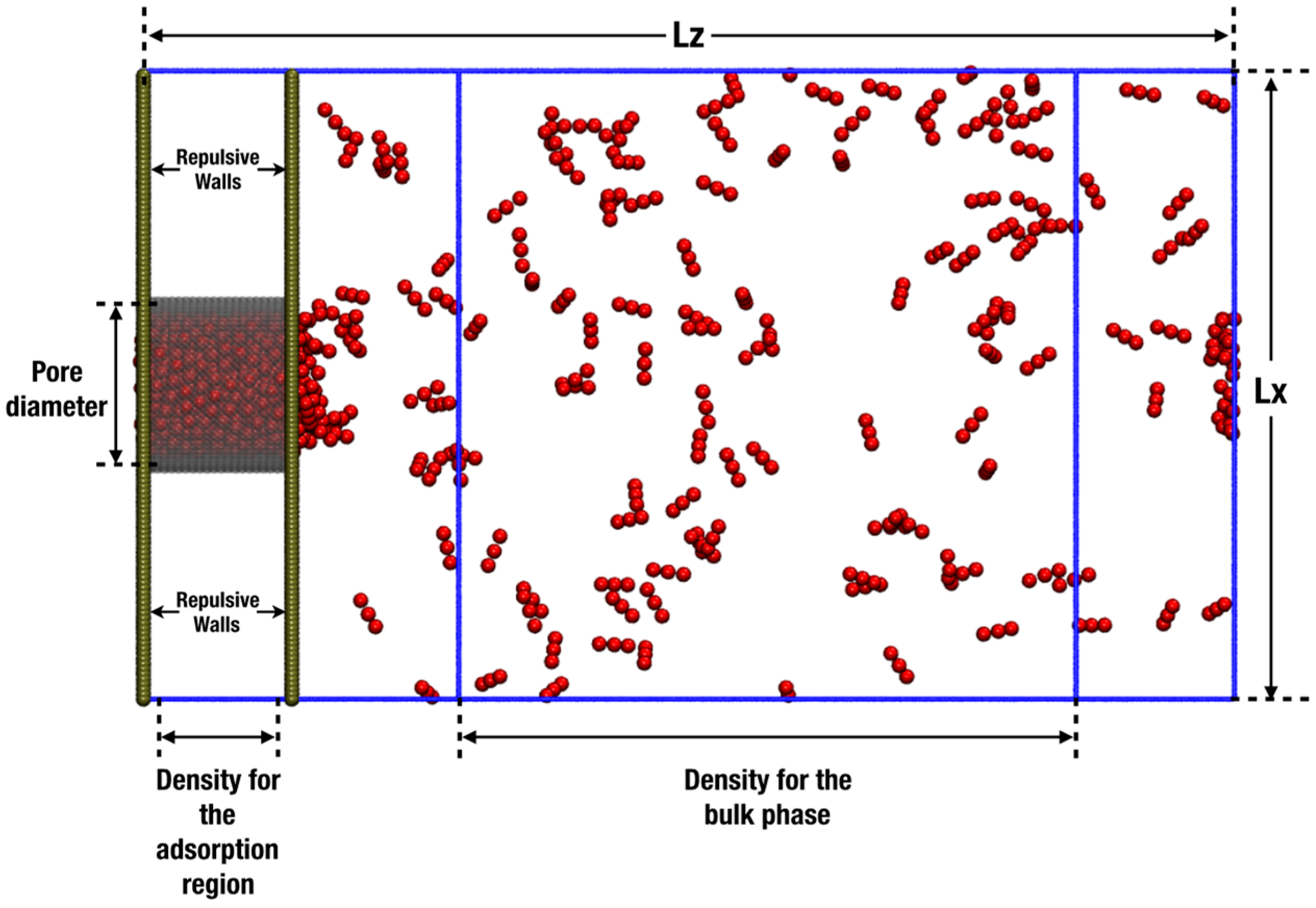
2. Simulation Details
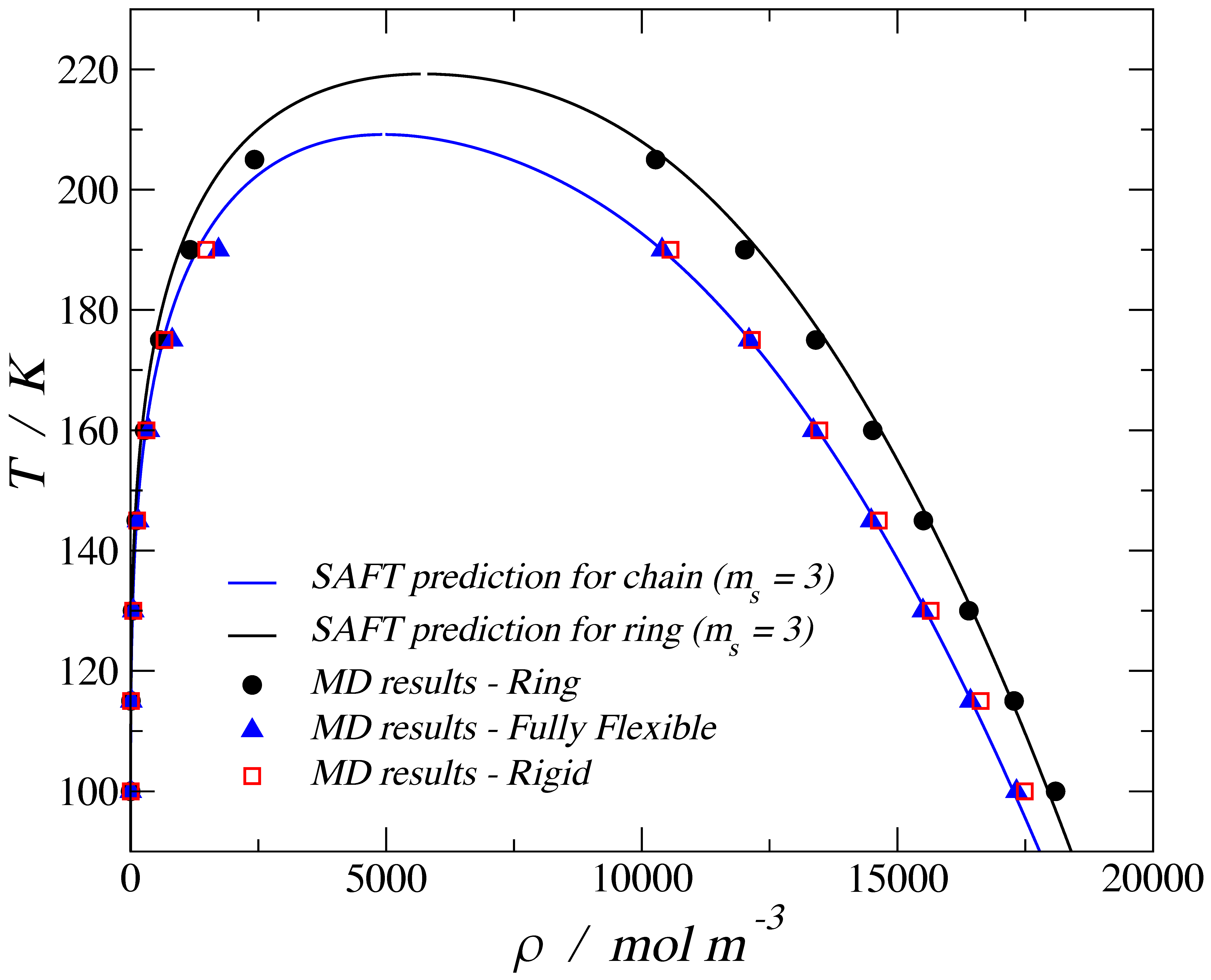
3. Results
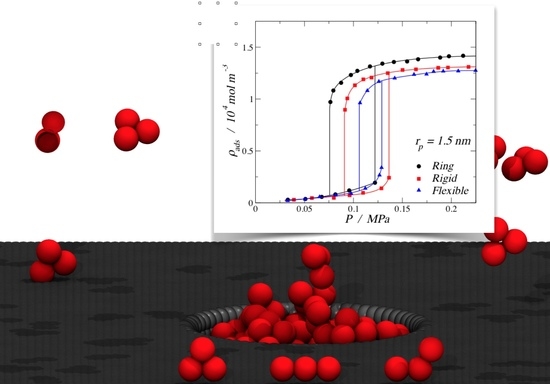
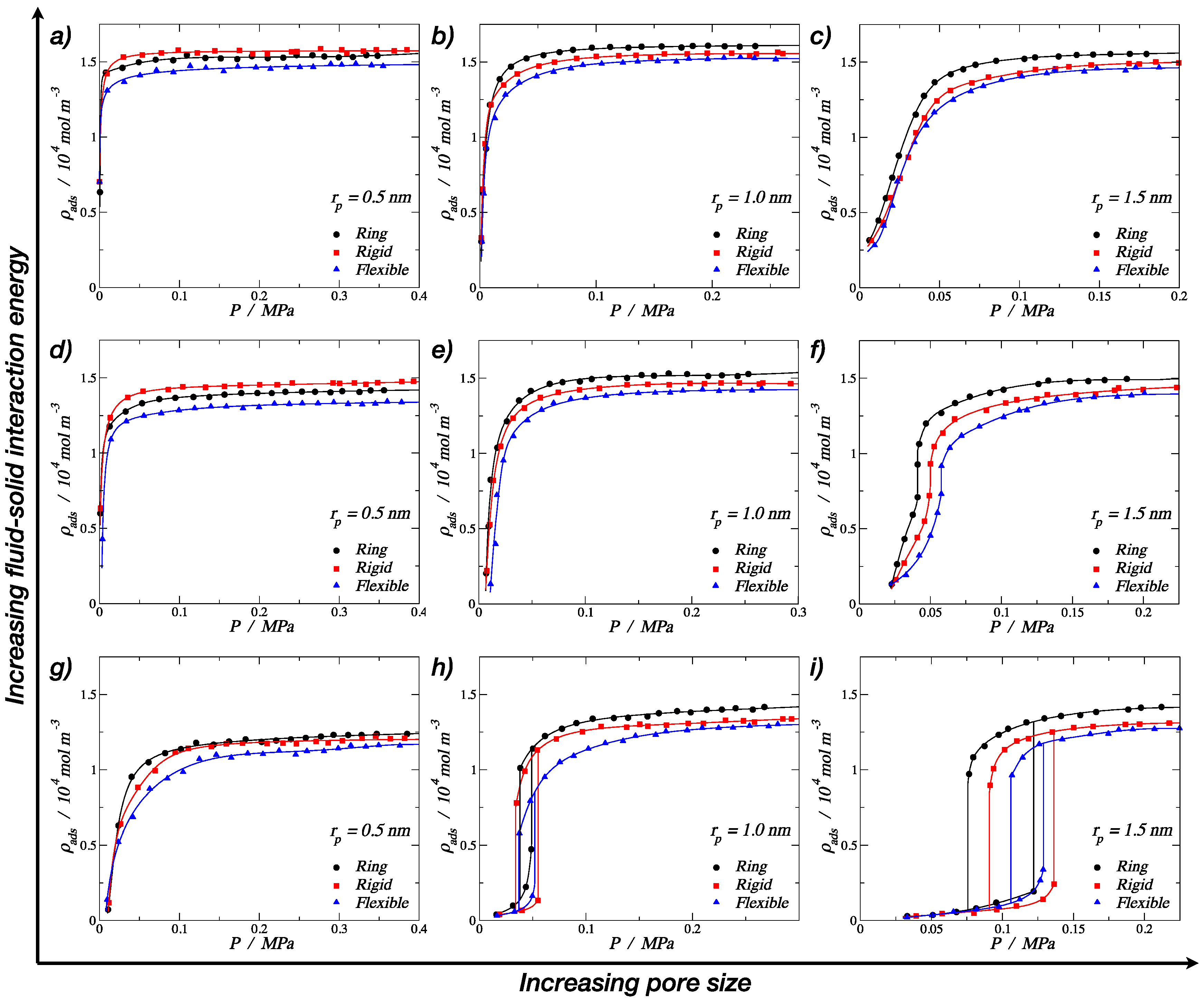
3.1. Adsorption of Trimers in Cylindrical Pores
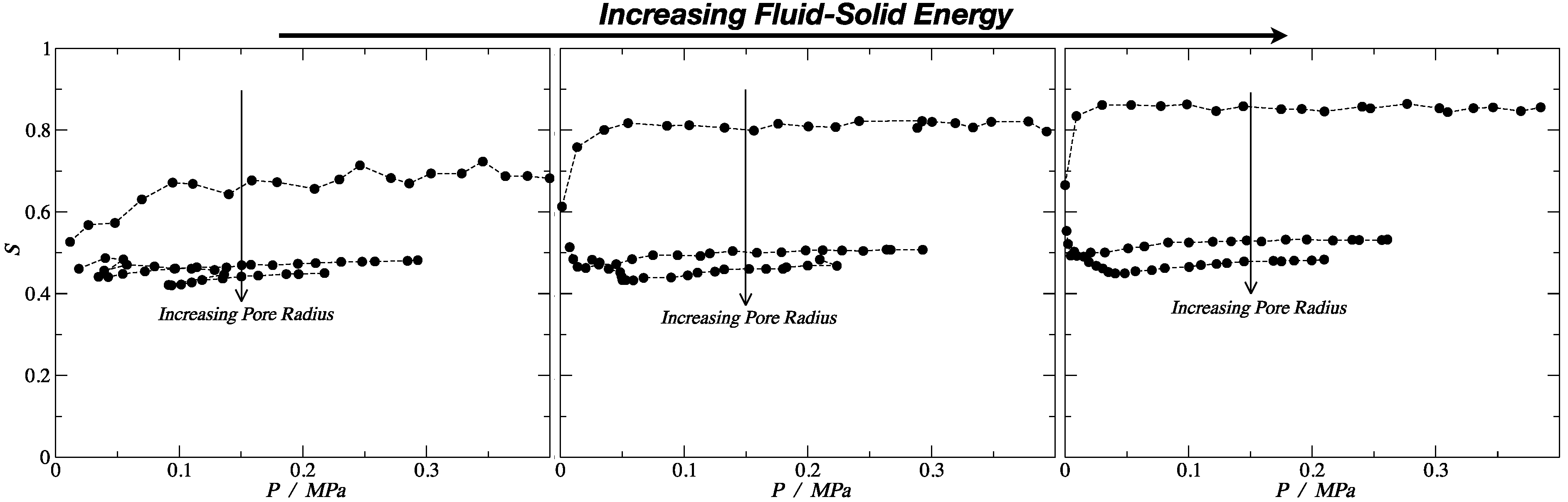
3.2. Nematic Order Parameter
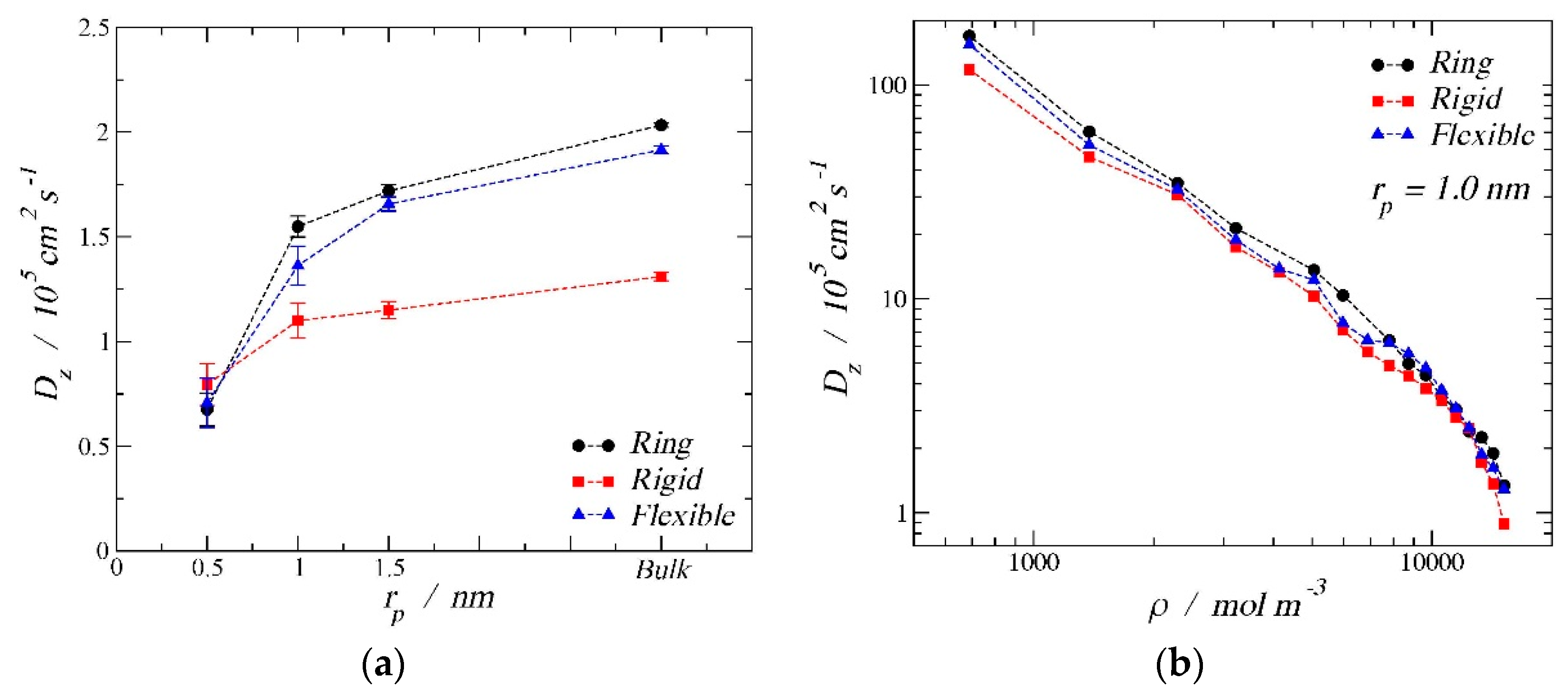
3.3. Diffusion Coefficients
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Urita, K.; Shiga, Y.; Fujimori, T.; liyama, T.; Hattori, Y.; Kanoh, H.; Ohba, T.; Tanaka, H.; Yudasaka, M.; lijima, S.; et al. Confinement in Carbon Nanospace-Induced Production of KI Nanocrystals of High-Pressure Phase. J. Am. Chem. Soc. 2011, 133, 10344–10347. [Google Scholar] [CrossRef] [PubMed]
- Nair, R.; Wu, H.; Jayaram, P.; Grigorieva, I. Unimpeded Permeation of Water Through Helium-Leak–Tight Graphene-Based Membranes. Science 2012, 335, 442–444. [Google Scholar] [CrossRef] [PubMed]
- Höfling, F.; Franosch, T. Anomalous transport in the crowded world of biological cells. Rep. Prog. Phys. 2013, 76, 046602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alba-Simionesco, C.; Coasne, B.; Dosseh, G.; Dudziak, G.; Gubbins, K.E.; Radhakrishnan, R.; Sliwinska-Bartkowiak, M. Effects of confinement on freezing and melting. J. Phys-Condens. Mat. 2006, 18, R15–R68. [Google Scholar] [CrossRef]
- Valiullin, R.; Kärger, J. Confined Fluids: NMR Perspectives on Confinements and on Fluid Dynamics. In Diffusion NMR of Confined Systems; Valiullin, R., Ed.; Royal Society of Chemistry: Cambridge, MA, USA, 2016; pp. 390–434. [Google Scholar]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Oliver, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 89, 1051–1069. [Google Scholar] [CrossRef]
- Monson, P.A. Understanding adsorption/desorption hysteresis for fluids in mesoporous materials using simple molecular models and classical density functional theory. Micropor. Mesopor. Mat. 2012, 160, 47–66. [Google Scholar] [CrossRef]
- Sarkisov, L.; Monson, P.A. Modeling of Adsorption and Desorption in Pores of Simple Geometry using Molecular Dynamics. Langmuir 2001, 17, 7600–7604. [Google Scholar] [CrossRef]
- Horikawa, T.; Do, D.D.; Nicholson, D. Capillary condensation of adsorbates in porous materials. Advan. Coll. Interf. Sci. 2011, 169, 40–58. [Google Scholar] [CrossRef]
- Kruk, M.; Jaroniec, M.; Sayari, A. Adsorption study of surface and structural properties of MCM-41 materials of different pore sizes. J. Phys. Chem. B 1997, 101, 583–589. [Google Scholar] [CrossRef]
- Thommes, M.; Cychosz, K.A. Physical adsorption characterization of nanoporous materials: progress and challenges. Adsorption 2014, 20, 233–250. [Google Scholar] [CrossRef]
- Smit, B.; Maesen, T.L.M. Molecular simulations of zeolites: Adsorption, Diffusion, and Shape Selectivity. Chem. Rev. 2008, 108, 4125–4184. [Google Scholar] [CrossRef] [PubMed]
- Smit, B.; Siepmann, J.I. Simulating the Adsorption of Alkanes in Zeolites. Science 1994, 264, 1118–1120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schenk, M.; Smit, B.; Vlugt, T.J.H.; Maesen, T.L.M. Shape Selectivity in Hydrocarbon Convertion. Angew. Chemie Inter. Ed. 2001, 40, 736–739. [Google Scholar] [CrossRef]
- Jiang, J.; Sandler, S.I. Monte Carlo simulation for the Adsorption and Separation of linear and branched alkanes in IRMOF-1. Langmuir 2006, 22, 5702–5707. [Google Scholar] [CrossRef] [PubMed]
- Sarkisov, L.; Düren, T.; Snurr, R.Q. Molecular modelling of adsorption in novel nanoporous metal-organic materials. Mol. Phys. 2006, 102, 211–221. [Google Scholar] [CrossRef]
- Kärger, J.; Ruthven, D.M. Diffusion in Zeolites and Other Microporous Materials; John Wiley and Sons: New York, NY, USA, 1992. [Google Scholar]
- Kärger, J.; Freude, D.; Haase, J. Diffusion in Nanoporous Materials: Novel Insights by Combining MAS and PFG NMR. Processes 2018, 6, 147. [Google Scholar] [CrossRef]
- Krishna, R. Diffusion in porous crystalline materials. Chem. Soc. Rev. 2012, 41, 3099. [Google Scholar] [CrossRef] [PubMed]
- Van Baten, J.M.; Krishna, R. Entropy effects in adsorption and diffusion of alkane isomers in mordenite: An investigation using CBMC and MD simulations. Micropor. Mesopor. Mat. 2005, 84, 179–191. [Google Scholar] [CrossRef]
- Johnson, J.K.; Müller, E.A.; Gubbins, K.E. Equation of state for Lennard-Jones chains. J. Phys. Chem. 1994, 98, 6413–6419. [Google Scholar] [CrossRef]
- Galindo, A.; Vega, C.; Sanz, E.; MacDowell, L.G.; de Miguel, E.; Blas, F.J. Computer simulation study of the global phase behavior of linear rigid Lennard-Jones chain molecules: Comparison with flexible models. J. Chem. Phys. 2004, 120, 3957–3968. [Google Scholar] [CrossRef]
- Van Westen, T.; Oyarzún, B.; Vlugt, T.J.H.; Gross, J. An analytical equation of state for describing isotropic-nematic phase equilibria of Lennard-Jones chain fluids with variable degree of molecular flexibility. J. Chem. Phys. 2015, 142, 244903. [Google Scholar] [CrossRef] [PubMed]
- Müller, E.A.; Mejía, A. Extension of the SAFT-VR Mie EoS to model homonuclear rings and its parametrization based on the principle of corresponding states. Langmuir 2017, 33, 11518–11529. [Google Scholar] [CrossRef] [PubMed]
- Lafitte, T.; Apostolakou, A.; Avendaño, C.; Galindo, A.; Adjiman, C.S.; Müller, E.A.; Jackson, G. Accurate statistical associating fluid theory for chain molecules formed from Mie segments. J. Chem. Phys. 2013, 139, 154504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, S.K.; Sinha, A.; Deo, G.; Singh, J.K. Vapor-Liquid phase coexistence, critical properties, and surface tension of confined alkanes. J Phys. Chem. C 2009, 113, 7170–7180. [Google Scholar] [CrossRef]
- Müller, E.A.; Jackson, G. Force-Field Parameters from the SAFT-γ Equation of State for Use in Coarse-Grained Molecular Simulations. Annu. Rev. Chem. Biomol. Eng. 2014, 5, 405–427. [Google Scholar] [CrossRef] [PubMed]
- Ervik, Å.; Mejía, A.; Müller, E.A. Bottled SAFT: A Web App Providing SAFT-γ Mie Force Field Parameters for Thousands of Molecular Fluids. J. Chem. Inf. Model. 2016, 56, 1609–1614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herdes, C.; Totton, T.S.; Müller, E.A. Coarse grained force field for the molecular simulation of natural gases and condensates. Fluid Phase Equilibr. 2015, 406, 91–100. [Google Scholar] [CrossRef] [Green Version]
- Jaeger, F.; Matar, O.K.; Müller, E.A. Bulk viscosity of molecular fluids. J. Chem. Phys. 2018, 148, 174504. [Google Scholar] [CrossRef]
- Herdes, C.; Ervik, Å.; Mejía, A.; Müller, E.A. Prediction of the water/oil interfacial tension from molecular simulations using the coarse-grained SAFT-γ Mie force field. Fluid Phase Equilib. 2018, 476, 9–15. [Google Scholar] [CrossRef]
- Herdes, C.; Petit, C.; Mejía, A.; Müller, E.A. Combined Experimental, Theoretical, and Molecular Simulation Approach for the Description of the Fluid-Phase Behavior of Hydrocarbon Mixtures within Shale Rocks. Energ. Fuel. 2018, 32, 5750–5762. [Google Scholar] [CrossRef]
- Barreda, D.; Pérez-Mas, A.M.; Silvestre-Albero, A.; Casco, M.E.; Rudić, S.; Herdes, C.; Müller, E.A.; Blanco, C.; Santamaria, R.; Silvestre-Albero, J.; et al. Unusual flexibility of mesophase pitch-derived carbon materials: An approach to the synthesis of graphene. Carbon 2017, 115, 1–7. [Google Scholar] [CrossRef]
- Lafitte, T.; Pérez-Mas, A.M.; Silvestre-Albero, A.; Casco, M.E.; Rudić, S.; Herdes, C.; Muller, E.A.; Blanco, C.; Santamaria, R.; Silvestre-Albero, J.; et al. SAFT-γ force field for the simulation of molecular fluids: 3. Coarse-grained models of benzene and hetero-group models of n−decylbenzene. Mol. Phy. 2012, 110, 1189–1203. [Google Scholar] [CrossRef]
- Krishna, R. Describing the Diffusion of Guest Molecules Inside Porous Structures. J. Phys. Chem. C 2009, 113, 19756–19781. [Google Scholar] [CrossRef]
- Jover, J.; Haslam, A.J.; Galindo, A.; Jackson, G.; Müller, E.A. Pseudo Hard-Sphere potential for use in continuous molecular-dynamics simulation of spherical and chain molecules. J. Chem. Phys. 2012, 137, 144505. [Google Scholar] [CrossRef] [PubMed]
- Van der Spoel, D.; Lindahl, E.; Hess, B.; Groenhof, G.; Mark, A.E.; Berendsen, H.J. GROMACS: Fast, flexible, and free. J. Comput. Chem. 2005, 26, 1701–1718. [Google Scholar] [CrossRef] [PubMed]
- Rowlinson, J.S.; Widom, B. Molecular Theory of Capillarity; Clarendon Press: Oxford, UK, 1982. [Google Scholar]
- Low, R.J. Measuring order and biaxiality. Eur. J. Phys. 2002, 23, 111–117. [Google Scholar] [CrossRef] [Green Version]
- Wijmans, J.G.; Baker, R.W. The solution-diffusion model: A review. J. Memb. Sci. 1995, 107, 1–21. [Google Scholar] [CrossRef]
Sample Availability: Not available. |
| σ [nm] | ε/kb [K] | λr | λa | |
|---|---|---|---|---|
| Ccylinder,1-LJfluid | 0.25 | 25.00 | 11.0 | 4.0 |
| Ccylinder,2-LJfluid | 0.25 | 19.30 | 11.0 | 4.0 |
| Ccylinder,3-LJfluid | 0.25 | 13.80 | 11.0 | 4.0 |
| LJfluid | 0.30 | 100.00 | 12.0 | 6.0 |
| εfluid-wall/kb[K] | 13.80 | 19.30 | 25.00 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| rp [nm] | 0.5 | 1.0 | 1.5 | 0.5 | 1.0 | 1.5 | 0.5 | 1.0 | 1.5 |
| S | 0.69 | 0.477 | 0.45 | 0.815 | 0.506 | 0.47 | 0.86 | 0.53 | 0.48 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cárdenas, H.; Müller, E.A. Molecular Simulation of the Adsorption and Diffusion in Cylindrical Nanopores: Effect of Shape and Fluid–Solid Interactions. Molecules 2019, 24, 608. https://doi.org/10.3390/molecules24030608
Cárdenas H, Müller EA. Molecular Simulation of the Adsorption and Diffusion in Cylindrical Nanopores: Effect of Shape and Fluid–Solid Interactions. Molecules. 2019; 24(3):608. https://doi.org/10.3390/molecules24030608
Chicago/Turabian StyleCárdenas, Harry, and Erich A. Müller. 2019. "Molecular Simulation of the Adsorption and Diffusion in Cylindrical Nanopores: Effect of Shape and Fluid–Solid Interactions" Molecules 24, no. 3: 608. https://doi.org/10.3390/molecules24030608